ABSTRACT
Eighty-two nest and roosting cavities of Syrian Woodpeckers Dendrocopos syriacus were documented over a period of 25 years (1994–2018). Fourteen different tree species were used, the majority being non-native in Hungary. Cavities were found in both main trunks and in larger limbs. The median cavity height was 3.0 m and ranged from 1 to 7 m. The cavity entrance orientation was non-random with a preference for a south-southeast orientation.
The Syrian Woodpecker Dendrocopos syriacus is distributed within the Palearctic from Asia Minor, the Middle East, the Caucasus, the Balkans, Central Europe as far west as Austria and the Czech Republic, and as far north as Poland and Russia (Gorman Citation2004). In the twentieth century, the species expanded its range significantly into Europe, but in recent years range expansion in a westerly direction seems to have slowed but steadily continues towards the north and east (Zavialov et al. Citation2008). In Central Europe, including Hungary, Syrian Woodpeckers inhabit drier, open, fragmented and lightly wooded deciduous stands, rarely using conifers or venturing into dense forests of any type (Gorman Citation2004). It is the most synanthropic member of the family across its range, typically found in ‘secondary’ anthropogenic wooded habitats in urban, suburban and village environments, such as parks, gardens, orchards, groves, vineyards, allotments, school grounds and cemeteries. In rural areas, it inhabits woodland edges, copses and roadside lines of trees (Glutz von Blotzheim & Bauer Citation1994, Winkler et al. Citation1995, Gorman, Citation2004). The global population of the Syrian Woodpecker is not known precisely but is considered stable overall. About 90% of the population is estimated to be in Europe and its International Union for Conservation of Nature (IUCN) Red List category is Least Concern (BirdLife International Citation2019).
Very few data exist on the orientation of Syrian Woodpecker nest cavities. Previous studies on the species have focused mainly on its relationship to the Great Spotted Woodpecker Dendrocopos major, breeding biology, range expansion and habitat preferences (Al-Safadi Citation2004, Aghanajafizadeh et al. Citation2011, Michalczuk & Michalczuk Citation2016b, Figarski & Kajtoch Citation2018). A study in southwest Iran that compared Syrian Woodpecker and Middle Spotted Woodpecker Dendrocoptes medius nest holes, found no preference in hole entrance direction (Shafaeipour et al. Citation2019). The main aims of this study were to characterize cavity trees used by Syrian Woodpeckers including the tree species, its state of decay, the cavity locations (whether they were on trunks or limbs), cavity height and the position and orientation of cavity entrances. Tree diameter was not measured. This study seeks to fill in a gap in the published knowledge on Syrian Woodpecker cavity orientation.
In Hungary, the similar-sized Great Spotted Woodpecker Dendrocopos major, which has a comparable ecology (Glutz von Blotzheim & Bauer Citation1994), is often sympatric with the Syrian Woodpecker (Gorman Citation2014). This presented a potential pitfall as it was not straight-forward to establish whether cavities found were created by Syrian Woodpeckers or by their relative, or indeed any other woodpecker. Therefore, only those cavities which Syrian Woodpeckers were observed making, or those in which the species was seen nesting or roosting, were documented. As the study was conducted over 25 years and across a wide geographic area it is very unlikely that cavities were created by the same individuals over those years and thus the individual preferences of a few birds probably do not bias the patterns presented here. Although the species was occasionally observed on wooden utility-poles with cavities, these cavities were not included in the study as the possibility that they had been excavated by Great Spotted Woodpeckers could not be excluded.
From 1994 to 2018 any cavity being used (whether for breeding, roosting or both) by Syrian Woodpeckers found during fieldwork in several counties in western, central and northeast Hungary was characterized. Data were collected in the months of March, April and May as this is the period when cavity excavation is most prevalent. Most of the cavities were being used for nesting (60 out of 82; 73.17%). Ultimate use of the remaining cavities was either as roosts or unclear. I recorded the tree species, whether the tree was alive or dead and the state of decay. For each cavity, I recorded the height of the entrance above the ground estimated (using trigonometry), its orientation using a compass and whether it was in the main trunk or a limb. Cavities were searched for in all wooded habitats, but only found in urban and suburban areas. This was not surprising as the species is highly synanthropic. Cavities were found by visiting and visually searching known areas of occurrence and by listening for calls and drumming.
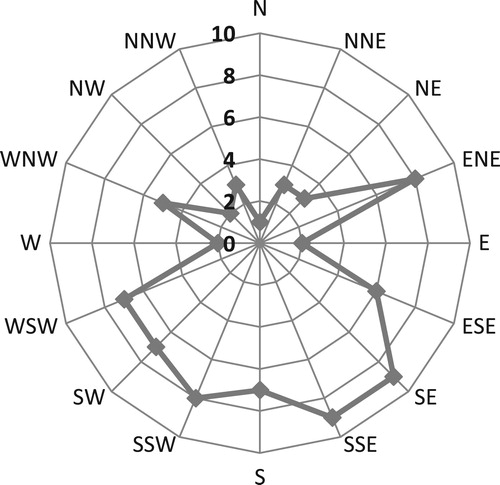
Cavity orientation was recorded with a compass using 16 standard points (N, NNE, NE, ENE, etc.). I tested for non-random orientation using a Rayleigh test (Pewsey et al. Citation2013) as implemented in the package ‘circular’ in R (Lund & Agostinelli Citation2011, R Development Core Team Citation2015). This provides a statistic, r, with value between 0 and 1, where zero indicates a random distribution and 1 represents perfect alignment to one direction. The package also provides a mean orientation.
This paper summarizes some characteristics of 82 Syrian Woodpecker cavities in Hungary over a period of 24 years (online Table S1). All cavities documented were newly excavated: none were from previous years that were being reused. New cavities can be recognized by their entrances having clean edges with no renewed tree growth, and light-coloured wood (Gorman Citation1995). Cavity trees were in open wooded areas in urban and suburban areas and rural villages. Cavities were found in 14 different tree species (number of times used in brackets): False Acacia Robinia pseudoacacia (25), Walnut Juglans regia (13), Poplar hybrid Populus x euramericana (8), Sour Cherry Prunus cerasus (7), White Willow Salix alba (7), Apple Malus x domestica (6), Plum Prunus spp. (4), Horse Chestnut Aesculus hippocastanum (3), Pear Pyrus c. communis (3), Plane Platanus spp. (2), Japanese Pagoda Tree Sophora japonica (1), Mulberry Morus spp.(1), Small-leaved Lime Tilia cordata (1), Sycamore Acer pseudoplatanus (1). All cavities were in broadleaved trees with none in coniferous species. There was a clear dominance by one tree species, False Acacia Robinia pseudoacacia (also known as Black Locust). False Acacia accounted for 25 (30.5%) of the 82 cavities documented. The second most frequently used tree was Walnut Juglans regia, another introduced and cultivated tree, which was used 13 times (15.9%). Of the 14 tree species used only three are indigenous to Hungary (White Willow, Small-leaved Lime, Sycamore), with the remaining 11 being introduced and/or cultivated. Indigenous trees accounted for just nine (11.0%) of all cavity trees documented.
All cavities were in living trees, but consistently located in sections of those trees with fungal rot and/or damage. It was not always possible to verify the existence or extent of decay on cavity trees, however, 69 (84.1%) were obviously affected. A recent study of cavities of Grey-headed Woodpecker Picus canus in Hungary produced similar results (Gorman Citation2019). Forty-three cavities (52.4%) were positioned in trunks and 39 (47.6%) in limbs. Syrian Woodpeckers frequently excavate cavities on the ‘underside’ of angled limbs with the entrance facing downwards towards the ground (Gorman Citation1995). In the present study, 31 (79.5%) of the 39 cavity entrances in limbs were positioned in this way: some angled downwards by approximately 35 degrees. Cavities in trunks generally faced outwards or slightly downwards.
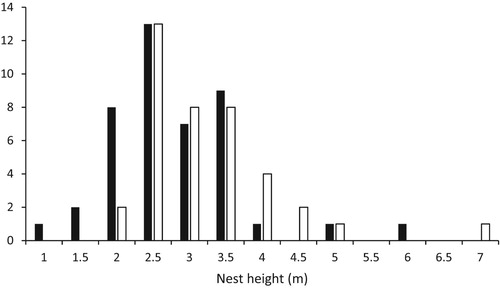
All cavities were positioned on clear, foliage-free areas of trees, below canopy level, with a clear flyway to the entrance. The heights of cavity entrances ranged from 1 to 7 m with an overall median of 3.0 m. Nests in limbs were slightly higher than those in the trunks (; trunk nests, median height = 2.5 m, range 1–6 m, n = 43; limb nests, median height = 3.0 m range 2–7 m, n = 39; Mann–Whitney U = 1854, P = 0.03). shows that the cavity orientation was non-random (Rayleigh test r = 0.30, P = 0.0007, n = 82) with a mean direction of SSE (170 degrees clockwise from north).
Figure 1. The frequency distribution of Syrian Woodpecker cavity heights. Solid bars, nests in trunks (n = 43); open bars, nests in limbs (n = 39).
Many woodpeckers reuse their cavities, using them as nest sites in subsequent years, however, Syrian Woodpeckers do not seem to do so as often as, for example, Great Spotted Woodpeckers. In Poland, 3.8% of Great Spotted Woodpecker nests were in re-used cavities (Wesolowski & Tomialojc Citation1986) and in southern England it was higher at 13.2% in re-used cavities with the rate of re-use strongly dependent on the tree species (Smith Citation1997). Syrian Woodpeckers will, however, use old cavities as overnight roosts (pers. obs.). The most frequently chosen tree for cavities was False Acacia, a tree indigenous to eastern North America which was introduced to Hungary in the eighteenth century and is now widespread, accounting for 23% of the country’s forest cover (Rédei et al. Citation2008). The fraction of nests found in False Acacia did not differ from that expected by chance given the high coverage of the species in the Hungarian landscape (χ2 = 2.25, df = 1, P = 0.134). The use of this non-native tree by Syrian Woodpeckers for the location of cavities is not surprising as its use of anthropogenic tree stands is well known. For example, a study in southeast Poland found that orchards are the most important habitat type for the species (Michalczuk & Michalczuk Citation2016a). In central Poland, as in Hungary, Walnut and planted fruit trees are often used (Figarski & Kajtoch Citation2018). In Israel, too, introduced trees are preferred for breeding (Orchan et al. Citation2013). In central Iran, Pistachio trees dominate as nesting sites although many other trees are present (Aghanajafizadeh et al. Citation2011). Elsewhere in Asia Minor and the Middle East, the Syrian Woodpecker occurs in settlements with planted trees, in olive groves, plantations of avocado, almond, pistachio, pecan and other cultivated trees (Mendelssohn & Yom-Tov Citation1999, Al-Safadi Citation2004). Interestingly, a study of nest sites of Great Spotted Woodpeckers in a riparian forest in Hungary also found that introduced tree species were used much more than indigenous ones (Ónodi & Winkler Citation2016). Clearly, these two related and often sympatric woodpeckers are ecologically very flexible and adaptable in terms of tree choice for cavities and are therefore probably able to coexist in non-forest habitats due to being able to utilize a broad range of tree types.
The question arises as to why Syrian Woodpeckers consistently used non-native trees over native species (from 14 tree species only three were native). Data on the relative frequencies of other trees at each site, compared to those used, were not available. Further research on tree occurrence may elucidate use versus availability. However, two basic factors, availability and suitability, are significant. Firstly, being a relatively new colonist to the region (only becoming established in central Europe in the mid-twentieth century), the Syrian Woodpecker occupied niches that were readily available (Glutz von Blotzheim & Bauer Citation1994). Today in Hungary, as elsewhere, it continues to persist in anthropogenic tree stands in often disturbed urban environments. Ultimately, the Syrian Woodpecker is an adaptable species which, despite its frequent use of False Acacia in Hungary as a cavity tree, is not attached to any specific tree species as indicated in studies from other countries (Aghanajafizadeh et al. Citation2011, Michalczuk & Michalczuk Citation2016a). It seems to be indiscriminate in tree selection, creating cavities for nesting and/or roosting in what is available and suitable wherever it occurs, with factors such as tree health, location, height and prevailing wind direction being important. Often, the cavity tree used appeared to be the only suitable one in the vicinity. It is likely that Syrian Woodpeckers cannot afford to be demanding in this respect, as territories are probably established first and then cavity sites within the territory chosen later, as is the case for some other European woodpecker species (Kosinski & Winiecki Citation2004).
Secondly, for most woodpeckers, including the Syrian Woodpecker, it is ease of excavation and security of the nest that are important when selecting a cavity location rather than any affinity to specific tree species. Most woodpeckers invest significant time and effort in making cavities, however, despite being morphologically adapted to excavate wood, they tend to select trees, and parts of trees, which are decayed or dead, and which are thus easier to work upon but not so soft that the nest is liable to collapse or be vulnerable to predators. The condition of trees is important: dead, dying or living trees with weak and soft sections due to fungal rot, insect infestation or wounds from lightning, wind, frost and the like, provide convenient opportunities for cavity placement. Trees affected with fungal rot are often chosen as they are generally easier to excavate than healthy ones (Zahner et al. Citation2012). At least 69 (84.1%) of the 82 trees in this study were clearly affected by rot or damage in the section that housed the cavity. It is probably true that Syrian Woodpeckers prefer damaged trees, but due to insufficient data about the share of such trees in the vicinity of the cavity trees in this study, it is not possible to test this statistically. Trees such as False Acacia, which become dry, brittle, and subject to decay when they mature, can provide ideal locations. Many woodpeckers, including Syrian Woodpeckers, favour soft timber over hard for the same reason (Michalczuk & Michalczuk Citation2016b, Figarski & Kajtoch Citation2018).
Ultimately, providing the surrounding habitat offers satisfactory foraging opportunities, almost any tree in a given area that is easy to excavate can be selected for cavities. This finding is in accordance with the conclusions of some other studies, including one on two European species, the Great Spotted and Middle Spotted Woodpeckers (Kosinski & Winiecki Citation2004).
Syrian Woodpeckers in this study showed a clear preference for the nest hole to be orientated to the SSE. A study in Iran (Shafaeipour et al. Citation2019) and one in Israel (Ar et al. Citation2004) both found random orientation, although the latter only included 19 nests. A meta-analysis of cavity entrance orientation from 80 populations of 23 species of woodpecker throughout the Northern Hemisphere, concluded that cavity entrance orientation was influenced by regional climatic forces and was typically non-random (Landler et al. Citation2014). As yet, there have been no studies showing the adaptive advantages of particular nest orientations, but it is thought that they must exist. Landler et al. (Citation2014) included data from 12 studies on Great Spotted Woodpeckers, a close relative of the Syrian Woodpecker and one which is often sympatric. All but three of the studies found random orientation, the exceptions being studies in Great Britain (Tracy Citation1938), Poland (Hebda Citation2009) and China (Wan et al. Citation2008).
In the present study of Syrian Woodpecker cavities across Hungary, a general alignment and southerly orientation of entrances, with an easterly component, clearly prevailed. The reasons for this are not entirely clear and several factors are likely to be involved. The Syrian Woodpecker is regarded as a thermophilic species (Glutz von Blotzheim & Bauer Citation1994) and thus temperature and sunlight possibly influence the orientation of their cavities. Those facing south and east receive more sun, hence illumination and warmth, in the morning hours. In the breeding season this is the time when adults emerge from a night inside the nesting chamber and begin to forage and provision their brood. The high proportion of entrances facing southwards seems to suggest that early morning warming is preferred, however, Landler et al. (Citation2014) highlights the paucity of data for the Syrian Woodpecker. Thermophilic benefits from southerly orientation of nest holes in the Northern Flicker Colaptes auratus in North America (Wiebe Citation2001) were found to be associated with increased egg productivity but this did not translate to hatching or fledging success. However, Landler et al. (Citation2014) identified a stronger southerly orientation selection for North American over Eurasian woodpeckers, so factors other than latitude may influence selection in Hungary. For instance, prevailing wind direction in Hungary, which is from the northwest (Hungarian Meteorological Service Citationundated), may be a contributing factor. Yet, as with the positioning and location of cavities on trees, a trade-off may sometimes be at work. Local circumstances and conditions may result in certain factors overriding others. For example, the southern sides of trees may not always be the sunniest due to the surrounding environment: other trees or buildings may create shade. In addition, woodpeckers may disregard compass direction when excavating a cavity if doing so involves substantial excavation energy output. A part of the tree where a cavity can be more easily excavated may be chosen, although it may not be ideal in terms of orientation. Cavities facing away from the south may be chosen because of ease of excavation.
Acknowledgements
The author would like to thank Prof. Dr Hans Winkler for his expert comments and advice, Daniel Alder for many suggestions and help in preparing the figures, Ken Smith for comments on an early draft and help with the statistics, and two anonymous reviewers.
References
- Aghanajafizadeh, S., Heydari, F., Naderi, G. & Hemami, M.R. 2011. Nesting hole site selection by the Syrian Woodpecker, Dendrocopos syriacus, in Yazd province. Iran. Zool. Middle East 53: 3–6. doi: 10.1080/09397140.2011.10638494
- Al-Safadi, M.M. 2004. On the breeding biology of the Syrian Woodpecker, Dendrocopos syriacus, in the Gaza Strip. Zool. Middle East 32: 7–12. doi: 10.1080/09397140.2004.10638038
- Ar, A., Barnea, A., Yom-Tov, Y. & Mersten-Katz, C. 2004. Woodpecker cavity aeration: a predictive model. Respir. Physiol. Neurobiol. 144: 237–249. doi: 10.1016/j.resp.2004.04.019
- BirdLife International. 2019. Species factsheet: Dendrocopos syriacus.
- Figarski, T. & Kajtoch, L. 2018. Differences in habitat requirements between two sister Dendrocopos Woodpeckers in urban environments: implication for the conservation of Syrian Woodpecker. Acta Ornithol. 53 (1): 23–36. doi: 10.3161/00016454AO2018.53.1.003
- Glutz von Blotzheim, U.N. & Bauer, K.M. (eds). 1994. Handbuch der Vögel Mitteleuropas. Band 9. AULA-Verlag Gmbh, Wiesbaden.
- Gorman, G. 1995. Identifying the presence of woodpecker (Picidae) species on the basis of their holes and signs. Aquila 102: 61–67.
- Gorman, G. 2004. Woodpeckers of Europe – a Study of the European Picidae . Bruce Coleman. Chalfont St Peter, Bucks.
- Gorman, G. 2014. Woodpeckers of the World: the complete guide. Christopher Helm, London.
- Gorman, G. 2019. Characteristics of Grey-headed Woodpecker (Picus canus) cavities in Hungary. Aquila 126: 33–39.
- Hebda, G. 2009. Nesting sites of the Great Spotted Woodpecker Dendrocopos major L. in Poland: analysis of nest cards. Pol. J. Ecol. 57: 149–158.
- Hungarian Meteorological Service. Undated. Wind conditions of Hungary. https://www.met.hu/en/eghajlat/magyarorszag_eghajlata/altalanos_eghajlati_jellemzes/szel/. Date accessed 16/5/2019.
- Kosinski, Z. & Winiecki, A. 2004. Nest-site selection and niche partitioning among the Great Spotted Woodpecker Dendrocopos major and Middle Spotted Woodpecker Dendrocopos medius in riverine forest of Central Europe. Ornis Fenn. 81: 145–156.
- Landler, L., Jusino, M.A., Skelton, J. & Walters, J.R. 2014. Global trends in woodpecker cavity entrance orientation: latitudinal and continental effects suggest regional climate influence. Acta Ornithol. 49: 257–266. doi: 10.3161/173484714X687145
- Lund, U. & Agostinelli, C. 2011. Package ‘circular’: circular statistics. R package version 0.4-3.
- Mendelssohn, H. & Yom-Tov, Y. 1999. A report of birds and mammals which have increased their distribution and abundance in Israel due to human activity. Isr. J. Zool. 45: 35–47.
- Michalczuk, J. & Michalczuk, M. 2016a. Habitat preferences of Picidae woodpeckers in the agricultural landscape of SE Poland: is the Syrian Woodpecker (Dendrocopos syriacus) colonizing a vacant ecological niche? North-West J. Zool. 12: 14–21.
- Michalczuk, J. & Michalczuk, M. 2016b. Nesting preferences of Syrian Woodpeckers Dendrocopos syriacus in the agricultural landscape of SE Poland. Acta Ornithol. 51: 71–81. doi: 10.3161/00016454AO2016.51.1.006
- Orchan, Y., Chiron, F., Shwartz, A. & Kark, S. 2013. The complex interaction network among multiple invasive bird species in a cavity-nesting community. Biol. Invasions 5: 429–445. doi: 10.1007/s10530-012-0298-6
- Ónodi, G. & Winkler, D. 2016. Nest site characteristics of the Great Spotted Woodpecker in a bottomland riparian forest in the presence of invasive tree species. Ornis Hung. 24: 81–95. doi: 10.1515/orhu-2016-0005
- Pewsey, A., Neuhauser, M. & Ruxton, G.D. 2013. Circular Statistics in R. OUP, Oxford.
- R Development Core Team. 2015. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
- Rédei, K., Osváth-Bujtás, Z. & Veperdi, I. 2008. Black locust (Robinia pseudoacacia L.) improvement in Hungary: a review. Acta Silv. Lign. Hung. 4: 127–132.
- Shafaeipour, A., Fathinia, B. & Mohamadian, F. 2019. Comparison of nest holes between Syrian Woodpecker (Dendrocopos syriacus) and Middle Spotted Woodpecker (Dendrocoptes medius) around Yasouj city in Southwestern of Iran. Iran. J. Anim. Biosyst. 15 (1): 99–105.
- Smith, K. 1997. Nest site selection of the Great Spotted Woodpecker Dendrocopos major in two oak woods in southern England and its implications for woodland management. Biol. Conserv. 80: 283–288. doi: 10.1016/S0006-3207(96)00038-9
- Tracy, N. 1938. Great Spotted Woodpecker. Beitr. Fortpfl. Biol. Vog. 14: 41–48.
- Wan, T., Hu, J.-F., Jiao, Z.-B., Wen, J.-B. & Luo, Y.-Q. 2008. Nest-cavity characteristics of the Great Spotted Woodpecker Dendrocopos major in shelter plantations of west Inner Mongolia. For. Stud. China 10: 36–40. doi: 10.1007/s11632-008-0010-1
- Wesolowski, T. & Tomialojc, L. 1986. The breeding ecology of woodpeckers in a temperate primeaval forest – preliminary data. Acta Ornithol. 22: 1–21.
- Wiebe, K.L. 2001. Microclimate of tree cavity nests: is it important for reproductive success in Northern Flickers? The Auk 118: 412–421. doi: 10.1093/auk/118.2.412
- Winkler, H., Christie, D. & Nurney, D. 1995. Woodpeckers: a guide to the woodpeckers, piculets and wrynecks of the world. Pica Press, Robertsbridge.
- Zahner, V., Sikora, L. & Pasinelli, G. 2012. Heart rot as a key factor for cavity tree selection in the Black Woodpecker. For. Ecol. Manage. 271: 98–103. doi: 10.1016/j.foreco.2012.01.041
- Zavialov, E., Tabachishin, V.G. & Mosolova, E.Y. 2008. Expansion of Syrian Woodpecker in European Russia and Ukraine. Dutch Bird. 30: 236–238.