ABSTRACT
Capsule
Barn Swallow Hirundo rustica nestlings exhibited higher local recruitment when the body mass was high and when raised in an artificial nest early in the breeding season.
Aims
To determine natal dispersal and recruitment of Barn Swallows breeding in artificial nests and natural nests in an urban habitat.
Methods
During the breeding seasons of 2012–2018 a total of 3907 Barn Swallow nestlings were ringed and in the breeding seasons of 2016–2019 adult Barn Swallows were recaptured to assess natal dispersal distances and local recruitment.
Results
Female Barn Swallows dispersed from their natal nest sites on average 2.5 times further than the males. Nestling mass predicted future recruitment probability, and the mean nestling body mass of recruits was higher than the mean body mass of their non-recruited siblings. A significantly larger than expected number of recruits originated from first broods, reflecting that the timing of Barn Swallow breeding is an important factor for future recruitment. Neither nest type nor brood type affected natal dispersal. Barn Swallows raised in artificial nests were recruited more than birds raised in natural nests.
Conclusion
Natal dispersal of Barn Swallows in urban habitats was sex-biased, with females dispersing further than males. A Barn Swallow nestling raised early in the season (1st broods), in a clean artificial nest, and with good competitive skills for food, had a high probability of survival and local recruitment. For a declining passerine species, such as the Barn Swallow, artificial nests may be a possible conservation option in urban habitats.
Natal dispersal is an important aspect in ecological studies since the movement of individuals contributes to genetic flow between populations (Greenwood & Harvey Citation1982, Clobert et al. Citation2001, Balbontín et al. Citation2009). Natal dispersal of individuals is defined as the movement between a natal site and the site of first breeding (Greenwood & Harvey Citation1982, Clobert et al. Citation2001). The underlying factors promoting dispersal of individuals may be related to intra-specific competition for resources, such as food and nesting sites (Gowaty Citation1993, Negro et al. Citation1997, Teglhøj Citation2017, Citation2018), competition for mates (Dobson & Jones Citation1985, Møller Citation1994), the avoidance of ectoparasites (Brown & Brown Citation1992, Saino et al. Citation2014) and inbreeding avoidance (Wheelwright & Mauck Citation1998, Daniels & Walters Citation2000). In birds the female is usually the dispersing sex, while the opposite is usually the case in mammals (Greenwood Citation1980, Johnson & Gaines Citation1990, Pusey Citation1987, Clark et al. Citation1997, Wolff & Plissner Citation1998). Dispersal of yearling Barn Swallows has been studied in different rural areas in Europe and North America and has shown a higher degree of philopatry in males than in females (Shields Citation1984, Balbontín et al. Citation2009, Scandolara et al. Citation2014).
Natural nest sites can be a limiting resource and may influence natal dispersal decisions in passerines. Re-use of old nests is common among Barn Swallows, where most pairs re-use old nests for their first breeding attempt (Safran Citation2006). The re-use of an old nest may spare individuals the energetic costs linked to building a new nest (Teglhøj Citation2018), lead to earlier egg-laying of first clutches (Barclay Citation1988, Shields et al. Citation1988, Safran Citation2006, Teglhøj Citation2018), and provide important settlement cues for first-year breeders (Safran Citation2004). Artificial nesting options, such as nest boxes, may offer additional breeding sites for species where there is a high level of intra-specific competition for natural nest sites (Newton Citation1994, Corrigan et al. Citation2011, Libois et al. Citation2012). The significance of nest box use on breeding phenology and reproductive success has been studied in several passerine species (Major & Kendal, Citation1996, Purcell et al. Citation1997, Ramstack et al. Citation1998, Lorenzón & Quiroga Citation2012, Rhim et al. Citation2013); however, few studies have examined these effects in Barn Swallows using artificial nest boxes (Mercadante & Stanback Citation2011, Teglhøj Citation2018). In urban habitats, Barn Swallows using artificial nest boxes – or old natural nests – breed earlier, lay more eggs and produce more nestlings and fledglings than pairs breeding in new natural nests (Teglhøj Citation2018).
Nestling body mass may predict survival and future recruitment in passerines, and body mass can be an indicator of individual condition and competitive ability within broods (Both et al. Citation1999, Saino et al. Citation2012). In House Sparrows Passer domesticus, nestlings that had a greater body mass at 1 and 11 days old were more likely to fledge and recruit (Cleasby et al. Citation2010). In Brown Thornbills Acanthiza pusilla, the probability of reaching independence was higher for heavier nestlings (Green & Cockburn Citation2001). In Great Tits Parus major, survival of juveniles was positively correlated with fledging body mass, and the probability of local recruitment increased with fledging body mass (Naef-Daenzer et al. Citation2001, Monrós et al. Citation2002).
The timing of reproduction may impact on future recruitment in passerine species. In Barn Swallows, late-brood fledglings had a lower probability of returning to breed the following year than early-brood fledglings (Møller Citation1994, Citation2002, Raja-Aho et al. Citation2017). Possible changes in the timing of reproduction may arise from changes in migration patterns, food availability and other factors, and differences in the timing of reproduction among breeding sites may result in sites with early reproduction contributing more to local recruitment (Møller Citation2008). Barn Swallow reproductive output is determined primarily by two parental decisions: the timing of breeding and the timing of family break-up (Grüebler & Naef-Daenzer Citation2008). Differences in these decisions may lead to differential survival of second-brood fledglings and consequently affect future recruitment prospects (Grüebler & Naef-Daenzer Citation2008).
So far, no studies have addressed natal dispersal patterns of Barn Swallows in urban habitats, and the significance of the nest types used and nestling condition for future recruitment. The objectives of this study were, therefore, to investigate to what extent:
natal dispersal of Barn Swallows occurs within an urban habitat, additionally quantifying potential differences in natal dispersal between the sexes.
dispersal distances could be linked to natal nest type and the timing of reproduction.
nestling mass could predict future recruitment.
If Barn Swallow nestlings raised in an urban habitat disperse in a similar pattern to nestlings from rural areas, males should be more philopatric and females should show a greater degree of natal dispersal.
If the timing of the nestling period is linked to survival and future recruitment, first brood fledglings should be more likely to recruit than second brood fledglings.
If the nest type has no effect on the survival and fledging of nestlings, the recruitment to local urban breeding populations should resemble the fractions of ringed nestlings from the different nest types.
If nestling mass at the time of ringing (10–15d) is a reliable measure of nestling condition and a predictor of survival and future recruitment, recruiting individuals should, on average, be heavier than their siblings.
Methods
Study area
Svendborg (55°3′34″N 10°36′30″E) is an old Danish town, situated in the southern part of Fyn, Denmark. The centre of the town is dominated by old buildings some of which have gates where swallows may build their nests. The town periphery is dominated by open pastures, scattered buildings, sports fields and other recreational areas. Before the breeding season in 2012 some 164 artificial nests, made of woodcrete (Vivara®), were placed at existing and potentially new breeding sites within a radius of 5.5 km of the town centre. For further details see Teglhøj (Citation2017, Citation2018).
Study organism
The Barn Swallow Hirundo rustica is a socially monogamous, aerial insectivorous passerine, mainly breeding semi-colonially in rural areas, where nests are placed in barns and sheds (Møller Citation1994, Balbontín et al. Citation2009). Breeding Barn Swallows have also been reported in smaller European towns (Teglhøj Citation2017, Citation2018) and larger cities in Asia (Turner Citation2006, Osawa Citation2015). The female Barn Swallow usually lays 1–2 clutches per breeding season, with each clutch consisting of 4–5 eggs. The incubation period is approximately 15 days and the nestlings hatch over a period of 1–3 days (Møller Citation1994). The nestling period lasts 19–21 days (Møller Citation1994, Teglhøj Citation2017) and fledglings are fed by the parents for 1–2 weeks after fledging (Møller Citation1994). The Barn Swallow undertakes diurnal transequatorial long-distance migration and the Danish population migrates directly south across the Alps or southwest across the Iberian Peninsula and the Sahara to winter quarters in Zambia, Namibia and South Africa (Møller Citation1994).
Ringing of nestlings and capture/recapture of adults
During the breeding seasons of 2012–2018 a total of 3907 Barn Swallow nestlings was ringed at the age 10–15 days and individually weighed using a Satrue® SDA Series High precision scale (±0.01 g). In the breeding seasons of 2016–2019 adult Barn Swallows were caught at 46 different breeding sites within the study area, using mist nests. Breeding sites were defined as locations within the study area where one or more active Barn Swallow nests were found. Most breeding pairs bred solitarily or in small colonies of 2–5 pairs. Distances between individual breeding sites varied between 0.05 and 10.5 km. All captured adult swallows were either ringed or the ring number of recaptured birds noted. The individuals were sexed using the absence (males) or presence (females) of a brood patch, the length of the two outermost tail feathers (T6), the length of the tail fork (distance between T1 and T6) and the length of the white spot on T6 (Svensson Citation1992, van den Brinch Citation2011). All measures (to the nearest 0.5 mm) were obtained using a rule with a zero stop. The capture of male and female recruits was biased, and only 26.2% of the caught individuals were females. This bias may be explained by a female-biased natal dispersal pattern and the general lower annual survival rates of female Barn Swallows (25.5%) compared to male Barn Swallows (28.4%) (Møller Citation1994). Furthermore, some recruiting individuals may have been missed due to difficulties catching all of the adults in the study area; some of the breeding sites inside and around the town of Svendborg are difficult to access using mist nets. For ethical reasons no other catching techniques were used.
Dispersal distances
To calculate the straight-line dispersal distances between the natal nest sites and the breeding sites of recruits, Google Maps (maps.google.com, Citation2020) was used. Philopatric recruits were defined as non-dispersing (0 km) individuals breeding at their natal nest site. Dispersers were defined as individuals moving more than 0 km from their natal nest site to breed (min–max: 0.3–8.7 km).
Statistical analyses
All statistical tests were carried out using JMP Version 10 (SAS Citation2012) and SOCS (Citation2020). A Kolmogorov–Smirnov test was used on all data sets to test for normality. A match-paired t-test was used to test for differences between the mass of recruits and the mean mass of their non-recruited siblings. A linear mixed effect model (LMM) was used to support the match-paired t-test by controlling for date, nestling year and nest site (random factors), and using the nestling body mass of the recruits as the dependent variable. A χ2 test for goodness of fit was used to test whether the observed frequency distributions of recaptured recruits originating from different nest types matched the expected frequency distribution of ringed nestlings from different nest types. Odds ratios for recruits from different nest types were calculated using the calculator developed by Georgiev (Citation2020). Finally, the effects of sex (male, female), year, nest type (natural new, natural old, artificial) and brood order (first, second brood) on natal dispersal distance were tested using a linear mixed effect model (LMM). The LMM was designed to control for the non-independence of observations because 44.4% of the recruiting breeding sites recruited more than one individual.
Results
Recruitment
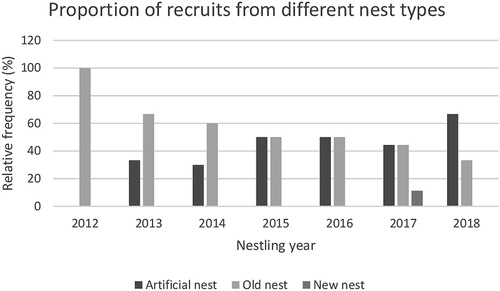
During the breeding seasons of 2016–2019, 719 adult Barn Swallows were captured (). Of these birds, 65 (9%) were local recruits, ringed as nestlings during 2012–2018, and their origins, with respect to brood and nest types, are summarised in . Of these recruits, 60 (92.3%) dispersed from their natal nest sites, whereas only 5 (7.7%) showed philopatry; the philopatric individuals were all male (). A comparison of observed and expected numbers of recruits from different nest types revealed significant differences (χ2 = 9.73, df = 2, P = 0.008), and the frequency of recruits from artificial nests was higher than expected by chance. Calculated odds ratios confirmed a higher than expected recruitment from artificial nests (odds ratio = 2.10, 95% confidence interval, CI: 1.29–3.44, P = 0.0031), and a lower than expected recruitment from old natural nests (Odds ratio = 0.59, 95% CI: 0.36–0.96, P = 0.035). Recruits from new natural nests tended to be lower than expected; however, the trend was not significant (odds ratio = 0.39, 95% CI: 0.09–1.62, P = 0.198). Likewise, significant differences were seen in observed and expected recruits from first and second broods (χ2 = 5.08, df = 1, P = 0.024), where birds from first broods recruited to a greater extent than expected by chance ().
Figure 1. Frequency distributions of the dispersal distances of male and female Barn Swallows. Class ‘0’ indicates the relative frequency of philopatric individuals. Class ‘1’: >0–1 km, Class ‘2’: 1–2 km, Class ‘3’: 2–3 km, Class ‘4’: 3–4 km, Class ‘5’: 4–5 km, Class ‘6’: 5–6 km, Class ‘7’: 6–7 km, Class ‘8’: 7–8 km and Class ‘9’: 8–9 km. N = 48 males, 17 females.
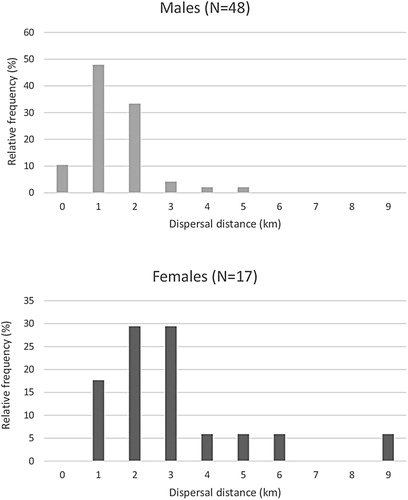
Table 1. Number of adult Barn Swallows ringed and recaptured during the breeding seasons 2016–2019.
Table 2. Brood and nest origin of ringed Barn Swallow nestlings (2012–2018) and recruits (2016–2019).
The mean nestling body mass of recruits (20.88 ± 0.47 g) was significantly higher than the mean body mass of their non-recruited siblings (20.16 ± 0.44 g; t = −2.67, df = 32, P = 0.012). There was no significant difference between the mean body mass of chicks reared early (first broods) and later (second broods) in the breeding season (F = 0.02, df = 1, P = 0.90; ).
Table 3. Effect of sibling mass, date, nest type and year on Barn Swallow recruitment mass. N = 33 broods.
Effect of sex, nest type and brood on natal dispersal
Evaluation of the effects of sex, year, nest type and brood order on natal dispersal distance showed that only the sex of Barn Swallows affected natal dispersal distance: females dispersed significantly further than males (). Distributions of the dispersal distances of male and female Barn Swallows are shown in . Mean (±SE) natal dispersal distances and minimum–maximum ranges (min–max:) of males (N = 48) and females (N = 17) in the urban habitat were 1.0 ± 0.13 km (min–max: 0–3.5 km) and 2.5 ± 0.5 km (min–max: 0.3–8.7 km), respectively (). Approximately 10% of males were philopatric whereas all females dispersed from their natal nest sites.
Table 4. Effect of sex, year, nest type and brood order (first, second brood) on Barn Swallow natal dispersal. N = 65 recruits.
Discussion
The main findings of this study were that all of the female Barn Swallows recruits that were subsequently recaptured had dispersed from their natal nest sites. On average, the female natal dispersal distance was 2.5 times greater than that of the recaptured males. Approximately 10% of the male recruits exhibited philopatry. Nestling mass predicted future recruitment probability, and the mean nestling mass of recruits at the time of ringing was higher than the mean mass of their non-recruited siblings. Neither nest type nor brood type significantly affected natal dispersal distance. A significantly larger than expected number of recruits originated from first broods, suggesting that the timing of Barn Swallow breeding is an important factor for future recruitment. Finally, the study showed that recruits raised in artificial nests constituted a significantly higher proportion than expected.
Dispersal and recruitment
The recapture rate of adult Barn Swallows ringed as nestlings was 1.7%, which is similar to the recapture rate of 1.5% reported by Shields (Citation1984) for a Barn Swallow population in North America. Scandolara et al. (Citation2014) reported 7–12% of ringed Barn Swallow nestlings were recaptured in a study in southern Switzerland. The natal dispersal of Barn Swallows in urban habitats showed a low degree of philopatry, with 96% of birds breeding at different sites to their site of birth. These findings are in accordance with Shields (Citation1984), who found that none of the recaptured Barn Swallows ringed as nestlings bred at their natal colonies. Scandolara et al. (Citation2014) reported philopatric frequencies of 24.4% for males and 7.7% for females. None of the females recaptured in this study were philopatric whereas 10.4% of the males bred at their natal site. Balbontín et al. (Citation2009) found a somewhat lower (5.5%) rate of male philopatry in a Danish rural population of Barn Swallows, but a somewhat higher proportion (1.3%) for females. The higher proportion of philopatric males in the urban habitat population may be explained by resource limitations in nearby rural areas: for example, a lack of appropriate nesting sites due to stable closure and a reduction in the number of cattle farms (Teglhøj Citation2017, Citation2018).
Dispersal distances of male and female recruits in the urban habitat averaged 1.0 and 2.5 km, respectively. These distances are somewhat lower than those reported by Scandolara et al. (Citation2014), who found dispersal distances for male and female Barn Swallows in rural habitats of 1.8 and 3.3 km respectively. The mean distances recorded were shorter than median dispersal distances of 6.3 km reported for an American population of Barn Swallows Hirundo rustica erythrogaser (Shields Citation1984), and shorter than the 2.4 km (males) and 8.4 km (females) reported for Tree Swallows Tachycineta bicolor (Winkler et al. Citation2005). The generally longer natal dispersal distance of females is consistent with findings in other passerines, where females disperse further than males (Greenwood Citation1980, Pusey Citation1987, Johnson & Gaines Citation1990, Clark et al. Citation1997, Wolff & Plissner Citation1998, Winkler et al. Citation2005).
Forty-nine percent of the recaptured recruits were yearlings, whereas 51% were recaptured for the first time as breeding adults two or more years after they were ringed as nestlings. This may be explained by the fact that the ringing of nestlings started in 2012, whereas recapture of breeding adult swallows was initiated in 2016. This is considered to be of minor importance because Barn Swallows show a very high degree of breeding site fidelity, with birds breeding at the same site for consecutive years if they manage to survive (Møller Citation1994, Balbontín et al. Citation2009). Personal observations supported this, since no breeding adults moved to other breeding sites within the study period. Recaptured adult recruits and Barn Swallows ringed as adults at the breeding sites were only recaptured in consecutive years at the same breeding site.
Recruitment from first and second broods
Recruited Barn Swallows belonged predominately to the group of birds raised early in the breeding season, reflecting higher general survival rates of first brood fledglings, whereas the second brood fledglings contribute less to local recruitment. Møller (Citation1994) found that early-nesting Barn Swallows in a rural area were better at producing fledglings than late-nesting birds, and Raja-Aho et al. (Citation2017) determined that late brood Barn Swallow fledglings had a 39% lower probability of returning to breed the following year, due to less efficient fuelling before migration departure and a younger age when departing, compared to early brood fledglings.
Natal nest type, recruitment and dispersal
Nestlings from artificial nests recruited more to the local population than expected by chance, whereas recruitment from old and new natural nests was lower than expected (). Barn Swallows breeding in artificial nests and old natural nests have previously been shown to fledge more young than pairs breeding in new natural nests (Teglhøj Citation2018), which supports the return frequencies of recruits from the various nest types. Tropical Fowl Mites Ornithonyssus bursa and other ectoparasites may cause severe fitness consequences for their Barn Swallow hosts (Møller Citation1994), which may contribute to individual dispersal decisions. Barn Swallow nestlings raised in old nests may experience a heavier burden of ectoparasites compared to birds raised in clean artificial nests and so may contribute to a potential larger dispersal distance from such nest sites. Visual inspection of the used artificial nests indicated low infestation rates; however, no quantification of ectoparasites was conducted in this study. The proportion of birds recruiting from artificial nests tended to increase over the years 2012–2018 () with an opposing decline in birds recruited from old natural nests, supporting the hypothesis that fewer parasites in artificial nests promoted better recruitment. Brown & Brown (Citation1992) demonstrated that Cliff Swallow Hirundo pyrrhonota nestlings that were relatively heavily parasitised by fleas and other ectoparasites dispersed to non-natal colonies to breed the following year, as opposed to only lightly parasitised individuals that predominately bred in their natal colony.
Nestling mass and recruitment
Nestling body mass can be used as a measure of nestling condition and has been used to predict the probability of future recruitment. Support for this was found in this study: nestling body mass of Barn Swallow recruits was significantly higher than the mean body mass of their non-recruiting siblings. In European Starlings Sturnus vulgaris, growth in nestling body mass was linked with lipid content: nestlings raised early in the season tended to have higher lipid levels, indicating the quality of individuals (Ardia Citation2005). Both et al. (Citation1999) found that Great Tit nestlings recruited relatively well if they were heavier compared to other fledglings. Similarly, Monrós et al. (Citation2002) reported that the probability of local recruitment of Great Tits increased with fledging mass. Møller (Citation1994) found similar associations between local recruitment of Barn Swallow nestlings, nestling mass and the time of fledging. Barn Swallow nestlings with superior immunity and body mass (females only) relative to their siblings enjoy greater longevity, implying that within-brood competition for food resources may have long-term effects on viability (Mock & Parker Citation1997, Saino et al. Citation2012). Furthermore, minor hatching asynchrony has been linked to a mass and size hierarchy of Barn Swallows that is maintained throughout the nestling period (Saino et al. Citation2012). This hierarchy may have long-term fitness consequences, predominately for females which are competitively inferior to males in Barn Swallow broods (Saino et al. Citation2012). In addition, late-hatching Barn Swallow nestlings develop their body mass and skeleton at the expense of wing feathers. This may have negative consequences, since this type of resource distribution may impair predator avoidance and foraging ability after fledging (Mainwaring et al. Citation2009).
Conclusion
This study is the first to highlight recruitment and natal dispersal patterns of Barn Swallows raised in different nest types in an urban habitat. Female natal dispersal distance was approximately 2.5 times greater than male natal dispersal, and males showed a higher degree of philopatry. Birds that recruited to the local breeding population originated predominately from first broods, which may be explained by better survival probabilities of chicks raised during the first part of the breeding season. Nestling body mass was a good predictor of future recruitment probability, and mean body mass at ringing was higher in recruits than in their non-recruited siblings. A greater than expected proportion of the recruits originated from artificial nests, whereas the proportion of recruits from natural nests was lower than expected. This implies an overall better survival of fledglings from artificial nests, which may be linked to an earlier initiation of egg-laying (Teglhøj Citation2018), differences in ectoparasite loads across nest types, and possible differences in nest microclimate that may have influenced chick condition.
From a conservation perspective, artificial nests may be used to counteract the decline of Barn Swallows in Europe and North America. Artificial nests may save the breeding birds time and energy linked to nest-building, especially in dry springs where access to mud is limited, and these nests have been shown to have both higher reproductive output (Teglhøj Citation2018) and higher local recruitment than natural nests, as documented in this study.
Acknowledgments
I thank The Danish Ornithological Association (DOF) for part-funding of the artificial nests used in the study; Jan Drachmann (DOF Scientific Committee) for constructive comments on the manuscript; the private citizens of Svendborg who allowed me to place artificial nests at their properties and enabling nest visits and ringing of Barn Swallows; and The Ringing Administration at The Natural History Museum, University of Copenhagen, for providing the bird rings used in the study.
References
- Ardia, D.R. 2005. Super size me: an experimental test of the factors affecting lipid content and the ability of residual body mass to predict lipid stores in nestling European Starlings. Funct. Ecol. 19: 414–420.
- Balbontín, J., Møller, A.P., Hermosell, I.G., Marzal, A., Reviriego, M. & de Lope, F. 2009. Geographic patterns of natal dispersal in barn swallows Hirundo rustica from Denmark and Spain. Behav. Ecol. Sociobiol. 63: 1197–1205.
- Barclay, R.M.R. 1988. Variation in the costs, benefits, and frequency of nest reuse by Barn Swallows (Hirundo rustica). Auk 105: 53–60.
- Both, C., Visser, M.E. & Verboven, N. 1999. Density–dependent recruitment rates in Great Tits: the importance of being heavier. Proc. R. Soc. London Ser. B: Biol. Sci. 266: 465–469.
- Brown, C.R. & Brown, M.B. 1992. Ectoparasitism as a cause of natal dispersal in Cliff Swallows. Ecology 73: 1718–1723.
- Clark, A.L., Sæther, B.E. & Røskaft, E. 1997. Sex biases in Avian dispersal: A reappraisal. Oikos 79: 429–438.
- Cleasby, I.R., Nakagawa, S., Gillespie, D.O.S. & Burke, T. 2010. The influence of sex and body size on nestling survival and recruitment in the House Sparrow. Biol. J. Linn. Soc. 101: 680–688.
- Clobert, J., Danchin, E., Dhondt, A.A. & Nichols, J.D. 2001. Dispersal: Individual Population and Community. Oxford University Press, Oxford.
- Corrigan, R.M., Scrimgeour, G.J. & Paszkowski, C. 2011. Nest boxes facilitate local-scale conservation of Common Goldeneye (Bucephala clangula) and Bufflehead (Bucephala albeola) in Alberta. Canada Avian Conserv. Ecol. 6: 1.
- Daniels, S.J. & Walters, J.R. 2000. Inbreeding depression and its effects on natal dispersal in Red-cockaded Woodpeckers. Condor 102: 482–491.
- Dobson, F.S. & Jones, W.T. 1985. Multiple causes of dispersal. Am. Nat. 126: 855–858.
- Georgiev, G.Z. 2020. “Odds Ratio Calculator”, [online] Available at: https://www.gigacalculator.com/calculators/odds-ratio-calculator.php.
- Google Maps. 2020. https://www.google.dk/maps.
- Gowaty, P.A. 1993. Differential dispersal, local resource competition, and sex ratio variation in birds. Am. Nat. 141: 263–280.
- Green, D.J. & Cockburn, A. 2001. Post-fledging care, philopatry and recruitment in Brown Thornbills. J. Anim. Ecol. 70: 505–514.
- Greenwood, P.J. 1980. Mating systems, philopatry, and dispersal in birds and mammals. Anim. Behav. 28: 1140–1162.
- Greenwood, P.J. & Harvey, P.H. 1982. The natal and breeding dispersal of birds. Annu. Rev. Ecol. Syst. 13: 1–21.
- Grüebler, M.U. & Naef-Daenzer, B. 2008. Fitness consequences of pre- and post-fledging timing decisions in a double-brooded passerine. Ecology 89: 2736–2745.
- Johnson, M.L. & Gaines, M.S. 1990. Evolution of dispersal: theoretical models and empirical tests using birds and mammals. Annu. Rev. Ecol. Syst. 21: 449–480.
- Libois, E., Gimenez, O., Oro, D., Minguez, E., Pradel, R. & Sanz-Aguilar, A. 2012. Nest boxes: a successful management tool for the conservation of an endangered seabird. Biol. Conserv. 155: 39–43.
- Lorenzón, R.E. & Quiroga, M.A. 2012. Breeding Biology of the white-rumped Swallow (Tachycineta leucorrhoa; hirundinidae) in a wetland: A Comparative approach. Avian. Biol. Res. 5: 47–53.
- Mainwaring, M.C., Rowe, L.V., Kelly, D.J., Grey, J., Bearhop, S. & Hartley, I.R. 2009. Hatching asynchrony and growth trade-offs within Barn Swallow broods. Condor 111: 668–674.
- Major, R.E. & Kendal, C.E. 1996. The contribution of artificial nest experiments to understanding avian reproductive success: a review of methods and conclusions. Ibis 138: 298–307.
- Mercadante, A.N. & Stanback, M.T. 2011. Out of sight, Out of mind? Visual obstructions affect settlement patterns in Barn Swallows (Hirundo rustica). Auk 128: 230–236.
- Mock, D.W. & Parker, G.A. 1997. The Evolution of Sibling Rivalry. Oxford University Press, Oxford.
- Monrós, J.S., Belda, E.J. & Barba, E. 2002. Post-fledging survival of individual great tits: the effect of hatching date and fledging mass. Oikos 99: 481–488.
- Møller, A.P. 1994. Sexual Selection and the Barn Swallow. Oxford University Press, Oxford.
- Møller, A.P. 2002. North Atlantic Oscillation (NAO) effects of climate on the relative importance of first and second clutches in a migratory passerine bird. J. Anim. Ecol. 71: 201–210.
- Møller, A.P. 2008. Climate change and micro-geographic variation in laying date. Oecologia 155: 845–857.
- Naef-Daenzer, B., Widmer, F. & Nuber, M. 2001. Differential post-fledging survival of great and coal tits in relation to their condition and fledging date. J. Anim. Ecol. 70: 730–738.
- Negro, J.J., Hiraldo, F. & Donazar, J.A. 1997. Causes of natal dispersal in the Lesser Kestrel: inbreeding avoidance or resources competition? J. Anim. Ecol. 66: 640–648.
- Newton, I. 1994. The role of nest sites in limiting the numbers of hole-nesting birds: a review. Biol. Conserv. 70: 265–276.
- Osawa, T. 2015. Importance of farmland in urbanized areas as a landscape component for Barn Swallows (Hirundo rustica) nesting on concrete buildings. Environ. Manag. 55: 1160–1167.
- Purcell, K.L., Verner, J. & Oring, L.W. 1997. A comparison of the breeding ecology of birds nesting in boxes and tree cavities. Auk 114: 646–656.
- Pusey, A.E. 1987. Sex-biased dispersal and inbreeding avoidance in birds and mammals. Trends in Ecology & Evolution 2: 295–299.
- Raja-Aho, S., Eeva, T., Suorsa, P., Valkama, J. & Lehikoinen, E. 2017. Juvenile Barn Swallows Hirundo rustica L. from late broods start autumn migration younger, fuel less effectively and show lower return rates than juveniles from early broods. Ibis 159: 892–901.
- Ramstack, J.M., Murphey, M.T. & Palmer, M.R. 1998. Comparative reproductive biology of three species of swallows in a common environment. Wilson Bull. 110: 233–243.
- Rhim, S.J., Son, S.H., Kim, K.J. & Hwang, H.S. 2013. Breeding ecology of tits using artificial nest boxes in coniferous and deciduous forests in Mt. namsan, Seoul metropolitan, Korea. Forest Science and Technology 9: 147–150.
- Safran, R.J. 2004. Adaptive site selection rules and variation in group size of Barn Swallows: individual decisions predict population patterns. Am. Nat. 164: 121–131.
- Safran, R.J. 2006. Nest-site selection in the Barn Swallow Hirundo rustica: what predicts seasonal reproductive success? Can. J. Zool. 84: 1533–1539.
- Saino, N., Romano, M., Ambrosini, R., Rubolini, D., Boncoraglio, G., Caprioli, M. & Romano, A. 2012. Longevity and lifetime reproductive success of Barn Swallow offspring are predicted by their hatching date and phenotypic quality. J. Anim. Ecol. 81: 1004–1012.
- Saino, N., Romano, M., Scandolara, C., Rubolino, D., Ambrosini, R., Caprioli, M., Costanzo, A. & Romano, A. 2014. Brownish, small and lousy Barn Swallows have greater natal dispersal propensity. Anim. Behav. 87: 137–146.
- SAS. 2012. JMP Version 10.0. SAS Institute Inc, Cary, NC.
- Scandolara, C., Lardelli, R., Sgarbi, G., Caprioli, M., Ambrosini, R., Rubolini, D. & Saino, N. 2014. Context-, phenotype-, and kin-dependent natal dispersal of Barn Swallows (Hirundo rustica). Behav. Ecol. 25: 180–190.
- Shields, W.M. 1984. Factors affecting nest and site fidelity in adirondack Barn Swallows (Hirundo rustica). Auk 101: 780–789.
- Shields, W.M., Crook, J.R., Hebblethwaite, M.L. & Wiles-Ehmann, S.S. 1988. Ideal free colonility in the swallows pp. In C. N. Slobodchifoff. (ed) The Ecology of Social Behavior, 189–228. Academic Press, San Diego, CA.
- Svensson, L. 1992. Identification Guide to European Passerines. 4th edn. Stokholm, Lullula, S:ta Toras väg 28, S-260 93.
- Teglhøj, P.G. 2017. A comparative study of insect abundance and reproductive success of barn swallows Hirundo rustica in two urban habitats. J. Avian Biol. 48: 846–853.
- Teglhøj, P.G. 2018. Artificial nests for Barn Swallows Hirundo rustica: a conservation option for a declining passerine? Bird Study 65: 385–395.
- SOCS. 2020. Social Science Statistics website: https://www.socscistatistics.com.
- Turner, A. 2006. The Barn Swallow. T & AD Poyser, London.
- van den Brinch, B. 2011. The Zambian Barn Swallow (Hirundo rustica) project. Afring News 40: 27–29.
- Wheelwright, N.T. & Mauck, R.A. 1998. Philopatry, natal dispersal, and inbreeding avoidance in an island population of Savannah sparrows. Ecology 79: 755–767.
- Winkler, D.W., Wrege, P.H., Allen, P.E., Kast, T.L., Senesac, P., Wasson, M.F. & Sullivan, P.J. 2005. The natal dispersal of Tree Swallows in a continuous mainland environment. J. Anim. Ecol. 74: 1080–1090.
- Wolff, J.O. & Plissner, J.H. 1998. Sex biases in avian natal dispersal: an extension of the mammalian model. Oikos 83: 327–330.