ABSTRACT
Capsule
Monitoring of European Nightjar Caprimulgus europeaus nest sites over seven years produced no evidence of a negative effect of tail-mounted radio tag deployment on nest success.
Aims
To test whether nest success of European Nightjars was affected by radio tag deployment.
Methods
The breeding success of European Nightjars was monitored at the Brechfa West Wind Farm, Carmarthenshire, Wales, from 2013 to 2019. A total of 85 nests were located through a combination of capture and radio tracking of breeding individuals, and direct observation combined with focused searching. All located nests were subsequently monitored through a combination of visual checks and trail camera deployment until their natural conclusion.
Results
No evidence was identified to support a negative effect of tail-mounted radio tag deployment on the nest success of European Nightjars. However, nesting success (at least one chick fledged) was positively associated with mean temperature during the nesting period, although the strength of this effect varied through time.
Conclusion
The use of tail-mounted radio tags on European Nightjars had no significant negative effect on nest success.
The marking and tagging of birds are widespread and important methods that have informed studies of many aspects of animal ecology, including migration, foraging behaviour, and physiological ecology (Bodey et al. Citation2018). The techniques used for such marking are continuously evolving, and have been used in some form for many decades. The extra mass that these devices impose, the tag configuration, and attachment method used has, however, been a cause for concern, especially for relatively heavy devices such as radio tags, global positioning system (GPS) devices, and geolocators (Bowlin et al. Citation2010). The deployment of such devices has been shown in some cases to reduce survival, inhibit parental care (Bodey et al. Citation2018), induce potentially costly behavioural modifications (Vandenabeele et al. Citation2014), or reduce the probability of nesting (Barron et al. Citation2010). Several mechanisms for such effects have been identified including increased energetic costs of flight through drag (Bowlin et al. Citation2010), reduced foraging success (Wanless et al. Citation1988), impacts on young through reduced provisioning (Robert et al. Citation2006), and increased thermoregulatory costs due to feather loss and skin damage (Hines & Zwickel Citation1985). Although it is likely that such affects are, in many cases, species-specific, with other studies identifying few or no effects (Bell et al. Citation2017, Brlik et al. Citation2020).
In an attempt to overcome such device effects, the research community has adopted rules of thumb for the design of tagging studies, such as the ‘5% rule’. This dictates a maximum tag mass limit of 5% of a bird’s body mass (Brander & Cochran Citation1969). The figure of 5% has been considered too high by some authors or for some taxa; for example Kenward (Citation2001) suggested a limit of no more than 3%, supported by studies of nest abandonment in albatross and petrel species (Phillips et al. Citation2003, Casper Citation2009).
In recent years, further research has shown a simple percentage mass rule of thumb is likely to be over-simplified. For example, various studies have shown that factors such as device-induced drag (Vandenabeele et al. Citation2012), tag shape, and attachment location (Kay et al. Citation2019) are also critical considerations. These considerations, coupled with the apparently species-specific nature of tag effects, highlight the importance of testing for tagging impacts on individual species.
European Nightjars Caprimulgus europaeus (henceforth, Nightjar), breeding in Welsh upland coniferous forest, are difficult to monitor using conventional survey techniques due to their crepuscular nature, cryptic camouflage, and low-population density (Cross et al. Citation2005, Gilbert et al. Citation1998). Therefore, a combination of radio tracking and observational nest finding methods have generally been used together for such studies at upland sites in Wales.
Radio transmitters and GPS devices suitable for deployment on Nightjars have been available for some time, and have been widely used in breeding studies, most often as tail-mounted devices (Alexander & Cresswell Citation1990, Cross et al. Citation2005, Evens et al. Citation2018). Despite their widespread use in studies of breeding Nightjars (Sharps et al. Citation2015, Evens et al. Citation2017) there is, to our knowledge, no published study of the effects of such tag deployment on breeding success. It is, however, critical that such effects should be investigated so that risks can be evaluated and minimized (Wilson & Burnham Citation2006, Casas et al. Citation2015).
An additional challenge in evaluating tag effects is to distinguish them from environmental impacts on survival or breeding success due to factors such as habitat quality or weather. Previous studies on nest survival in Nightjars have identified probable effects of weather on nest survival (English et al. Citation2018) and similar effects are widely documented from studies in other species (Miller et al. Citation2017, Martin et al. Citation2017). It is, therefore, critical that studies of tag effects account for such variables to accurately gauge any evidence of effects. In the present study, we therefore considered tag effects together with a set of environmental variables that we hypothesized may influence Nightjar breeding success.
Here, we directly compare the observed nesting success of tagged birds and untagged birds, in order to investigate the potential effects of tag deployment and environmental variables on nesting Nightjars. These data have been collected as part of on-going ecological impact monitoring requirements associated with the Brechfa West Wind Farm development. The data set includes nest record data from the study site during the pre-development, construction, and operational phases of the wind farm.
Methods
Study species
Nightjars are ground nesting birds that typically lay two eggs (occasionally one egg) and usually produce two broods per breeding season (Holyoak Citation2001). The Nightjar is usually thought of as a heathland species, but in Wales they mainly breed in clear-fell forestry (i.e. recently felled forestry, before substantial re-planting/re-growth), check coupes (i.e. stands of stunted tree growth), and recently restocked conifer plantations (Conway et al. Citation2007). Male Nightjars establish breeding territories within the study area in May; females arrive in mid-May, and subsequently become paired with established territorial males.
Nightjars are of conservation concern due to historic population declines and range contraction (Balmer et al. Citation2013, Hagemeijer & Blair Citation1997). The Nightjar is Annex 1 species in the European Union (Council Directive 2009/147/EC) has Amber status in the United Kingdom (Bird of Conservation Concern; Eaton et al. Citation2015) and is listed under Section 7 of the Environment (Wales) Act 2016. The Nightjar population in Wales has been increasing since at least 1981 (Morris et al. Citation1994), possibly due to increased habitat availability following the maturation and felling of plantations that were planted in the 1950s.
Study site
This study uses nest data from Brechfa Forest (South Wales, UK, 51.967432° N 4.1964175° W), a commercial forestry plantation managed by Natural Resources Wales on behalf of the Welsh Government. The forest is dominated by dense Sitka Spruce Picea stitchensis blocks (coupes), interspersed with recently felled areas around wind turbines, and with semi-natural woodland along watercourses. Topography and forest age at this site have enabled observational nest finding to be relatively successful during recent commercial ecological monitoring work.
Nest data collection
The inclusion of the Nightjar in species protection legislation ensures that their nest locations are protected from damage/destruction under the Wildlife and Countryside Act (1981). Suitably licensed and experienced individuals undertook all tagging and nest monitoring visits completed in this study.
Territory identification
Active territories were located by systematic searches in areas of suitable habitat, and were confirmed by observation of pairs or of displaying males, which produce a distinctive ‘churring’ call (Ferguson-Lees et al. Citation2011).
Observational nest location
Nest searching commenced annually in late May and continued until August. Active territories were systematically watched on multiple occasions by multiple observers at dusk, and visual cues were used to guide follow-up nest searches (Langston et al. Citation2007). Subsequent nest searches consisted of detailed visual inspection in areas of observed Nightjar activity during dusk watches, with searchers aiming to pass within 3–4 m of any point within the search area.
Radio tracking nest location
Where observation of active Nightjar territories yielded little information, or nest searches were unsuccessful, or where pairs were considered likely to attempt a second brood, then these territories/pairs were targeted for radio tagging effort. Mist nets were set up in the vicinity of identified territories, and male Nightjars were then lured into the mist nets by playing the typical territorial song of the species (Squire & Alexander Citation1981). Song playback proved less effective at attracting incubating females. Females were caught by mist-netting at favoured feeding sites, or by trapping at known first brood nests (found by field observation) to allow radio tracking to second brood nests.
Captured birds were fitted (under licence) with PIP-3 radio-transmitters (from Lotek Ltd; Alexander & Cresswell Citation1990), attached to the base of one of the central tail feathers. Attaching the radio-transmitters in this way ensures that they are shed during post-breeding moult in the wintering grounds, and thus does not affect the birds during their spring migration. The tags used in this study each weighed 1.2 g, male Nightjars weighed between 60.2–87.0 g (n = 34), and females weighed between 69.0–100.8 (n = 23), so tags weighed 1.34–1.99% of male body weight, and 1.19–1.72% of female body weight.
Tags were deployed across the breeding season, with tagging dates ranging between the 3rd June and 24th July. The median tagging date was 25th June; the mean tagging date was the 25th June for females and 27th June for males. Tags were deployed both prior to and after nests were located; 19 of the 39 tagged females were tagged after their nest was located, as were 11 of the 25 tagged males.
Following the identification of active nests through either observation or radio tracking, all nests were monitored to their natural completion (fledging or nest failure) by an experienced Nightjar fieldworker, using regular (approximately weekly) nest site visits. Nests were classified as either successful or failed, based on a combination of the timing of nest visit records and available evidence at the nest site and within the territory (i.e. presence of flying young).
Weather data
In order to account for the influence of weather on nesting success, data from the closest available weather station (Pembrey; 51.7144117°N 4.366197°W, approximately 30 km south of the study site) was obtained using the GSODR package (Sparks et al. Citation2017) using R software version 3.6.1 (R Development Core Team Citation2019), implemented via R Studio (RStudio team Citation2018). The GSODR package provides automated downloading, parsing, and cleaning of Global Surface Summary of the Day (GSOD) (United States National Oceanic and Atmospheric Administration National Climatic Data Center) weather data. This provided daily rainfall (mm) and mean temperature (Tm, °C). Data manipulation and visualization were undertaken using the R libraries tidyverse (Wickham et al. Citation2019), lubridate (Grolemund & Wickham Citation2011), and ggplot2 (Wickham Citation2016). Mean temperature and mean precipitation were calculated for the active period of each nest (laying date to last known presence) and used in subsequent analysis.
Statistical analysis
We performed all statistical analyses in R 3.6.0 (R Core Team 2019). In order to account for the inherent bias in nest studies arising from the lower detection probability of failed nests (due to their shorter time available for potential observation), we estimated daily nest survival rates (DSR; Mayfield Citation1975, Dinsmore et al. Citation2002) using RMark version 2.2.7 and MARK (Laake Citation2013, White & Burnham Citation1999).
DSR were estimated and modelled with selected covariates using the R package RMark version 2.2.7 (Laake Citation2013). We undertook model selection of nest survival models using an information theoretical approach based on the second-order Akaike information criterion for small sample sizes (AICc; Burnham & Anderson Citation2002).
A set of 193 biologically plausible models was derived, including additive effects of Julian day, nest age (as estimated based on hatch date, if available, or if not then using estimates based on egg floatation (Westerskov Citation1950) or observational information), brood (first, second, third), year, mean rainfall within the relevant active nest period, mean temperature (Tm) within the relevant active nest period, the presence of windfarm construction activity (binary yes/no – nest active in year of construction activity), adult male tag status (tag status of the male associated with nest - binary yes/ no), adult female tag (tag status of the female associated with nest – binary yes/ no), and combined adult tag status (tag status of both adults associated with nest; binary yes/ no, i.e. both birds tagged or not). The candidate models also included the interaction between mean temperature and date, to help distinguish the effect of temperature from seasonality. The combined adult tag status variable was included to account for potential synergistic effects of tagging both parents. All covariates were scaled prior to analysis, to have a mean of zero and a standard deviation of one. The set of candidate models also included a global model (containing all candidate independent variables) and a null model (containing no independent variables). Co-linearity between variables was determined using Pearson’s correlation coefficient, and this identified low levels of correlation between candidate model variables. No candidate model variables exceeded the threshold correlation of 0.7 (Dorman et al. Citation2013) and all candidate variables were thus included in the analysis.
Models were ranked using AICc, and the ΔAICc values and Akaike weights (wi) were used to infer support for each of the candidate models (online Table S1). In our model selection analysis, no single model was clearly better than all others, and to account for model selection uncertainty, models within two AICc units of the top model were selected for model averaging, as this can provide a robust means of obtaining parameter estimates in such scenarios (Burnham & Anderson Citation2002, Grueber et al. Citation2011, Harrison et al. Citation2018). A weighted average of the parameter estimates (and 95% confidence limits) was calculated for all of the variables contained in the top models, using the package MuMIn (Grueber et al. Citation2011, Barton Citation2018, Mwangi et al. Citation2018) (). Parameters were considered statistically significant where their model-averaged 95% confidence limits did not span zero.
Overall nest survival was calculated from predictions DSR made by the final, averaged model. These were converted to the overall nest success by assuming a 36-day standard nesting period (DSR^36) from the median nest initiation date. Variance in the nest survival estimates were obtained using the delta method (Powell Citation2007).
The same suite of models was also re-run using a subset of the data representing the egg stage and chick stage respectively. Whilst this reduced the sample size for these models, it was considered to potentially provide greater insights into potential tag effects during the two different breeding stages, given the likely different energetic demands and behaviours associated with each stage. Due to convergence problems, because of small sample sizes, the chick stage models were run without the year parameter.
Results
Nest finding and monitoring
Eighty-five Nightjar nests were located over the course of the study (2013–2019); 61 of these were located through direct observation of adult behaviour, and 24 were located using radio tracking. Median nest initiation date was 16th June (range = 27th May–27th July). In total, 59 nests were confirmed first brood nests and 13 confirmed second brood nests. Two nest attempts were also recorded as ‘third brood’ nests, although these were a result of early failure of previous nesting attempts (first or second broods) and thus are replacement clutches; they have nevertheless been referred to as third brood nests for the ease of reference. Brood number could not be confirmed at 11 of the located nests.
We found nests at different stages of development: 52 (61.1%) during incubation and 33 (38.8%) were found during the nestling period. From all of the nests, 52 fledged at least one chick, whilst the remainder (33) failed, with 15 at the egg stage and 18 failing at the chick stage. A summary of nest success and the number of nests with attending tagged adults is provided in , whilst details the breakdown of nests attended by tagged adults, by adult sex, and brood number.
Table 1. Summary of outcomes for monitored Nightjar nests. Total number of successful nests (fledging one chick or more) and percentage success rates, with a breakdown by tag status of the attending adults and brood number.
Table 2. Summary of number of Nightjar nests attended by tagged parents, broken down by brood status.
Nest survival
In our model selection analysis, there were three models within 2 AIC units and they contained the following variables: nest age, female tag status, adult tag status, temperature, precipitation, and Julian day (). In order to account for model selection uncertainty, a conditional weighted average (averaged over only the models containing those parameters) and a full weighted average (all models using zero value for parameters not present) of the parameter estimates and 95% confidence limits was calculated for all of the variables contained in the top three models (conditional weighted averages in , and full weighted averages in ). Full weighted model average parameter estimates are reported below, along with the standard error (se).
Table 3. Conditional model averaged estimates (± se) of the effects of mean rainfall, mean temperature, nest age, time (days from 28th of May), construction year, adult female tag status, and adult male or female tag status, on daily nest survival rates (DSR) of Nightjars at Brechfa Forest. Model averaged parameter estimates were derived by weighted averaging across all models within 2 AICc units of the top model (). Parameters in bold are considered to have an important effect based on 95% CL.
Table 4. Full model averaged estimates (± se) of the effects of mean rainfall, mean temperature, nest age, time (days from 28th of May), construction year, adult female tag status, and adult male or female tag status, on daily nest survival rates (DSR) of Nightjars at Brechfa Forest. Model averaged parameter estimates were derived by weighted averaging across all models within 2 AICc units of the top model (). Parameters in bold are considered to have an important effect based on 95% CL.
Estimated average daily nest survival (± se), across all years and tag treatments, was 0.986 (± 0.008). This extrapolates over the 36-day nesting cycle to an average annual nest success rate of 0.63 (± 0.18).
The same suite of models run on subsets of the full data set for the egg stage of the nesting cycle failed to identify any parameters as having an important effect on DSR and identified no detectable difference between DSR for tagged nests versus untagged nests at either stage. Top selected models and model averaged coefficients for the identified top models are presented in online supplementary Table S2, S3, and S4. The same suite of models for the chick stage of the nesting cycle failed to converge due to low sample sizes.
Radio tag effects
There was no evidence for tags reducing nesting success. Although two of the three top models of DSR included either female tag status or adult tag status variables, these all indicated a positive relationship that was not significant: a result confirmed by the averaged model (β fm_tag = +0.158 ± 0.429; β f_tag = +0.445 ± 0.499).
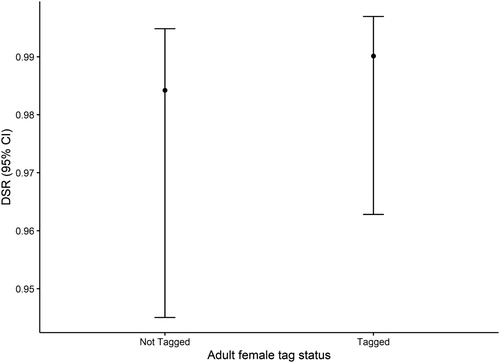
Overall DSR (± se) for untagged female attended nests and tagged female attended nests were 0.984 (± 0.010) and 0.990 (± 0.006), respectively (). Estimated DSR for untagged and tagged adult attended nests (male or female) were very similar at 0.986 (± 0.010) and 0.991 (± 0.006), respectively ().
Figure 1. Relationship between daily survival rate (DSR) and radio tag deployment status of parental adult Nightjar at Brechfa Forest, Carmarthenshire, Wales, 2013–2019. Daily survival results are based on 85 nests pooled across 2013–2019. The points represent the estimated mean DSR values, and the bars represent the 95% confidence intervals.
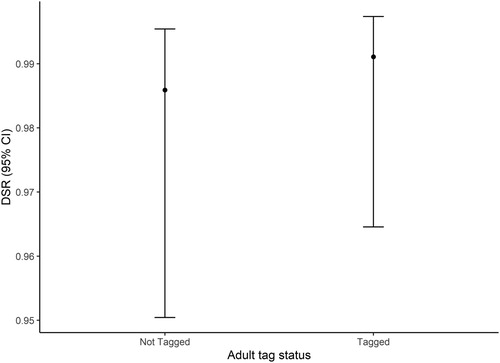
Figure 2. Relationship between daily survival rate (DSR) and radio tag deployment status of parental female adult Nightjar at Brechfa Forest, Carmarthenshire, Wales, 2013–2019. Daily survival results are based on 85 nests pooled across 2013–2019. The points represent the estimated mean DSR values, and the bars represent the 95% confidence intervals.
Nest age and Julian day
The top model of daily survival rate included significant effects of Julian day and nest age (initiation date; ). Nest survival rate of Nightjar decreased as the season progressed (model-averaged parameter ± se; β Julian day = −0.07 ± 0.023) but increased with the age of the nest (β nest initiation date = +0.072 ± 0.028). Over the nesting season, model averaged DSR ranged from 0.988 (± 0.012) on day 1 of the nesting season (28th May) to 0.986 (± 0.013) on day 81 (17th August).
Table 5. Top models (models within 2 AICc units of the top model) of Nightjar daily nest survival rates, for a set of models including mean rainfall (m_prcp2), average temperature (m_temp), nest age (NestAge), time (Julian day), adult female tag status (f_tag), year (2013–2019), and adult tag status (fm_tag).
Weather effects
Initial data exploration of weather data identified a weak positive correlation between relative humidity (surrogate for cloud cover) and minimum temperature (tau = 0.177), with a similar positive correlation noted between relative humidity (surrogate for cloud cover) and minimum temperature (tau = 0.219). As such, weather effects should be interpreted in this context.
The top models together provide good evidence that temperature has an important effect on nest success, as temperature was consistently selected in top models. Alternative models without this variable did not receive strong statistical support and were at least 2.7 AICc units from the top model.
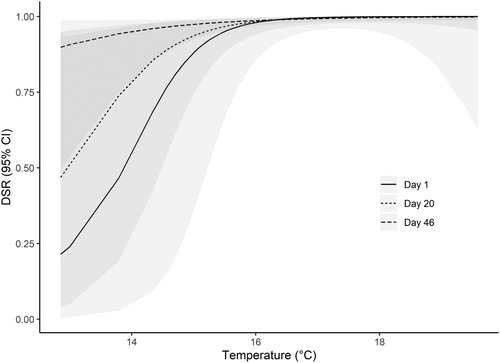
Average temperatures during active nest periods over the study years ranged from 12.8°C to 19.5°C, and model predictions showed a positive relationship with temperature (β m_temp = +2.501 ± 1.083; ). As confidence intervals did not include zero, this is considered a statistically significant effect. The top models also consistently incorporated an interactive effect between temperature and Julian day on DSR, and this interaction term appeared in all top models.
Model estimates show a negative parameter for the temperature x Julian day interaction term (β m_temp: Time = −0.035 ± 0.019; ). As confidence intervals include zero this is however not considered to be a statistically significant interaction. Despite this, the important effects of temperature on DSR must be viewed in the context of its relationship with time, as its inclusion in top models suggests that the magnitude of the positive effect temperature is potentially conditioned on Julian day. This interaction term describes how the effect of temperature varies through time, and indicates that the positive effect of temperature on DSR depends on the Julian day and decreases through the breeding season. This may be due to threshold effects of temperature, as temperature exhibits a non-linear relationship with time through the season, or could be due to further interactions with the stage of nest development – i.e. nests are more likely to have chicks later in the season.
Predicted DSR increased from 0.36 (95% confidence intervals, CI = 0.03–0.920) to 0.999 (95% CI 0.994–0.999) over the recorded temperature range (12.8–19.5°C), for a nest initiated on the 16th June (median date of nest initiation) assuming average values for the other covariates ().
Figure 3. Model averaged predicted daily nest survival rate in relation to mean temperature during the Nightjar nesting period in Brechfa Forest, Carmarthenshire, Wales, 2013-2019. Estimates (lines) and 95% confidence bands (shaded) are shown for day 1 of the season (28th May), day 20 (16th June–median nest initiation date), and day 46 (12th July – median hatch date), with other covariates fixed at mean values.
Mean daily rainfall during the active nest periods ranged from 0 to 10.55 mm, with a mean of 2.10 mm. No significant effect of precipitation on DSR was detected (β m_prcp 0.509 ± 0.382, ); confidence intervals for this estimate spanned zero, suggesting a lack of any statistically significant effect.
Discussion
Mean temperature, time and nest age were identified as important factors associated with annual reproductive success of Nightjars at the study site ( and ). No evidence for a negative effect of tagging was identified by the models of nest survival, and this is consistent with the raw data, where mean nest success across the seven years of the study was 61% for nests attended by one or two tagged parents, and 62% for nests attended by untagged parents. This provides good evidence that the continued use of radio tagging to facilitate nest finding is unlikely to impact nest survival.
Models identified no evidence that any of the other candidate variables affected nesting success, with no statistically significant effect noted for precipitation, brood, or year of construction. Previous studies of Nightjar nest success have focused on the effects of recreational disturbance (Langston et al. Citation2007, Lowe et al. Citation2014) and in general have identified a negative effect of such disturbance, but have not investigated relationships with tagging, time, or weather. Langston et al. (Citation2007) estimated overall nesting success to be 39% in the Dorset heathlands, whereas Lowe et al. (Citation2014) estimated success at 53% in Nottinghamshire plantation forestry sites. Overall nest success estimates of 61–62% from the upland forestry habitats of the Brechfa Forest study site thus compare favourably with reported nest success rates from other studies.
A significant effect of nest age on daily survival rate was identified, with DSR increasing with nest age within individual breeding attempts. Similar variation in chick/nest survival with age has been observed in other species (Grant et al. Citation2005, McDonald et al. Citation2016, English et al. Citation2018, Maziarz et al. Citation2019, Zhao et al. Citation2020). The positive pattern noted here could be due to older chicks having greater resilience to poor weather and being more able to overcome the nutritional and thermoregulatory burden of poor weather, as has been suggested for Northern Bobwhite chicks Colinus virginianus (Terhune et al. Citation2019).
The identified positive association between temperature and nest survival is unsurprising, as during periods of low temperature nests can fail due to chick starvation (pers. obs.) and similar positive effects of temperature have been made in a North American nightjar species the Whip-Poor-Will Antrostomus vociferous (English et al. Citation2018). In general, young, downy chicks are likely to be less able to thermally regulate effectively (Du Rant et al. Citation2012, Newberry & Swanson Citation2018), and thus may be particularly vulnerable to adverse weather and predation. Young chicks will repeatedly call when chilled; this advertisement is likely to increase predation risk as has been observed in other bird species (Dearborn Citation1999, Briskie et al. Citation1999, Ibanez-Alamo et al. Citation2012, Husby Citation2019, Goncharova & Klenova Citation2019), and may form part of the mechanism by which low temperature leads to nest failure. In addition, moth activity is generally positively correlated with temperature (Holyoak et al. Citation1997), so a direct negative effect of cold weather on nest success through reduced food availability, would be expected though direct impacts on provisioning at the chick stage, or indirectly through reduced incubation intensity at the egg stage. Similar effects of temperature on chick survival have also been noted in the Whip-Poor-Will (English et al. Citation2018) with higher chick survival recorded on warmer nights.
It is surprising, however, that rainfall did not show a negative effect on nest survival, as nest failure due to hypothermia/starvation has previously been recorded following protracted heavy rain (pers. obs.), and moth activity is generally negatively correlated with rainfall (Holyoak et al. Citation1997). One explanation may be the presence of a positive correlation between the minimum daily temperature (likely at night) and rainfall (tau = 0.177), as during cloudy conditions night-time temperatures are usually higher than under clear skies. This may be particularly relevant for the dawn foraging period for Nightjars, when at 300 m elevation (as at the study site), the temperature is often below 10°C following a night of clear skies during the main breeding season (online Figures S1 and S2). Hence it may be that extreme rainfall events have a negative effect by causing direct chick mortality, as has been shown in the White Stork Ciconia ciconia (Tobolka et al. Citation2015) and Northern Wheatear Oenanthe oenanthe (Oberg et al. Citation2015), but food availability is perhaps increased both when evenings are warm following sunny weather, and during cloudy, drizzly conditions, when both dusk and dawn foraging periods are relatively mild. This increase in food availability may lead to improved nest survival, as has been noted in other species (White Ibis Eudocimus albus, Herring et al. Citation2011; Eurasian Reed Warbler Acrocephalus scirpaceus, Vafidis et al. Citation2016). However, more work is needed in this area, including collecting insect abundance data, to try to unpick the relationships between weather, insect abundance and nest survival (Shewring et al. CitationForthcoming).
Wind farm construction had no observable effect on the daily nest survival rate, and the year of construction variable was not selected in any of the top models. It is, however, worth noting that any effects of construction disturbance are likely to be influenced by the proximity of individual nests to construction activity. Such detailed data were not available to inform the current study, but would certainly be recommended in future studies focused on the effects of construction disturbance. In addition, there were deliberate attempts to limit any effects of construction on the Nightjars at the Brechfa windfarm (e.g. by using disturbance exclusion buffers around located nests) and as such, this conclusion is only relevant to construction where such mitigation procedures are implemented. In light of this, we would advise that this aspect of the analysis be treated with the appropriate caution when interpreting the sensitivity of Nightjar to construction disturbance.
It should however be noted that nest survival is a single metric for impact identification of tagging, and other effects of tag deployment on Nightjar cannot be discounted based on the current study. It is certainly possible that tagging has affected foraging success and ranging behaviour, as has been noted in other species (Taylor et al. Citation2001, Phillips et al. Citation2003), but any such effects have not fed through to detectable effects on nest survival. As such, we would recommend further study of tag effects in Nightjar, especially where tagging is proposed for longer durations or where heavier tags are proposed.
In conclusion, the current study confirms the importance of weather effects on Nightjar nest survival, particularly the positive effect of temperature. It also confirms the lack of observable tagging effects on nest survival when using tail-mounted radio tags, and indicates that their continued use in nest finding studies is unlikely to have a negative impact on nest survival. Integrating these two conclusions leads us to recommend that future tagging studies adequately consider potentially confounding weather effects.
Acknowledgements
We thank RWE renewables UK Ltd for the help and support during fieldwork at Brechfa West Wind Farm and two anonymous reviewers for their insightful and helpful comments. Mike Shewring would also like to thank the Knowledge Economy Skills Scholarship (KESS2) and Wildlife Trust of South and West Wales for their financial support.
Additional information
Funding
References
- Alexander, I. & Cresswell, B. 1990. Foraging by Nightjars Caprimulgus europaeus away from their nesting areas. Ibis 132: 568–574.
- Balmer, D.E., Gillings, S., Caffrey, B.J., Swann, R.L., Downie, I.S. & Fuller, R.J. eds. 2013. Bird Atlas 2007–11: the breeding and wintering birds of Britain and Ireland. BTO Books, Thetford.
- Barron, D.G., Brawn, J.D. & Weatherhead, P.J. 2010. Meta-analysis of transmitter effects on avian behaviour and ecology. Methods Ecol. Evol. 1: 180–187.
- Barton, K. 2018. Multi-model inference. R Package version 1.15. 6. 2016. http.CRANR-project.org/package=MuMIn.
- Bell, S.C., Harouchi, M.E.L., Hewson, C.M. & Burgess, M.D. 2017. No short- or long-term effects of geolocator attachment detected in Pied Flycatchers Ficedula hypoleuca. Ibis 159: 734– 743. https://doi.org/10.1111/ibi.12493
- Bodey, T.W., Cleasby, I.R., Bell, F., Parr, N., Schultz, A., Votier, S.C. & Bearhop, S. 2018. A phylogenetically controlled meta-analysis of biologging device effects on birds: deleterious effects and a call for more standardized reporting of study data. Methods Ecol. Evol. 9: 946–955.
- Bowlin, M.S., Henningsson, P., Muijres, F.T., Vleugels, R.H.E., Liechti, F. & Hedenström, A. 2010. The effects of geolocator drag and weight on the flight ranges of small migrants. Methods Ecol. Evol. 1: 398–402.
- Brander, R.B. & Cochran, W.W. 1969. Radio location telemetry. In R. H. Giles Jr. (ed) Wildlife Management Techniques, 95–103. The Wildlife Society, Washington, DC.
- Briskie, J.V., Martin, P.R. & Martin, T.E. 1999. Nest predation and the evolution of nestling begging calls. Proc. R. Soc. London Ser. B Biol. Sci. 266: 2153–2159.
- Brlík, V., Koleček, J., Burgess, M., Hahn, S., Humple, D., Krist, M., Ouwehand, J., Weiser, E.L., Adamík, P., Alves, J.A. & Arlt, D. 2020. Weak effects of geolocators on small birds: a meta-analysis controlled for phylogeny and publication bias. J. Anim. Ecol. 89: 207–220.
- Burnham, K.P. & Anderson, D.R. 2002. Model Selection and Multimodel Inference: a practical information-theoretic approach, 2nd edn. Springer, Berlin.
- Casas, F., Benítez-López, A., García, J.T., Martín, C.A., Viñuela, J. & Mougeot, F. 2015. Assessing the short-term effects of capture, handling and tagging of sandgrouse. Ibis 157: 115–124.
- Casper, R. 2009. Guidelines for the instrumentation of wild birds and mammals. Anim. Behav. 78: 1477–1483.
- Conway, G., Wotton, S., Henderson, I., Langston, R., Drewitt, A. & Curriem, F. 2007. Status and distribution of European Nightjars Caprimulgus europaeus in the UK in 2004. Bird Study 54: 98–111.
- Cross, T., Lewis, J., Lloyd, J., Morgan, C. & Rees, D. 2005. Science for Conservation Management: European Nightjar Caprimulgus Europaeus Breeding Success and Foraging behaviour in Upland Coniferous Forests in Mid? Llandrindod Wells, Wales.
- Dearborn, D.C. 1999. Brown-headed cowbird nestling vocalizations and risk of nest predation. Auk 116: 448–457.
- Dinsmore, S.J., White, G.C. & Knopf, F.L. 2002. Advanced techniques for modeling avian nest survival. Ecology 83: 3476–3488.
- Dormann, C.F., Elith, J., Bacher, S., Buchmann, C., Carl, G., Carré, G., Marquéz, J.R.G., Gruber, B., Lafourcade, B., Leitão, P.J., Münkemüller, T., McClean, C., Osborne, P.E., Reineking, B., Schröder, B., Skidmore, A.K., Zurell, D. & Lautenbach, S. 2013. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36: 27–46.
- DuRant, S.E., Hopkins, W.A., Wilson, A.F. & Hepp, G.R. 2012. Incubation temperature affects the metabolic cost of thermoregulation in a young precocial bird. Funct. Ecol. 26: 416–422.
- Eaton, M., Aebischer, N., Brown, A., Hearn, R., Lock, L., Musgrove, A. & Gregory, R. 2015. Birds of Conservation concern 4: the population status of birds in the UK, Channel Islands and Isle of Man. Br. Birds 108: 708–746.
- English, P.A., Nocera, J.J. & Green, D.J. 2018. Nightjars may adjust breeding phenology to compensate for mismatches between moths and moonlight. Ecol. Evol. 8: 5515–5529.
- Evens, R., Beenaerts, N., Ulenaers, E., Witters, N. & Artois, T. 2018. An effective, low-tech drop-off solution to facilitate the retrieval of data loggers in animal-tracking studies. Ring. Migr. 33: 10–18.
- Evens, R., Beenaerts, N., Witters, N. & Artois, T. 2017. Study on the foraging behaviour of the European Nightjar Caprimulgus europaeus reveals the need for a change in conservation strategy in Belgium. J. Avian Biol. 48: 1238–1245.
- Ferguson-Lees, J., Castell, R. & Leech, D. 2011. A Field Guide to Monitoring Nests. Thetford: British Trust for Ornithology.
- Gilbert, G., Gibbons, D.W. & Evans, J. 1998. Bird Monitoring Methods. RSPB, Sandy.
- Goncharova, M.V. & Klenova, A.V. 2019. Siberian crane chick calls reflect their thermal state. Bioacoustics 28: 115–128.
- Grant, T.A., Shaffer, T.L., Madden, E.M. & Pietz, P.J. 2005. Time-specific variation in passerine nest survival: new insights into old questions. Auk 122: 661–672.
- Grolemund, G. & Wickham, H. 2011. Dates and times made easy with lubridate. J. Stat. Softw. 40: 1–25.
- Grueber, C.E., Nakagawa, S., Laws, R.J. & Jamieson, I.G. 2011. Multimodel inference in ecology and evolution: challenges and solutions. J. Evol. Biol. 24: 699–711.
- Hagemeijer, W.J. & Blair, M.J. 1997. The EBCC Atlas of European Breeding Birds. Poyser, London.
- Harrison, X.A., Donaldson, L., Correa-Cano, M.E., Evans, J., Fisher, D.N., Goodwin, C.E.D., Robinson, B.S., Hodgson, D.J. & Inger, R. 2018. A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ. 6: e4794.
- Hensler, G.L. & Nichols, J.D. 1981. The Mayfield method of estimating nesting success: a model, estimators and simulation results. Wilson Bull. 93: 42–53.
- Herring, G., Cook, M.I., Gawlik, D.E. & Call, E.M. 2011. Food availability is expressed through physiological stress indicators in nestling white ibis: a food supplementation experiment. Funct. Ecol. 25: 682–690.
- Hines, J.E. & Zwickel, F.C. 1985. Influence of radio packages on young blue grouse. J. Wildl. Manage. 49: 1050–1054.
- Holyoak, D.T. 2001. Nightjars and Their Allies: the Caprimulgiformes (Vol. 7). Oxford, UK: Oxford University Press.
- Holyoak, M., Jarosik, V. & Novak, I. 1997. Weather-induced changes in moth activity bias measurement of long-term population dynamics from light trap samples. Entomol. Exp. Appl. 83: 329–335.
- Husby, M. 2019. Nestling begging calls increase predation risk by corvids. Anim. Biol. 69: 137–155.
- Ibáñez-Álamo, J.D., Arco, L. & Soler, M. 2012. Experimental evidence for a predation cost of begging using active nests and real chicks. J. Ornithol. 153: 801–807.
- Kay, W.P., Naumann, D.S., Bowen, H.J., Withers, S., Evans, B., Wilson, R., Stringell, T., Bull, J., Hopkins, P. & Börger, L. 2019. Minimizing the impact of biologging devices: using computational fluid dynamics for optimizing tag design and positioning. Methods Ecol. Evol. 10: 1222–1233.
- Kenward, R.E. 2001. A Manual for Wildlife Radio Tagging. Academic Press, London, UK.
- Laake, J.L. 2013. RMark: An r interface for analysis of capture-recapture data with MARK. AFSC Processed Reports. 25p. Alaska Fisheries Science Center, NOAA, Natl. Mar. Fish. Serv., 7600. Sand Point Way NE, Seattle, WA.
- Langston, R.H.W., Liley, D., Murison, G., Woodfield, E. & Clarke, R.T. 2007. What effects do walkers and dogs have on the distribution and productivity of breeding European Nightjar Caprimulgus europaeus? Ibis 149: S27–S36.
- Lowe, A., Rogers, A.C. & Durrant, K.L. 2014. Effect of human disturbance on long-term habitat use and breeding success of the European Nightjar, Caprimulgus europaeus. Avian Conserv. Ecol. 9: 6.
- MacDonald, E.C., Camfield, A.F., Martin, M., Wilson, S. & Martin, K. 2016. Nest-site selection and consequences for nest survival among three sympatric songbirds in an alpine environment. J. Ornithol. 157: 393–405.
- Martin, K., Wilson, S., MacDonald, E.C., Camfield, A.F., Martin, M. & Trefry, S.A. 2017. Effects of severe weather on reproduction for sympatric songbirds in an alpine environment: interactions of climate extremes influence nesting success. Auk 134: 696–709.
- Mayfield, H. 1975. Suggestions for calculating nest success. Wilson Bull. 87: 456–466.
- Maziarz, M., Grendelmeier, A., Wesołowski, T., Arlettaz, R., Broughton, R.K. & Pasinelli, G. 2019. Patterns of predator behaviour and Wood Warbler Phylloscopus sibilatrix nest survival in a primaeval forest. Ibis 161: 854–866.
- Miller, M.W., Leech, D.I., Pearce-Higgins, J.W. & Robinson, R.A. 2017. Multi-state, multi-stage modeling of nest-success suggests interaction between weather and land-use. Ecology 98: 175–186.
- Morris, A., Burges, D., Evans, A., Smith, K. & Fuller, R. 1994. The status and distribution of Nightjars Caprimulgus europaeus in Britain in 1992. A report to the British Trust for Ornithology. Bird Study 41: 181–191.
- Mwangi, J., Ndithia, H.K., Kentie, R., Muchai, M. & Tieleman, B.I. 2018. Nest survival in year-round breeding tropical red-capped larks Calandrella cinerea increases with higher nest abundance but decreases with higher invertebrate availability and rainfall. J. Avian Biol. 49: e01645.
- Newberry, G.N. & Swanson, D.L. 2018. Elevated temperatures are associated with stress in rooftop-nesting Common Nighthawk (Chordeiles minor) chicks. Conserv. Physiol. 6: 010.
- Öberg, M., Arlt, D., Pärt, T., Laugen, A.T., Eggers, S. & Low, M. 2015. Rainfall during parental care reduces reproductive and survival components of fitness in a passerine bird. Ecol. Evol. 5: 345–356.
- Phillips, R.A., Xavier, J.C. & Croxall, J.P. 2003. Effects of satellite transmitters on albatrosses and petrels. Auk 120: 1082–1090.
- Powell, L.A. 2007. Approximating variance of demographic parameters using the delta method: a reference for Avian biologists. Condor 109: 949–954.
- R Development Core Team. 2019. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing.
- Robert, M., Drolet, B. & Savard, J.P.L. 2006. Effects of backpack radio-transmitters on female Barrow's Goldeneyes. Waterbirds 29: 115–120.
- RStudio Team. 2018. RStudio: integrated development for R. RStudio, Inc., Boston, MA. http://www.rstudio.com/.
- Sharps, K., Henderson, I.A.N., Conway, G., Armour-Chelu, N. & Dolman, P.M. 2015. Home-range size and habitat use of E uropean N ightjars C aprimulgus europaeus nesting in a complex plantation-forest landscape. Ibis 157: 260–272.
- Shewring, M., Vaughan, I.P. & Thomas, R.J. Forthcoming. Coniferous plantation woodland moth biomass and diversity - implications for aerial nocturnal insectivores.
- Sparks, A., Hengl, T. & Nelson, A. 2017. GSODR: global summary daily weather data in R. J. Open Source Softw. 2: 177.
- Squire, T. & Alexander, I. 1981. Capture techniques for full grown Nightjars. Ringers’ Bull. 5: 132.
- Taylor, G. & Thomas, A. 2002. Animal flight dynamics II. Longitudinal stability in flapping flight. J. Theor. Biol. 214: 351–370.
- Taylor, S.S., Leonard, M.L., Boness, D.J. & Majluf, P. 2001. Foraging trip duration increases for Humboldt penguins tagged with recording devices. J. Avian Biol. 32: 369–372.
- Terhune, T.M., Palmer, W.E. & Wellendorf, S.D. 2019. Northern bobwhite chick survival and effects of weather. J. Wildl. Manage. 83: 963–974.
- Tobolka, M., Zolnierowicz, K.M. & Reeve, N.F. 2015. The effect of extreme weather events on breeding parameters of the White Stork Ciconia ciconia. Bird Study 62: 377–385.
- Vafidis, J.O., Vaughan, I.P., Jones, T.H., Facey, R.J., Parry, R. & Thomas, R.J. 2016. The effects of supplementary food on the breeding performance of Eurasian reed warblers Acrocephalus scirpaceus; implications for climate change impacts. PLoS One 11: e0159933.
- Vandenabeele, S.P., Grundy, E., Friswell, M.I., Grogan, A., Votier, S.C. & Wilson, R.P. 2014. Excess baggage for birds: inappropriate placement of tags on gannets changes flight patterns. PLoS One 9: e92657.
- Vandenabeele, S.P., Shepard, E.L.C., Grogan, A. & Wilson, R.P. 2012. When three per cent may not be three per cent; device-equipped seabirds experience variable flight constraints. Mar. Biol. 159: 1–14.
- Wanless, S., Harris, M.P. & Morris, J.A. 1988. The effect of radio transmitters on the behavior of common murres and razorbills during chick rearing. Condor. 90: 816–823.
- Weidinger, K. 2008. Nest monitoring does not increase nest predation in open-nesting songbirds: inference from continuous nest-survival data. Auk 125: 859–868.
- Westerskov, K. 1950. Methods for determining the age of game bird eggs. J. Wildl. Manage. 14: 56–67.
- White, G.C. & Burnham, K.P. 1999. Program MARK: survival estimation from populations of marked animals. Bird Study 46: S120–S139.
- Wickham, H. (2016). ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag. ISBN 978-3-319-24277-4, https://ggplot2.tidyverse.org.
- Wickham, H., Averick, M., Bryan, J., Chang, W., McGowan, L.D.A., François, R., Grolemund, G., Hayes, A., Henry, L., Hester, J. & Kuhn, M. 2019. Welcome to the Tidyverse. J. Open Source Softw. 4: 1686.
- Wilson, R.P. & McMahon, C.R. 2006. Measuring devices on wild animals: what constitutes acceptable practice? Front. Ecol. Environ. 4: 147–154.
- Zhao, J.M., Yang, C., Lou, Y.Q., Shi, M., Fang, Y. & Sun, Y.H. 2020. Nesting season, nest age, and disturbance, but not habitat characteristics, affect nest survival of Chinese grouse. Curr. Zool. 66: 29–37.
