ABSTRACT
Capsule
Roosting behaviour during the pre-breeding season is an important part of the Eurasian Griffon Vulture Gyps fulvus life cycle. We demonstrate the importance of roost site selection and its relation to the breeding distribution of the Griffon Vulture.
Aims
To quantify the distribution, population size, age structure and roosting site selection of the Eurasian Griffon Vulture in the Eastern Rhodopes, Bulgaria during the pre-breeding season.
Methods
We used correlation analysis and generalized linear mixed models to examine the relationship between the vultures use of roost sites and their distribution in relation to topographic variables. We additionally used GPS telemetry data from 25 birds to study and compare the use of roosting cliffs.
Results
During the 14-year survey period the number of roosting Eurasian Griffon Vultures gradually increased from 25 birds in 2005 to 201 in 2018. We recorded 16 cliffs used by 124 ± 51 (sd) vultures for roosting. The number of non-adult vultures increased by 25% and reached 41% of all the recorded birds in the last year of the survey. We found that the number of breeding pairs correlated positively with the number of roosting vultures (r = 0.85) and that roosting cliff selection was determined by cliff length and height, distance to the nearest feeding site, and whether the cliff was used for breeding.
Conclusions
Cliffs regularly used by vultures in the pre-breeding season, were afterwards used for breeding. Thus, cliffs used in the pre-breeding season for roosting could be used as an index for the breeding distribution of the species, and should be protected to sustain the species in and out of the breeding season.
The Eurasian Griffon Vulture Gyps fulvus (hereafter Griffon Vulture) is a large Old World vulture, that scavenges carcasses of livestock and wild ungulates (DeVault et al. Citation2003). It is distributed from Portugal to the Himalayas (Ferguson-Lees et al. Citation2001). The species is listed as ‘Least Concern’ by the International Union for Nature Conservation, and is the most common vulture species in Europe (BirdLife International Citation2020). Over 85% of the European population of the Griffon Vulture is concentrated in Spain, where it has increased rapidly and is now considered to be at its highest global density (Del Moral Citation2009). In contrast, Griffon Vulture population size and range have decreased sharply in the Balkans due to poisonings and persecution (Andevski Citation2013, Demerdzhiev et al. Citation2014). In the past it was one of the most abundant vulture species breeding in Bulgaria, numbering probably over 1000 pairs (Demerdzhiev et al. Citation2014). It nearly went extinct in the mid-twentieth century, because of a mass poisoning campaign to exterminate large predators in Bulgaria (Baumgart Citation1974). However, in 1978 the Griffon Vulture was rediscovered breeding in the Eastern Rhodopes, representing the only wild native population of the species surviving in Bulgaria (Demerdzhiev et al. Citation2014). Since the late 1980s that population has been subject to regular monitoring; the number of occupied nests, number of incubating pairs, number of hatched chicks, breeding success and productivity have been recorded annually (Dobrev & Stoychev Citation2013). Nowadays, the species also breeds in the Balkan Mountain range and in the Pirin Mountains, as a result of reintroduction programs, and the national population exceeds 130 pairs distributed in five breeding nuclei (Demerdzhiev et al. Citation2014, Stoynov et al. Citation2018, Dobrev et al. Citation2019).
The breeding season of the Griffon Vulture varies depending upon the geographic latitude and extends between December and August (Donazar Citation1993, Xirouchakis Citation2010). In Bulgaria, egg laying starts in January and February, and juveniles fledge in July-August. During September and November the species undertakes migration and roaming behaviour (Griesinger Citation1998) and subsequently begins the pre-breeding season in late autumn (November–December), which is an important part of the life cycle when pair-bond formation, courtship flights, selection of nests and partners are initiated (Donazar Citation1993, Ferguson-Lees et al. Citation2001). Roost formation is typical for large, social, long-lived birds, such as vultures (Beauchamp Citation1999), and there are a number of hypotheses regarding their formation (Lack Citation1968, Ward & Zahavi Citation1973, Fuller & Mosher Citation1981, Engel et al. Citation1992, Kelly & Thorpe Citation1993). Griffon Vultures roost socially in groups of tens of birds (Ferguson-Lees et al. Citation2001) in either summer or winter, with different preferences towards cliff locations (Xirouchakis & Mylonas Citation2004). However, they can also use temporary communal roosts related to high food availability (Mateo-Tomas & Olea Citation2010a). Summer dynamics of communal roosting of Griffon Vultures and its ecological function have been well-studied in the Iberian Peninsula (Olea & Mateo-Tomas Citation2009, Mateo-Tomas & Olea Citation2010b, Mateo-Tomas & Olea Citation2011). In the Cantabrian Mountains the presence of domestic cows and sheep (Olea & Mateo-Tomas Citation2009) and game hunting activities (Mateo-Tomas & Olea Citation2010a) were directly related to the existence of vulture roosts. Moreover, the vultures can shift their foraging grounds to take advantage of food sources, and the number of immature birds at a roost increases during summer and autumn (Olea & Mateo-Tomas Citation2009).
Despite the major biological and ecological importance of pre-breeding roosting sites for Griffon Vultures (Ferguson-Lees et al. Citation2001, Mateo-Tomas & Olea Citation2010a), roost formation, dynamics, age-structure, roosting cliff selection, their significance to breeding pairs, and the role of the non-adult birds, are not entirely understood and have not been surveyed in Bulgaria, the Balkans or Eastern Europe. The objectives of the this study were to: (1) reveal the pre-breeding season distribution, population size and age structure of the Griffon Vulture during roosting; (2) establish the relationship between Griffon Vulture pre-breeding congregations and the distribution of breeding pairs (3) analyse the topographic features that shape roost cliff selection; and (4) compare the use of the cliffs for roosting in the pre-breeding and breeding seasons.
Methods
Study population
The Griffon Vulture is listed as ‘Endangered’ in the Red Data Book of Bulgaria and is strictly protected by legislation (Golemanski Citation2015). The breeding population in the Eastern Rhodopes, which is the subject of the current study, has one of the highest reproductive rates recorded in Europe (Demerdzhiev et al. Citation2014). The population has seen a gradual recovery as a result of intensive conservation actions over the last 35 years in the study area and the population numbered 94 pairs in 2018.
Study area
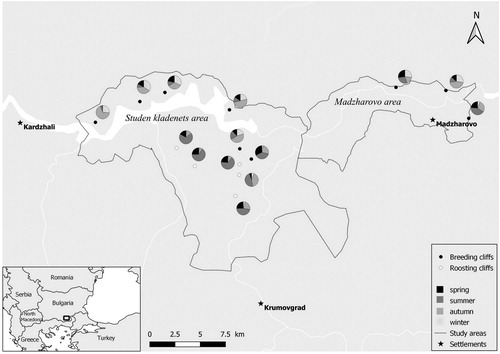
Monitoring of the communal roosts of Griffon Vultures in the pre-breeding season was conducted in the Eastern Rhodope Mountains, Bulgaria. The study area was subdivided into Madzharovo and Studen kladenets following Demerdzhiev et al. (Citation2014) and represents the core breeding area for the Griffon Vulture in the Eastern Rhodope Mountains and in Bulgaria (). This low mountainous region is approximately 5900 km2 in area and is characterized by a high diversity of natural habitats, rich biodiversity and a low density human population (Kopralev Citation2002). The mean daily temperature in winter is between 1°С and 2°С, while the summers can be relatively hot and dry, with mean daily temperatures of 24–25°С. The annual rainfall is between 450 and 900 mm. There are up to 20 days with permanent snow cover annually (Kopralev Citation2002). The area retains the highest diversity of breeding raptor species in Bulgaria (n = 23 species) and sustains 171 breeding bird species (Stoychev et al. Citation2004).
Fieldwork procedures
The roost counts of vultures were conducted annually in November–December for 14 years (2005–2018). We conducted a single count per year and monitored up to 16 cliffs in the area. The observations began at noon and were done simultaneously within one or two days. The study was done under suitable weather conditions by experienced observers with spotting scopes (20 × 60) and binoculars (10 × 50) from elevated viewpoints opposite the monitored cliffs. Roosting sites were identified based on the monitoring data of breeding sites, but also the monitoring of other raptors, where we have checked all cliffs for occupancy (for details see Demerdzhiev et al. Citation2014). Additionally, since 2016 onwards we used global positioning system (GPS) data from 25 Griffon Vultures tagged with GPS transmitters to check and locate unknown roosting sites and to compare the use of the cliffs for communal roosting at different times of year. Vultures were captured using a walk-in trap or at their nests before fledging and were fitted with GPS tags (E-Obs, www.e-obs.de; Ecotone, http://www.ecotone-telemetry.com; Microwave telemetry, www.microwavetelemetry.com) attached as backpacks with 11.2 mm Teflon ribbon. All Griffon Vultures were marked with a coloured wing tag, standard metal ring and plastic colour ring to aid their identification in the wild. The transmitter harness, rings and wing tag did not exceed 3% of a bird’s body mass, in accordance with the recommended limits (Bodey et al. Citation2018). The GPS fixes acquired at night were filtered and selected for this study.
For comparison with previous studies (Mateo-Tomas & Olea Citation2010a, Mateo-Tomas & Olea Citation2010b, Olea & Mateo-Tomas Citation2009) and to match and explain those results with the species-specific breeding stages (Donazar Citation1993), we defined seasons by the winter and summer solstices and spring and autumn equinox dates. We included five roosting cliffs not used for breeding during 2016–2018, to compare their pattern of use with the breeding cliffs.
The numbers and the ages of vultures roosting at each cliff site were recorded. The birds were grouped into two age classes for the analysis: adults (over 6 calendar years) and non-adults (group consists of subadults, 4–5 calendar years; immatures, 2–3 calendar years; and juveniles, under 1 calendar year) and unaged (Forsman Citation1999). The observations lasted until dusk (Gibbons & Gregory Citation2006). No supplementary feeding was carried out before the census, to avoid artificially inflating the number of roosting birds and to allow an unbiased estimate of natural dispersal and roosting habitat selection by the vultures.
Statistical procedures
We used Student’s t-test for dependеnt samples to compare the number of the Griffon Vultures roosting in the two main areas: Madzharovo and Studen kladenets. We used Spearman’s rank correlation (rho) to explore the relationship between the number of the breeding pairs and the number of roosting birds in the pre-breeding season. To do so, we measured the relative variability of the first variable against the number of birds in the pre-breeding season by the determination coefficient (R2). We used a non-parametric chi-Squared test (χ2) to compare differences in age group composition: adults versus non-adults. All cliffs where vultures were recorded roosting only once were excluded from further analysis. The relationship between Griffon Vultures and cliff selection was surveyed between 2010 and 2018 only, due to monitoring gaps in earlier years.
We used generalized linear mixed models (GLMM) with a log link function and Poisson error distribution to explore roosting cliff selection by Griffon Vultures. Prior to building the model we used Spearman’s rank correlation (rho) to explore the inter-correlations of the tested variables. When the coefficient of a pair of correlated variables was >0.6, one of the variables was removed and the most important was retained (Green Citation1979). The dependent variable was the number of the roosting vultures at each count, with year included as a random effect. The explanatory variables were: cliff breeding status (used for breeding or not), cliff type (monolith or heterogeneous), cliff aspect, cliff height, cliff length, and distance to the nearest feeding site. We used Akaike Information Criterion corrected for small sample sizes (AICc) for model selection. Models were then ranked and those with the lowest AICc (AICc < 2) from the set of our candidate models were considered the best models (ΔAICc) (Burnham & Anderson Citation2002). We used AIC weights (wi) for the best models to assess their relative support for the data.
We used QGIS (QGIS Development Team Citation2016) to measure the respective topographic variables and obtain their values. The statistical significance was set to P < 0.05. Means are presented ± standard deviation (SD) (Fowler & Cohen Citation1992). All analyses were performed using Statistica for Windows Release 7.0 (StatSoft Inc. Citation2004) and R v.3.6.1 (R Core Team Citation2012).
Results
Overall, 16 cliffs were used for roosting by Griffon Vultures between 2010 and 2018 in the study area, and five were used during the whole study period (). Two of the regularly used cliffs were situated in the area of Madzharovo and the other three at Studen kladenets. We found a significant difference in the number of the Griffon Vultures roosting in the two main areas, as most of the birds preferred to roost at Studen kladenets (t-test, t = 4.53, df = 8, P < 0.05). In only one case did the number of vultures roosting at Madzharovo outnumber those at Studen kladenets; in 2010 when 26 vultures were recorded at Studen kladenets and 33 at Madzharovo. The mean number of recorded roosting Griffon Vultures at Madzharovo was 55 ± 14.4, whereas it was 96 ± 29.3 at Studen kladenets.
The number of the roosting Griffon Vultures gradually increased from 25 individuals in 2005 to 201 individuals in 2018. Thеsе results coincided with the substantial increase in the breeding population in the Eastern Rhodopes, from 35 pairs in 2005 to 94 pairs in 2018 (). We observed 124 ± 51 roosting birds on average for all years.
Figure 2. Comparison between the number of the roosting Griffon Vultures (adults – white bars; non-adults – grey dotted bars) and the number of the territorial pairs (black bars) within the study area.
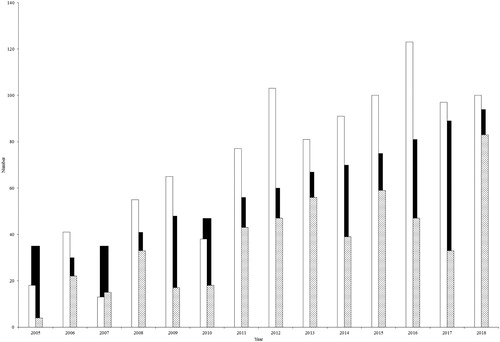
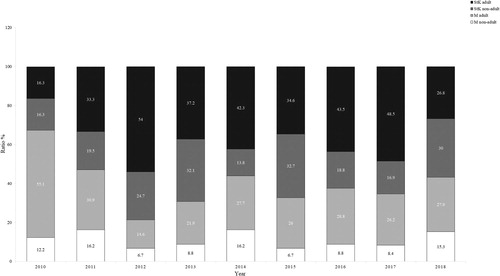
We observed changes in the age structure and composition of the roosting Griffon Vultures. In 2005 the proportion of non-adult vultures formed 16% of all birds, while in 2018 it increased to 41%; however, this variation was not statistically significant (χ2 = 0.65, df = 12, P > 0.05). Across the whole survey 35 ± 22.5% of the counted vultures were non-adults ().
Figure 3. Ratio of the adult and non-adult Griffon Vultures at Madzharovo (M) and Studen kladenets (StK) between 2010 and 2018.
There was a strong positive correlation (Spearman’s rank correlation, rho = 0.85, P < 0.05) between the number of the roosting vultures and the number of territorial pairs recorded during the survey. The determination coefficient revealed that up to 77% of the variance in the number of occupied nests was explained by the variance in the number of roosting vultures in the pre-breeding season (P < 0.05). This result was supported by the GPS data of tracked vultures, which showed that breeding Griffon Vultures roosted on their breeding cliffs, on average for 38 ± 11.6% of nights in winter and for 29 ± 8.9% of nights in autumn. This percentage was lowest in summer, when only 12 ± 7.9% of the roosting birds were recorded at the breeding cliffs.
The GLMM analysis of the roosting cliffs selection included two top-ranked candidate models with ΔAIC < 2 (). The best model (ΔAIC = 0.00, wi = 0.59) included cliff height, cliff length, distance to the nearest feeding site and breeding occupancy of the cliff as the best predictors for roost site selection in the pre-breeding season. Distance to the nearest feeding site (Wald. Stat. = 200.08, P < 0.05) and cliff length (Wald. Stat. = 157.21, P < 0.05) were the most significant predictors in the model. Cliff aspect was not a significant predictor in the model (Wald. Stat. = 1.25, P = 0.26). Therefore, the second-ranked model did not show different effects in comparison to the best model ().
Table 1. Results from the top-ranked GLMM used for the analysis of different topographic factors in respect to the selection of roosting cliffs by Griffon Vultures in the pre-breeding season between 2010 and 2018.
No previously unknown roosting sites were located from the GPS data. In autumn and winter, vultures were mostly roosting on breeding cliffs (80.1 ± 24.2% and 88 ± 24.8%, respectively) while in the spring this percentage dropped to 59.4 ± 25.3%, and was lowest in the summer 45.8 ± 24.8% (). Additionally, we found that in 5.2 ± 3.9% of cases, vultures were roosting on trees.
Discussion
This is the first study in Bulgaria and the Balkans to reveal Griffon Vulture roosting characteristics and dynamics in the pre-breeding season. A similar study to ours considered only roosting behaviour during the breeding season, surveying the relationship of seasonal livestock grazing and usage of three summer roosts by Griffon Vultures in Spain (Olea & Mateo-Tomas Citation2009). The increased number of roosting Griffon Vultures in the recent survey can be explained by the increase in population size in Bulgaria (Demerdzhiev et al. Citation2014, Stoynov et al. Citation2018). Furthermore, vultures from neighbouring populations might also have contributed to the increased number of birds recorded during our study; however, the majority of the vultures were not individually marked, so we did not know their origin (Deng et al. Citation2003). In our study, the Studen kladenets area was preferred over Madzharovo. This may be explained by the distribution and abundance of livestock and the availability of foraging habitats (Arkumarev et al. Citation2020). Additionally, the main flight corridor between the breeding and feeding sites, on the Greek and the Bulgarian side of the mountain, passes through this area (Arkumarev et al. Citation2021).
Roosting Griffon Vultures used five cliffs throughout the survey period and 11 cliffs intermittently. We suspect that the larger population and its increase during the study years may have forced some breeding pairs and birds of low rank in the population hierarchy to disperse along the smaller cliffs (Delov Citation2016, Sergio et al. Citation2003, Sergio & Newton Citation2003). Our telemetry data revealed that vultures can spend 5.2 ± 3.9% of nights roosting in trees. We suggest that this might occur when weather conditions are unsuitable for soaring flight, or when the birds have found an unpredictable food resource. Thus, birds will roost nearby and take advantage of it immediately the following day, saving energy costs and gaining resources before competition increases as vultures from elsewhere arrive later to feed.
Age structure and composition of the roosting Griffon Vultures changed gradually during the study period with increasing numbers of both age groups. Non-adult birds comprised up to 41% of all birds in 2018, and some of the cliffs were used for roosting by up to 53% of non-adult birds solely (Authors, unpubl. data). In Spain, higher numbers of immature birds are usually recorded in the autumn (Olea & Mateo-Tomas Citation2009). The age ratio might provide important evidence for changes in mortality rates, productivity and immigration within populations (Ferrer et al. Citation2003, Griesinger Citation1998). We suggest that the increase in the proportion of non-adults resulted from a population size increase, rather than from increased mortality of older birds (Demerdzhiev et al. Citation2014). Social life and relationships, especially among immature birds, are major factors in colonization of new territories (Mateo-Tomas & Olea Citation2011). Thus, cliffs often used by more vultures are afterwards used for breeding (Mateo-Tomas & Olea Citation2010a, Mateo-Tomas & Olea Citation2011).
We found that cliffs occupied by breeding pairs held a higher number of roosting vultures in the pre-breeding season. Roosting in the breeding colonies can play a significant role in gaining copulations and for the selection and recruitment of partners (Blanco et al. Citation2009, Carrete et al. Citation2007) and is a major driver in the pair formation process (Beauchamp Citation1999, Ward & Zahavi Citation1973, Zahavi Citation1971). However, we revealed that non-adult birds preferred roosting on cliffs where there were fewer adult vultures and breeding pairs. Breeders are usually experienced birds with a much higher tendency to occupy territories (Mateo-Tomas & Olea Citation2011) and are dominant over non-breeders, the majority of which are immature birds. We suggest that immature birds might congregate at less favourable roosting sites because of competition from older, breeding birds (Coombs Citation1961).
The Griffon Vulture is a colonial raptor and is highly selective in terms of availability of rocky habitats (Xirouchakis & Mylonas Citation2005). Therefore, characteristics such as the height, length and aspect are of major importance for site selection, as shown by our results. Height and length are important for the Griffon Vultures in terms of taking off and landing, but also in nest site selection and occupancy, as demonstrated by other studies (Xirouchakis & Mylonas Citation2004, Xirouchakis & Mylonas Citation2005). The aspect of the cliff was not selected among the best predictors, despite having been shown to be important for avoiding adverse weather conditions (Xirouchakis & Mylonas Citation2005). However, in our area cliffs were exclusively heterogeneous and thus this factor might not play a significant role once birds can find shelter among different cavities and ledges that are protected in this respect. In the current survey, the cliffs used most often for roosting were next to feeding sites, which is probably a response to the limited food resources during this period of the year (Amezian & El Khamlichi Citation2016, Ward & Zahavi Citation1973). Therefore, we hypothesize that such food sources have a high impact on the selection and establishment of the roosting sites used by Griffon Vultures (Botha & Krüger Citation2012). This is confirmed by other surveys that provided evidence for how roosting in large numbers increased foraging efficiency (Rabenold Citation1986). Griffon Vultures that use communal roosts exchange information on the availability of food and navigate together, with naïve birds following the most skilled birds (Harel et al. Citation2017). This behaviour may increase competition but benefits greatly the inexperienced vultures by saving energy through increased foraging efficiency (Sweeney Citation1984, Marzluff et al. Citation1996, Bijleveld et al. Citation2010). This can explain the higher number of roosting vultures at cliffs closest to the feeding sites in our study (Olea & Mateo-Tomas Citation2009), particularly in the pre-breeding season when soaring conditions can be limited. However, this can affect the age structure, preferences and roost formation (Moreno-Opo et al. Citation2015).
The number of roosting vultures varied significantly between cliffs and years; however, our survey confirmed that some cliffs are preferred more consistently than others. We found that in autumn and winter, Griffon Vultures roosted mostly on their breeding cliffs or on cliffs with breeding pairs. This coincides with the period when vultures are mating, displaying, defending nest sites and nest building (Grubach Citation2014). The opposite tendency was observed during spring and especially in summer, when vultures were mostly roosting on cliffs which were not occupied by breeding pairs, and which were located at higher altitudes. We suggest this might be a response to the distribution of the free-ranging livestock in the area (Olea & Mateo-Tomas Citation2009). On the other hand, behaviours related to chick rearing (Mendelssohn & Leshem Citation1983), feeding (Fernandez Citation1975) and post-fledging dependence (Xirouchakis & Mylonas Citation2007) might also have an impact on the selection of roosting cliffs.
Our findings provide the very first results of a long-term study on the roosting strategies of Griffon Vultures. Apart from other demographic factors, this is an important component of their annual biology and ecology. We showed the relationship between roosting behaviour in the pre-breeding season and the following breeding season, demonstrated the importance of different topographic features related to roost selection, and highlighted cliff usage by vultures. As such, identification and conservation of the roosting cliffs within and outside breeding grounds is strongly recommended for this rare and threatened scavenger species. Our baseline survey underlines the need for more research on this topic. Along with the development of GPS tracking technology and increasing the number of the marked individuals, it would also be useful to consider other important elements, such as courtship behaviour and pair bond formation, the relationship between roosting behaviour and foraging dispersion, and information transfer mechanisms at the roost. Finally, our results highlight the need for further research on this topic in Bulgaria and elsewhere.
Acknowledgements
The current research was financed by National program Young scientists and Postdoctoral Candidates funded by the Bulgarian Ministry of Education and Science. This work was additionally supported by the LIFE project Conservation of Black and Griffon Vultures in the cross-border Rhodopes mountains (LIFE14 NAT/NL/000901) funded by the European Union and co-funded by Rewilding Europe. We would like to express our gratitude to Hristo Hristov, Marin Kurtev, Desislava Kostadinova, Nikolay Terziev, Dimitar Plachyiski, Georgi Popgeorgiev, Nedko Nedyalkov, Stanislav Dyulgerov, Stoycho Stoychev, Ivan Pandakov, Pencho Pandakov, Atanas Delchev, Stoyan Nikolov, Vanya Angelova, Iana Gocheva, Ivaylo Angelov, Stoyan Ch. Nikolov and all the BSPB/Birdlife Bulgaria volunteers who supported the study. Thanks to Robert Burnside for providing valuable comments on a previous draft of the manuscript and the English proof of the text.
Additional information
Funding
References
- Amezian, M. & El Khamlichi, R. 2016. Significant population of Egyptian Vulture Neophron percnopterus found in Morocco. Ostrich 87: 73–76.
- Andevski, J. 2013. Vulture Conservation in the Balkan Peninsula and Adjacent Regions: 10 Years of Vulture Research and Conservation. Vulture Conservation Foundation, Skopje.
- Arkumarev, V., Dobrev, D., Stamenov, A., Terziev, N., Delchev, A. & Stoychev, S. 2020. Using GPS and ACC data to study diet of a top avian scavenger. Bird Study 67(3): 300–310.
- Arkumarev, V., Dobrev, D., Stamenov, A., Terziev, N., Delchev, A. & Stoychev, S. 2021. Seasonal dynamics in the exploitation of natural carcasses and supplementary feeding stations by a top avian scavenger. J. Ornithol. doi:10.1007/s10336-021-01865-1.
- Baumgart, W. 1974. Wie steht es um Europas Geier? Der Falke 8: 258–267.
- Beauchamp, G. 1999. The evolution of communal roosting in birds: origin and secondary losses. Behav. Ecol 10: 675–687.
- Bijleveld, A., Egas, M., Gils, J. & Piersma, T. 2010. Beyond the information centre hypothesis. Communal roosting for information on food, predators, travel companions and mates? Oikos 119: 277–285.
- BirdLife International. 2020. Species factsheet: Gyps fulvus. Downloaded from http://www.birdlife.org on 12/04/2020.
- Blanco, G., Pais, J.L., Fargallo, J.A., Potti, J., Lemus, J.A. & Davila, J.A. 2009. High proportion of non breeding individuals in an isolated Red-billed Chough population on an oceanic island (La Palma, Canary Islands). Ardeola 56: 229–239.
- Bodey, T.W., Cleasby, I.R., Bell, F., Parr, N., Schultz, A. & Votier, S.C. 2018. A phylogenetically controlled meta-analysis of biologging device effects on birds: deleterious effects and a call for more standardized reporting of study data. Methods Ecol. Evol. 9: 946–955.
- Botha, A. & Krüger, S. 2012. New cape Vulture Gyps coprotheres roosts in the free State Province, South Africa. Vulture News 62: 42–45.
- Burnham, K.P. & Anderson, D.R. 2002. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. 2nd ed. Springer, New York.
- Carrete, M., Grande, J., Tella, J., Sanchez-Zapata, J., Donazar, J., Dıaz-Delgado, R. & Romo, A. 2007. Habitat, human pressure, and social behavior: partialling out factors affecting large-scale territory extinction in an endangered vulture. Biol. Conserv. 136: 143–154.
- Coombs, C.J.F. 1961. Rookeries and roosts of the Rook and Jackdaw in south west Cornwall. Part III. Roosting. Bird Study 8: 55–70.
- Delov, V. 2016. Notes on behavior of the Griffon Vultures (Gyps fulvus) during recovery of the species in Kresna gorge and Kotlenska planina. Annuaire de l’Université de Sofia “St. Kliment Ohridski” Faculte de Biologie. First National Conference of Reintroduction of Conservation-reliant Species, University Press, Sofia.
- Del Moral, J.C. 2009. El buitre Leonado en Espana. Poblacion Reproductora en 2008 y Metodo de Censo. SEO/BirdLife, Madrid.
- Demerdzhiev, D., Hristov, H., Dobrev, D., Angelov, I. & Kurtev, M. 2014. Long-term population status, breeding parameters and limiting factors of the Griffon Vulture (Gyps fulvus) population in Eastern Rhodopes, Bulgaria. Acta Zool. Bulg. 66: 373–384.
- Deng, W.H., Wei, G. & Guang-Mei, Z. 2003. Nest and roost habitat characteristics of the Grey-faced Buzzard in northeastern China. J. Raptor Res. 37: 228–235.
- Dobrev, D. & Stoychev, S. 2013. Vulture conservation in Bulgaria. Proceedings of the Griffon Vulture Conference, 6–8 March 2013, Limassol.
- Dobrev, D., Arkumarev, V., Skartsi, T., Stamenov, A. & Kret, E. 2019. Griffon Vulture population trend and size in the Eastern Rhodopes at the crossroads to Asia. Poster, European vulture conference, Algarve, Portugal.
- Donázar, J.A. 1993. Los buitres ibéricos. Biología y conservación. Reyero, Madrid.
- DeVault, T.L., Rhodes, O.E. & Shivik, J.A. 2003. Scavenging by vertebrates: behavioral, ecological, and evolutionary perspectives on an important energy transfer pathway in terrestrial ecosystems. Oikos 102: 225–234.
- Engel, K.A., Young, L.S., Steenhof, K., Roppe, J.A. & Kochert, M.N. 1992. Communal roosting of the Common Ravens in southwestern Idaho. Wilson Bull. 104: 105–121.
- Ferguson-Lees, J., Christie, D.A., Franklin, K., Mead, D. & Burton, P. 2001. Raptors of the World. Houghton Mifflin Company, New York.
- Fernandez, J.A. 1975. Comportamiento del Buitre Leonado (Gyps f. fulvus) en nido. Ardeola 22: 29–54.
- Ferrer, M., Penteriani, V., Balbontin, J. & Pandolfi, M. 2003. The proportion of immature breeders as a reliable early warning signal of population decline: evidence from the Spanish Imperial Eagle of Doñana. Biol. Conserv. 114: 463–466.
- Fowler, J. & Cohen, L. 1992. Statistics for ornithologists. 2nd ed. BTO, London.
- Forsman, D. 1999. The raptors of Europe and the Middle East. A handbook of field identification. T & A D Poyser, London.
- Fuller, M.R. & Mosher, J.A. 1981. Methods of detecting and counting raptors: a review. Avian Biol. Res. 6: 235–248.
- Gibbons, D. & Gregory, R. 2006. Birds. In W. Sutherland. (ed) Ecological Census Techniques a Handbook, 2nd ed., Vol. 2: 308–350. Cambridge University Press, Edinburgh.
- Golemanski, V. 2015. Red Data Book of Bulgaria. Vol. 2. Animals. IBER – BAS & MIEW, Sofia.
- Green, R. 1979. Sampling Design and Statistical Methods for Environmental Biologists. John Wiley and Sons, New York.
- Griesinger, J. 1998. Juvenile dispersion and migration among Griffon Vultures Gyps fulvus in Spain. In R. D. Chancellor, B. U. Meyburg & J. J. Ferrero. (ed) Holarctic Birds of Prey, 613–621. Merida, ADENEX and World Working Group on Birds of Prey and Owls.
- Grubach, B. 2014. The Griffon Vulture Gyps fulvus. Nature conservation institute Serbia, Belgrade, Serbia. (in Serbian).
- Harel, R., Spiegel, O., Getz, W.M. & Nathan, R. 2017. Social foraging and individual consistency in following behaviour: testing the information centre hypothesis in free-ranging vultures. Proc. R Soc. B 284: 20162654. doi:10.1098/rspb.2016.2654.
- Kelly, G.M. & Thorpe, J.P. 1993. A communal roost of Peregrine Falcons and other raptors. Brit. Birds 86: 49–52.
- Kopralev, I. 2002. Geography of Bulgaria. Physical and Socio-Economic Geography. Forkom, Sofia.(in Bulgarian).
- Lack, D. 1968. Ecological Adaptations for Breeding in Birds. Methuen, London.
- Marzluff, J.M., Heinrich, B. & Marzluff, C.S. 1996. Raven roosts are mobile information centres. Anim. Behav. 51: 89–103.
- Mateo-Tomas, P. & Olea, P.P. 2010a. Anticipating knowledge to inform species management: predicting spatially explicit habitat suitability of a colonial vulture spreading its range. PLoS ONE 5: e12374.
- Mateo-Tomás, P. & Olea, P. 2010b. When hunting benefits raptors: a case study of game species and vultures. Eur. J. Wildl. Res. 56: 519–528.
- Mateo – Tomas, P. & Olea, P.P. 2011. The importance of social information in breeding site selection increases with population size in the Eurasian Griffon Vulture Gyps fulvus. Ibis 153: 832–845.
- Mendelssohn, H. & Leshem, Y. 1983. Observation on reproduction and growth of old world vultures. In S. Wilbur & J. Jakson. (ed) Vulture Biology and Management, 214–241. University of California Press, Berkeley, Los Angeles-London.
- Moreno – Opo, R., Trujillano, A., Arredondo, Á, Garcia, L. & Margalida, A. 2015. Manipulating size, amount and appearance of food inputs to optimize supplementary feeding programs for European vultures. Biol. Conserv. 181: 27–35.
- Olea, P. & Mateo-Tomás, P. 2009. The role of traditional farming practices in ecosystem conservation: the case of transhumance and vultures. Biol. Conserv. 142: 1844–1853.
- QGIS Development Team. 2016. QGIS: Geographic Information System. Open Source Geospatial Foundation Project. http://qgis.osgeo.org.
- Rabenold, P. 1986. Family associations in communally roosting Black vultures. Auk 103: 32–41.
- R Development Core Team. 2012. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/.
- Sergio, F. & Newton, I. 2003. Occupancy as a measure of territory quality. J Anim. Eocol. 72: 857–865.
- Sergio, F., Pedrini, P. & Marchesi, L. 2003. Adaptive selection of foraging and nesting habitat by Black Kites (Milvus migrans) and its implications for conservation: a multi-scale approach. Biol. Conserv. 112: 351–362.
- StatSoft Statistica. 2004. STATISTICA (data analysis software system), version 7. www.statsoft.com.
- Stoychev, S., Hristov, H., Iankov, P. & Demerdzhiev, D. 2004. Birds in the Bulgarian part of the Eastern Rhodopes. In P. Beron & A. Popov. (ed) Biodiversity of Bulgaria. 2. Biodiversity of Eastern Rhodopes (Bulgaria and Greece), 881–894. Pensoft & Natural Museum of Natural History, Sofia.
- Stoynov, E., Biro, E., Stoyanov, G., Peshev, H., Ivanov, I., Stoev, I., Bonchev, L., Vangelova, N., Nikolova, Z., Iankov, L., Parvanov, D. & Grozdanov, A. 2018. Population boost of the Griffon Vulture Gyps fulvus (Hablizl, 1783) (Accipitridae) in Bulgaria based on reintroductions. Acta Zool. Bulg. Suppl. 12: 59–65.
- Sweeney, T.M. 1984. Black and Turkey Vulture roost dynamics, marking, morphology and nesting in Virginia. Virginia. Doctoral dissertation, Virginia Polytechnic Institute and State University.
- Ward, P. & Zahavi, A. 1973. The importance of certain assemblages of birds as “information centres” for food finding. Ibis 115: 517–534.
- Xirouchakis, S. 2010. Breeding biology and reproductive performance of Griffon Vultures Gyps fulvus on the island of Crete (Greece). Bird Study 57: 213–225.
- Xirouchakis, S. & Mylonas, M. 2004. Griffon Vulture (Gyps fulvus) distribution and density in Crete. Isr. J. Zool. 50: 351–354.
- Xirouchakis, S. & Mylonas, M. 2005. Selection of breeding cliffs by Griffon Vultures Gyps fulvus in Crete (Greece). Acta Ornithol. 40: 155–161.
- Xirouchakis, S. & Mylonas, M. 2007. Breeding behavior and parental care in the Griffon Vulture Gyps fulvus on the island of Crete (Greece). Ethol. Ecol. Evol. 19: 1–26.
- Zahavi, A. 1971. The function of pre-roost gatherings and communal roosts. Ibis 113: 106–109.