ABSTRACT
Using a novel method for testing the effect of gaze direction on flight initiation distances of European Herring Gulls Larus argentatus, we found that distances were significantly shorter for an averted gaze treatment than for a direct gaze. There was no difference between adult and immature gulls in their response, suggesting that from an early age Herring Gulls consider the orientation of a human’s eye in their anti-predator responses.
Predation can create considerable selection pressures on populations of prey species (Kotrschal et al. Citation2017). For a predator to be successful, it must first find prey in its environment, employ a suitable method of pursuit and capture, and then consume the caught prey item. Consequently, prey species have developed a wide array of anti-predator mechanisms which interrupt steps in the chain of predation. Anti-predator mechanisms involve the combined use of physical adaptations and behavioural responses and can be grouped into three main strategies (Kavaliers & Choleris Citation2001). The first group of strategies aims to reduce detection from predators. The second set of strategies includes adaptations that allow prey to fight or ward off a predator. Lastly, the third group of strategies involves avoiding predation by fleeing from a predator once detected. A critical component of these strategies is the ability of a potential prey individual to identify a predator, assess the threat it poses, and then carry out an appropriate anti-predator behaviour (Beránková et al. Citation2014).
Under optimal escape theory, an animal should only flee when the risk is such that the benefit of fleeing will outweigh the costs of lost foraging or mating opportunities (Ydenberg & Dill Citation1986). If flight is initiated too frequently, the substantial loss of energy and time acquiring resources results in a fitness cost (Samia et al. Citation2013). Prey species must therefore consider a wide range of variables before determining the distance from a predator at which to flee (flight initiation distance, FID) (Samia et al. Citation2016). One such variable is the behaviour of the potential predator. As a predator is required to catch prey to consume it, predatory intent can be determined through analysing a predator’s gaze cues and system of approach (Bateman & Fleming Citation2011, Møller & Tryjanowski Citation2014). Gaze cues refer to the presence, direction and motion of the head or eyes. Species which are capable of identifying and reacting to the gaze cues of another individual are referred to as ‘gaze sensitive’ (Davidson & Clayton Citation2016).
Currently, the mechanisms by which gaze sensitivity develops within individuals are poorly understood, with hypotheses suggesting that gaze sensitivity is either an innate trait of certain species or that gaze sensitivity is an example of associative learning within individuals, with a higher probability of occurring within some species (Davidson & Clayton Citation2016). Gaze research within birds has illustrated that juvenile hand-reared Common Ravens Corvus corax display gaze sensitivity. At four months old, Common Ravens gain the ability to track the gaze of others, while adults demonstrate flexibility in their gaze responses and habituate to repeatedly meaningless gaze cues (Schloegl et al. Citation2007). Other bird species such as the Hadeda Ibis Bostrychia hagedash and European Herring Gull Larus argentatus (hereafter Herring Gull) have displayed aversive gaze responses to human head direction (Bateman & Fleming Citation2011, Goumas et al. Citation2019). Furthermore, Herring Gulls have larger FIDs when exposed to a forward facing human head with forward gaze, than to a forward facing head with a downward gaze (Goumas et al. Citation2020). Investigating adults and immature gulls could help determine when gaze sensitivity develops.
Herring Gulls are a good model species for investigating the development of gaze responses in wild populations, as both adults and recently fledged individuals display gaze aversion (Goumas et al. Citation2019, Citation2020), have distinct plumage stages with age (Dwight Citation1920), and are an accessible species to sample as they are frequently found in large numbers at coastal cities across Northern Europe (Rock Citation2005). They are also frequently habituated to human presence, allowing relatively close approaches before they initiate flight; key when determining whether they pay attention to subtle cues such as gaze direction or not. Aberdeen, Scotland is one such city; an urban area larger than previous study sites that hosts a large population of Herring Gulls.
First, we aimed to determine whether Herring Gulls respond to the orientation of the eyes from a human’s gaze. We developed a novel FID sampling method comparing a ‘direct-gaze’ treatment to an ‘averted gaze’ treatment (). As Herring Gulls have previously displayed aversion to human gaze, we expected that Herring Gulls exposed to the direct-gaze treatment would exhibit a longer FID. Secondly, we compared the gaze responses between adult and immature Herring Gulls, as differences between adult and immature birds would provide insight into the development of gaze responses. We predicted that adult gulls would exhibit a longer FID than immature gulls, as they would have learned through experience to be averse to a human’s gaze.
Figure 1. Method of tangent approach and treatments. Thick arrow line represents fieldworker approach direction, thin arrow line represents the fieldworkers head direction, dashed line represents the passing distance between the fieldworker and the gull. Direct-gaze paper eyes (upper left). Averted gaze paper eyes (lower left).
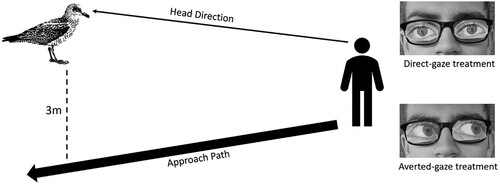
To create our direct-gaze and averted-gaze treatments, we used a pair of glasses with attachable images of eyes (made from paper) either pointing directly forwards (the direct-gaze treatment) or pointing off to the side (the averted gaze treatment, ). The paper eyes on the glasses had two small holes in the centre for the wearer to retain vision. The use of paper eyes allowed for a single field worker to safely carry out fieldwork and provided standardized treatments.
We carried out a pilot study to establish whether gulls differentiate between real eyes and paper eyes. We compared direct-gaze paper eyes to a direct gaze with real eyes using the same FID method as the main study. Thirty gulls were sampled in the FID pilot study. No significant difference was found in FID between the direct-gaze real eyes (mean ± SD = 12.84 ± 6.38 m) and direct-gaze paper eyes treatments (mean ± SD = 14.66 ± 6.33 m; two-sample t-test: t27.998 = −0.785, P = 0.438). Therefore, we determined that we could use paper eyes in the main study.
We carried out sampling on a length of beach at Aberdeen, United Kingdom (57.143°N 2.069°W to 57.172°N 2.078°W) on ten days from 15th October 2021 to 22nd December 2021, outside of the breeding season of Herring Gulls. On each day, the experimenter collected FID samples on one 3500 m transect, following the shoreline at Aberdeen beach. When daylight allowed, we commenced sampling just before low tide, when the largest area of sand for gulls to forage was available. When low tide did not coincide with daylight, we sampled during the lowest tide level that occurred during daylight. All sampling occurred between 08:30 and 16:00. Clothing colour (trousers: grey, brown or blue. Jacket: blue or navy) was randomized, as according to the species confidence hypothesis, birds may be attracted or repelled by colours similar to their own or dissimilar to their own respectively (Gould et al. Citation2004, Zhou & Liang Citation2020).
The beach is separated into 30 segments (mean length = 116m) by groyne fences. The experimenter walked along the transect, going from the southernmost to the northernmost groyne on the beach, sampling lone gulls encountered on the transect. A maximum of one gull per groyne segment was sampled so that gulls were not influenced by the others fleeing and as it enabled us to record the location of gulls we had just tested to avoid approaching them again immediately after. We allocated observed gulls into an age category, as either immature (1st to 3rd winter birds), or adult (4th winter or older), using the plumage traits described by Dwight (Citation1920).
The treatment each target gull received was randomized in statistics package R 4.1.1 (R Core Team Citation2021) prior to sampling. In each of the treatments, while wearing the glasses, the experimenter (LL) walked towards the target gull, at a pace of one step every two seconds, facing the bird directly with their head and passing at a tangent of 3 m (). We considered a direct approach angle as too aggressive, as pilot study trials indicated that it was likely to elicit very long FIDs. As the experiment aimed to determine whether gulls respond to eye gaze, we deemed it critical for head direction to be facing the gull, doing so ensured that any differences between the treatments were likely to be caused by a gaze response to eyes rather than head orientation. We considered flight to be sustained movement (approximately three seconds) away from the experimenter. FID was recorded by measuring the distance between the tip of the experimenter’s footprint and the gulls flight location to the nearest cm. We sampled a total of 39 gulls (adult averted gaze, n = 9; adult direct gaze, n = 12; immature averted gaze, n = 7; immature direct gaze, n = 11).
Statistical analysis was carried out using R. We fitted treatment (direct or averted gaze), age (immature or adult) and their interaction as fixed effects using the lme4 package (Bates et al. Citation2015). Adults and averted gaze were the reference categories. We met assumptions of normality by log transforming the FID variable. We fitted date as a random effect to account for multiple measures on the same days. As gulls were not marked, we could not account for the possibility that we recorded multiple measures on the same individuals across different days. Using the drop1 function we tested the interaction for statistical significance with a likelihood ratio test. If it was not statistically significant then we removed it from the model and proceeded to test the main effects for statistical significance, also with likelihood ratio tests, starting with the variable with the higher P value and removing it from the model if the P value was greater than 0.05 (stepwise regression; Crawley Citation2007).
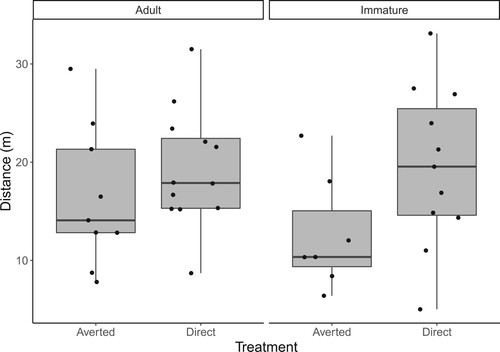
The effect of the treatment did not differ between adult and immature gulls (linear mixed effect model: treatment x age interaction β = 0.098, se = 0.278, χ2 = 0.148, P = 0.701). Adult and immature gulls did not differ in their FIDs (main effect of age β = −0.174, se = 0.131, χ2 = 1.783, P = 0.182). Gulls in the direct eye gaze treatment had longer FIDs (mean ± SD = 19.40 ± 6.95 m) than gulls in the averted gaze treatment (mean ± SD = 14.74 ± 6.69 m; main effect of treatment β = 0.305, se = 0.138, χ2 = 4.830, P = 0.028; ). The among date variance (0.042) was relatively small compared to the residual variance (0.147), indicating FIDs were not greatly different among days.
Figure 2. Boxplots illustrating distribution of Herring Gull flight initiation distance of each treatment in each age group. Jitter dots represent individual samples. Horizontal bars represent the medians, grey boxes represent interquartile ranges, and whiskers represent minimum and maximum values.
We found that Herring Gulls exposed to the averted gaze treatment exhibited a shorter FID, confirming our prediction. However, we also predicted adult gulls would have a longer FID than immature gulls, and we did not find that to be the case, neither did the response to the direction of eye gaze differ between adults and immature gulls. This study provides further evidence to support the idea that Herring Gulls consider the orientation of the eyes, as well as head direction, when determining a gaze response and corroborates the findings of previous research (Goumas et al. Citation2019), as well as extending the examination of this behaviour to a much larger urban area than considered previously (Aberdeen has a population of around 200,000, much larger than any site in west Cornwall; Goumas et al. Citation2020). However, as the Herring Gulls were not marked, we may have measured the same gull multiple times without controlling for this statistically. This is a form of pseudoreplication that may have inflated the significance of the results. Nevertheless, since our results match those of previous research (Goumas et al. Citation2020), it seems likely our conclusions are robust.
We found no difference between immature and adult gulls, concurring with Goumas et al. (Citation2020) and suggesting that gulls develop an aversive response to a direct gaze before maturity. In their natural habitat, they are often victims of kleptoparasitism from three skua species Stercorarius spp. and Great Black-Backed Gulls Larus marinus (Brockmann & Barnard Citation1979), and are preyed upon by Peregrine Falcons Falco peregrinus (Sutton et al. Citation2017) and even occasionally Marsh Harriers Circus aeruginosus (Graves Citation2014). Developing gaze sensitivity at an early age would allow immature gulls to identify when they are the target of predation or kleptoparasitism from both conspecifics and heterospecifics, allowing them to respond appropriately. Alternatively, as both immature and adult gulls from multiple gull species partake in kleptoparastism (Källander Citation2006, Steele & Hockey Citation1995), then perhaps gaze sensitivity could confer advantages for kleptoparasitism by allowing gulls to pick targets oblivious to their presence or intention.
We counted immature gulls in this study as individuals in their first to third winters. Therefore, the lack of difference between adult and immature gulls may be explained by older immature gulls behaving in a manner more similar to adult gulls, obscuring a difference between younger gulls and adults. Indeed, while there was no significant difference between the ages, the immature averted gaze category had the lowest flight initiation distance, perhaps skewed through younger, more naïve individuals. Additionally, we found the difference between averted and direct-gaze treatments was larger in immature gulls than adults, perhaps as younger gulls display a slightly more aversive response to a direct gaze, as they may be target to more kleptoparasitism or predation than adults. Further research could use a marked population, to test known individuals of more precisely known ages and to prevent pseudoreplication.
While we and others have found Herring Gulls, as well as other birds and mammals (Kaminski et al. Citation2005, Rosati & Hare Citation2009) are responsive to gaze, both Common Ravens and Chimpanzees Pan troglodytes, species known for their cognitive abilities, do not respond to gaze cues from eyes alone (Schloegl et al. Citation2008, Tomasello et al. Citation2007). This variation in response to gaze may be due to eye structure. Gaze sensitive species such as Herring Gulls, Western Jackdaws Coloeus monedula (von Bayern & Emery Citation2009) and humans show high contrast between the pupil and the iris/sclera, making eye orientation visible; Herring Gulls have black pupils and white to pale yellow irises with bright yellow to orange orbital rings, while Western Jackdaws have dark pupils and pale blue irises. The contrast between the pupil and the iris/sclera may facilitate the development of conspecific communication via eye orientation (von Bayern & Emery Citation2009, Davidson et al. Citation2014). Chimpanzees and Common Ravens both have completely dark eyes, making the determination of eye orientation difficult, potentially explaining their insensitivity to gaze direction (Davidson et al. Citation2014, Tomasello et al. Citation2007). However, some species with dark eyes, like the Common Starling Sturnus vulgaris, can be sensitive to eye gaze (Carter et al. Citation2008). Future research should test whether species with colour contrasts between the pupil and surrounding structures are more sensitive to gaze direction than species with little or no contrast. Further, presumably there are benefits for individuals in communicating the direction of their gaze and so the role gaze sensitivity plays in conspecific interactions needs to be explored.
Acknowledgements
We thank Harper Eagles for providing numerous useful comments and suggestions on an earlier draft, and two reviewers who made constructive comments. We have no conflicts of interest.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Bateman, P.W. & Fleming, P.A. 2011. Who are you looking at? Hadeda Ibises use direction of gaze, head orientation and approach speed in their risk assessment of a potential predator. J. Zool. 285: 316–323.
- Bates, D., Maechler, M. Bolker, B., & Walker, S. 2015. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 67: 1-48.
- Beránková, J., Veselý, P., Sýkorová, J. & Fuchs, R. 2014. The role of key features in predator recognition by untrained birds. Anim. Cogn. 17: 963–971.
- Brockmann, H.J. & Barnard, C.J. 1979. Kleptoparasitism in birds. Anim. Behav. 27: 487–514.
- Carter, J., Lyons, N.J., Cole, H.L. & Goldsmith, A.R. 2008. Subtle cues of predation risk: Starlings respond to a predator’s direction of eye-gaze. Proc. Royal Soc. B: Biol. Sci. 275: 1709–1715.
- Crawley, M.J. 2007. The R Book. John Wiley & Sons Ltd. Chichester, UK.
- Davidson, G.L., Butler, S., Fernández-Juricic, E., Thornton, A. & Clayton, N.S. 2014. Gaze sensitivity: function and mechanisms from sensory and cognitive perspectives. Anim. Behav. 87: 3–15.
- Davidson, G.L. & Clayton, N.S. 2016. New perspectives in gaze sensitivity research. Learn. Behav. 44: 9–17.
- Dwight, J. 1920. The plumages of gulls in relation to age as illustrated by the Herring Gull (Larus argentatus) and other species. Auk 37: 262–268.
- Gould, M.L., Green, L., Altenau, B. & Blumstein, D.T. 2004. A study of the species-confidence hypothesis with Spiny-cheeked Honeyeaters (Acanthagenys rufogularis). Emu Austral Ornithol. 104: 267–271.
- Goumas, M., Burns, I., Kelley, L.A. & Boogert, N.J. 2019. Herring Gulls respond to human gaze direction. Biol. Lett. 15: 20190405.
- Goumas, M., Collins, T.R., Fordham, L., Kelley, L.A. & Boogert, N.J. 2020. Herring Gull aversion to gaze in urban and rural human settlements. Anim. Behav. 168: 83–88.
- Graves, G.R. 2014. Western Marsh Harrier preys on Herring Gull. J. Raptor Res. 42: 191–192.
- Källander, H. 2006. Iakttagelser i södra Sverige av fyra måsarters stöld av föda från andra arter. Ornis Svecica 16: 127–149.
- Kaminski, J., Riedel, J., Call, J. & Tomasello, M. 2005. Domestic Goats, Capra hircus, follow gaze direction and use social cues in an object choice task. Anim. Behav. 69: 11–18.
- Kavaliers, M. & Choleris, E. 2001. Antipredator responses and defensive behavior: ecological and ethological approaches for the neurosciences. Neurosci. Biobehav. Rev. 25: 577–586.
- Kotrschal, A., Deacon, A.E., Magurran, A.E. & Kolm, N. 2017. Predation pressure shapes brain anatomy in the wild. Evol. Ecol. 31: 619–633.
- Møller, A.P. & Tryjanowski, P. 2014. Direction of approach by predators and flight initiation distance of urban and rural populations of birds. Behav. Ecol. 25: 960–966.
- R Core Team. 2021. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
- Rock, P. 2005. Urban gulls: Problems and solutions. Br. Birds 98: 338–355.
- Rosati, A.G. & Hare, B. 2009. Looking past the model species: diversity in gaze-following skills across primates. Curr. Opin. Neurobiol. 19: 45–51.
- Samia, D.S.M., Blumstein, D.T., Stankowich, T. & Cooper, W.E. 2016. Fifty years of chasing lizards: New insights advance optimal escape theory. Biol. Rev. 91: 349–366.
- Samia, D.S.M., Nomura, F. & Blumstein, D.T. 2013. Do animals generally flush early and avoid the rush? A meta-analysis. Biol. Lett. 9: 20130016.
- Schloegl, C., Kotrschal, K. & Bugnyar, T. 2007. Gaze following in Common Ravens, Corvus corax: ontogeny and habituation. Anim. Behav. 74: 769–778.
- Schloegl, C., Kotrschal, K. & Bugnyar, T. 2008. Modifying the object-choice task: is the way you look important for ravens? Behav. Process. 77: 61–65.
- Steele, W.K. & Hockey, P.A.R. 1995. Factors influencing rate and success of intraspecific kleptoparasitism among Kelp Gulls (Larus dominicanus). Auk 112: 847–859.
- Sutton, L.J., Burrell, R.A. & Loram, S. 2017. Spatio-temporal trends in the predation of large gulls by Peregrine Falcons (Falco peregrinus) in an insular breeding population. Slovak Raptor Journal 11: 103–109.
- Tomasello, M., Hare, B., Lehmann, H. & Call, J. 2007. Reliance on head versus eyes in the gaze following of great apes and human infants: the cooperative eye hypothesis. J. Hum. Evol. 52: 314–320.
- von Bayern, A.M.P. & Emery, N.J. 2009. Jackdaws respond to human attentional states and communicative cues in different contexts. Curr. Biol. 19: 602–606.
- Ydenberg, R.C. & Dill, L.M. 1986. The economics of fleeing from predators. Adv. Study Behav. 16: 229–249.
- Zhou, B. & Liang, W. 2020. Avian escape responses to observers wearing clothing of different colors: a comparison of urban and rural populations. Global Ecol. Conserv. 22: e00921.
