 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Capsule
Although the Great Spotted Woodpecker Dendrocopos major and Grey-headed Woodpecker Picus canus have overlapping ranges in South Korea, they occur in areas with different forest structures and the occurrence of each species is associated with their main foods.
Aims
To investigate the biotic factors related to the occurrence of the Great Spotted Woodpecker and the Grey-headed Woodpecker, two species that frequently inhabit overlapping regions in South Korea.
Methods
Correlations between the occurrence of the two woodpecker species, forest characteristics, and bird and insect species richness were evaluated based on large-scale public data from the National Ecosystem Survey and a Forest Type Map.
Results
The occurrence of Great Spotted Woodpeckers was associated with forest stands with at least 75% coniferous trees, whereas the occurrence of Grey-headed Woodpeckers was negatively associated with forested areas with canopy cover exceeding 50%. The occurrence of both species was strongly and positively correlated with the species richness of forest birds. The occurrence of Great Spotted Woodpeckers was correlated with the species richness of Coleoptera, while that of the Grey-headed Woodpecker was correlated with the species richness of Hymenoptera and Diptera.
Conclusion
The co-occurrence of the two woodpecker species within shared habitats can be explained by low competition due to differences in preferred forest characteristics and in the food sources used by each species.
Identifying factors that determine species occurrence is a prerequisite for successful species preservation (Luck Citation2002, Oppel et al. Citation2004) and a key goal in ecological research, as it improves our understanding of fitness under various environmental and ecological conditions (Vickery et al. Citation2001, Atkinson et al. Citation2005, Buckingham & Peach Citation2005, Sexton et al. Citation2009). Models predicting the probability of species occurrence are commonly used owing to their ability to incorporate a number of variables (Carvalho & Gomes Citation2003). Such models are advantageous with respect to time and cost efficiency for the differentiation of positive and negative variables related to the occurrence of a species (Guisan & Zimmermann Citation2000), including abiotic (e.g. temperature, precipitation, climate, and altitude; Porej et al. Citation2004, Van Buskirk Citation2005) and biotic factors (e.g. vegetation, competition, predation, and mutualism; Holbrook & Schmitt Citation1988, Lima & Dill Citation1990, Haila et al. Citation1996, Araújo & Luoto Citation2007, Hof et al. Citation2012, Wisz et al. Citation2013). To date, species occurrence has generally been viewed as a function of abiotic factors (Rahbek & Graves Citation2001, Hawkins et al. Citation2003); however, many studies have demonstrated that biotic factors are also powerful determinants of species distribution (Matthews et al. Citation2011, Giannini et al. Citation2013, de Araújo et al. Citation2014, Freeman & Mason Citation2015, Andradas et al. Citation2019).
Strategies for coexistence are needed when multiple species inhabit a single macro habitat (Leibold Citation1995). In particular, competition can occur between species which have similar ecological niches, such as overlapping food or breeding site requirements (Pianka Citation1976). A mechanism for maximizing resource availability while avoiding competition ensures that highly correlated biotic factors do not overlap with each other (Bastolla et al. Citation2005). This is possible because there are differences between species in their ability to respond to biotic factors (Chesson Citation2000, Leibold & McPeek Citation2006). Therefore, information on biotic factors can clearly identify whether species inhabiting the same region compete (Arlettaz et al. Citation2000, Kusch & Schmitz Citation2013), and might be important for conservation strategies (Nie et al. Citation2019).
Most woodpeckers live in forests and frequently occur in areas with dynamic forest elements, such as old trees, dead wood, structural diversity, natural edges, openings, etc. (Mikusiński & Angelstam Citation1997). The cavities on trees created by woodpeckers provide nesting and roosting sites for many secondary cavity users (birds, mammals, amphibians, reptiles, and invertebrates), thus supporting the role of woodpeckers as keystone species in the forest ecosystem (Gorman Citation2014). Acquiring reliable population information is facilitated by easily-detected unique woodpecker behaviours, such as drumming, pecking, and excavation (Drever et al. Citation2008). Among 11 native species of woodpecker (NIBR Citation2022) in South Korea, the Great Spotted Woodpecker Dendrocopos major and the Grey-headed Woodpecker Picus canus are the most common medium-sized residents, distributed throughout the country (Park Citation2014). The National Ecosystem Survey on birds for 2006–2018 reported approximately 40% habitat overlap for the two species, with cohabitation in 666 of 1668 total grids. When multiple species of woodpeckers coexist in a region, competition is often reduced by differentiation of food sources or tree characteristics (e.g. height and diameter at breast height, DBH) (Török Citation1990, Kruszyk Citation2003). However, only one study has evaluated the variables related to the occurrence of the Great Spotted Woodpecker in South Korea (Lee Citation2013), and that identified the two key variables as forest naturalness and the normalized difference vegetation index (NDVI). The habitat use of the Grey-headed Woodpecker in South Korea has not been evaluated to date, which limits our understanding of the co-occurrence of these two species. Moreover, the model developed by Lee (Citation2013) did not include biotic factors other than forest characteristics.
Therefore, this study aimed to determine the biotic factors influencing the occurrence of Great Spotted and Grey-headed Woodpeckers in South Korea using large-scale public data, including forest characteristics, incidence of other forest bird species in various mutual relationships with woodpeckers, and insects, which form much of their main food source. This analysis provides insight into the coexistence of the two species and the role of reduced competition in their distribution.
Methods
Climate and forest characteristics of South Korea
South Korea is located in the mid-latitude temperate climate zone and has four distinct seasons. In summer, the weather is hot due to the high temperature and humidity of the North Pacific High, while in winter, it is cold and dry due to the influence of cold and dry continental high pressure. In spring and autumn, there are many clear and dry days due to the influence of migratory anticyclones (KMA Citation2023).
The forests of South Korea are characterized by steep slopes in the east and gentle slopes in the west, as the Taebaek Mountains lean towards the east. According to Lee (Citation2015), the country has a high proportion of forests, exceeding 60% of the national territory. Coniferous forests account for 40.5% of the total forests, while broad-leaved forests account for 27.0%. The Korean Red Pine Pinus densiflora dominates the vegetation community and accounts for 27.9% of land cover, followed by the Mongolian Oak Quercus mongolica with 21.0% (Lee Citation2015).
Identification of occurrence sites of target species
The data were obtained from the National Ecosystem Survey (NES) conducted by the Ministry of Environment for the period 2006–2018. In accordance with the Natural Environment Conservation Act, the NES divided the country of South Korea into 5–10 year cycles, and the landform, vegetation, terrestrial flora and fauna (flora, mammals, birds, herptiles, and insects), aquatic fauna (fish and benthic invertebrates) are investigated annually (NIE Citation2019). They are investigated at a 1:25,000 scale using topographic maps (map size 11.3 km × 13.9 km) with each map divided into a grid of nine rectangles of 3.8 km × 4.6 km (Kwon et al. Citation2020). In the case of birds, since they inhabit almost all habitat types (Mardiastuti Citation2019), species and individuals are investigated seasonally in all habitat types in the grid using methods of total counts, line transects and point counts (NIE Citation2019).
Grids were selected that contained at least one species of forest bird. Grids including islands or coastal areas were excluded as possible migration routes. Owing to the lack of an official list of forest birds in South Korea, the habitats described by Lee et al. (Citation2020) were used for reference, and forests and woods were taken as relevant areas. The period of investigation varied slightly each year, although the survey was generally conducted between April and October. Only grids investigated in all three periods: spring (April–May), summer (June–August), and autumn (September–October), were selected for analysis. Among the selected grids, those coinciding with insect surveys as part of the NES in the same period were identified because the richness of the insect community is generally correlated with that of the avian community (Beşkardeş et al. Citation2018) and woodpeckers feed on insects throughout the year (Arslangundogdu Citation2010). For reference, NES insect surveys are performed twice a season, except for the winter season, to survey species and individuals using line transects, sweep netting and malaise traps diurnally, and light traps and pitfall traps nocturnally (NIE Citation2019).
Among grids satisfying all of the above criteria, the Great Spotted Woodpecker and the Grey-headed Woodpecker were present in 201 and 182 eligible grids respectively, with both species present in 43 eligible grids. In these grids, the occurrence data of the two species was not split according to season because woodpeckers are sedentary residents (Mikusiński et al. Citation2001, Virkkala Citation2006, Wisz et al. Citation2013, Park Citation2014).
Variable selection
The species richness was estimated for forest birds, Lepidoptera (LEPI), Coleoptera (COLE), Hymenoptera (HYME) and Diptera (DIPT). These insect taxa were included because they form much of the woodpeckers’ diet in South Korea (Fennell Citation1965). For grids investigated over multiple years, the cumulative species richness was estimated. Arc Map v.10.8 software (Environmental Systems Research Institute Inc., Redlands, CA, USA) was used to superimpose the actual vegetation map (polygons) from the NES for the period 2014–2018 and the 5th Forest Type Map (FTM) at a 1:25,000 scale for the period 2006–2010. The FTM is a forest theme map that shows the distribution of forests throughout South Korea, and provides information on various attributes, such as forest stands, diameter class, age class and canopy cover, in connection with the National Forest Inventory (Park et al. Citation2019). The number of vegetation populations composed of trees (TREE) were determined from the actual vegetation map (NIER Citation2012). The forest area (AREA), forest stands with 75% or more coniferous trees (CONI), forest stands with 75% or more deciduous trees (DECI), forest stands with over 50% medium- to large-diameter trees (≥18 cm DBH) (MELA), and forest stands with canopy cover greater than 50% (DEBC) were estimated from the 5th Forest Type Map for conversion to the grid unit area (%) (KOFPY Citation2017).
Scope determination and analysis
To identify factors that influence the occurrence of the Great Spotted Woodpecker and the Grey-headed Woodpecker, a binary logistic model was constructed. Binary logistic models predict the probability of occurrence of a target species based on independent factors (Sarhangzadeh et al. Citation2013). To ensure the accuracy of the model, a two-fold greater number of randomly selected non-occurrence grids were added to obtain a total of 730 and 623 grids for the Great Spotted Woodpecker and the Grey-headed Woodpecker, respectively (). Correlation analysis was performed to identify factors displaying high collinearity (Spearman’s r2 > 0.7; Zar Citation1999), all of which were included in the covariance analysis owing to a lack of correlation among variables. The explanatory power of variables related to the occurrence of target species was evaluated using Nagelkerke values, and the goodness of fit of the model was regarded as acceptable if the Hosmer–Lemeshow test indicated P > 0.05 (Hosmer & Lemeshow Citation2000). Statistical analysis was performed using the moonBook package in R v.4.1.2 (R Foundation, Vienna, Austria) and IBM SPSS Statistics v.20.0 software (IBM Corp., Armonk, NY, USA).
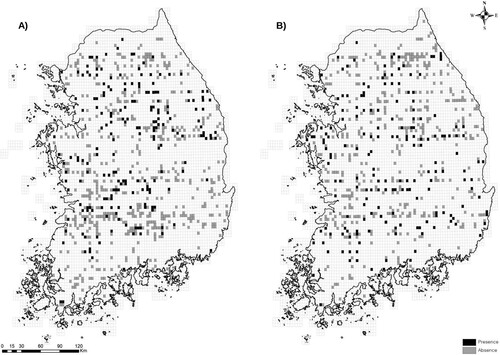
Figure 1. Distribution of the Great Spotted Woodpecker Dendrocopos major (A) and the Grey-headed Woodpecker Picus canus (B) in South Korea based on the National Ecosystem Survey conducted from 2006 to 2018. The grid squares and recording units were approximately 3.8 km × 4.6 km. Black indicates presence and grey indicates absence.
Results
The Hosmer-Lemeshow test for the binary logistic model revealed acceptable model fits for both the Great Spotted Woodpecker ( = 2.494, df = 8, P = 0.962) and the Grey-headed Woodpecker (
= 15.157, df = 8, P = 0.056). Nagelkerke
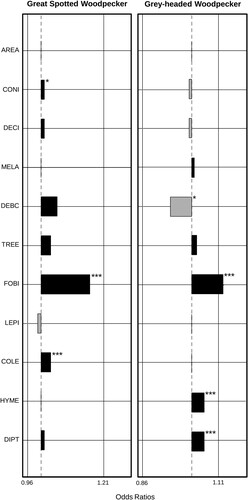
values (an indicator of explanatory power) for the investigated variables were 0.396 for the Great Spotted Woodpecker and 0.352 for the Grey-headed Woodpecker. Among forest characteristics, AREA, MELA and TREE were not significantly related to the occurrence of either species. The occurrence of the Great Spotted Woodpecker was positively correlated with CONI (β = 0.014, n = 730, P < 0.05), whereas the occurrence of the Grey-headed Woodpecker was negatively correlated with DEBC (β = −0.079, n = 623, P < 0.05). The species richness of forest birds was positively correlated with the occurrence of both the Great Spotted Woodpecker (β = 0.152, n = 730, P < 0.001) and the Grey-headed Woodpecker (β = 0.124, n = 623, P < 0.001). The species richness of LEPI showed no significant relationship with the occurrence of either woodpecker species. However, the occurrence of the Great Spotted Woodpecker was positively correlated with the species richness of COLE (β = 0.027, n = 730, P < 0.001) and that of the Grey-headed Woodpecker was positively correlated with the species richness of HYME (β = 0.045, n = 623, P < 0.001) and DIPT (β = 0.046, n = 623, P < 0.001) ().
Figure 2. Results of the binary logistic model based on large-scale public data for South Korea with the occurrence of the Great Spotted Woodpecker Dendrocopos major and the Grey-headed Woodpecker Picus canus as dependent variables and forest characteristics and species richness of forest birds and insects as independent factors. AREA: the forest area, CONI: forest stands with ≥ 75% coniferous trees, DECI: forest stands with ≥ 75% deciduous trees, MELA: forest stands with ≥ 50% medium- to large-diameter trees (≥18 cm diameter at breast height), DEBC: forest stands with canopy cover greater than 50%, TREE: the number of vegetation populations composed of trees, FOBI: the species richness of forest birds, LEPI: the species richness of Lepidoptera, COLE: the species richness of Coleoptera, HYME: the species richness of Hymenoptera, and DIPT: the species richness of Diptera. Black indicates a positive correlation and grey indicates a negative correlation. *P < 0.05, ***P < 0.001.
Discussion
Forest characteristics are an important determinant of the occurrence of woodpecker species (Stański et al. Citation2020) and preferences for forest attributes vary among species (MacArthur & MacArthur Citation1961). The occurrence of Great Spotted Woodpeckers in South Korea was significantly correlated with forested areas containing at least 75% coniferous trees, whereas that of the Grey-headed Woodpecker was not correlated with the abundance of coniferous or deciduous trees. The close association of the Great Spotted Woodpecker with coniferous trees has been reported in previous studies. Hogstad (Citation1978) claimed that coniferous trees comprised 96% of the feeding sites of the Great Spotted Woodpecker, and Stański et al. (Citation2020) reported that coniferous trees were used as feeding sites by Great Spotted Woodpeckers of both sexes, likely because these trees provide habitats for numerous insects found in the woodpecker’s diet (Hilszczański Citation2008). Great Spotted Woodpeckers frequently visit and forage on the surface of coniferous trees near the nest during the breeding season but display unique seasonal variation in food sources (Rolstad et al. Citation1995). During the summer and breeding season, they mainly feeds on insects, but can feed on seeds from pine cones during the winter by removing the cone from the branch, wedging it in an anvil and extracting the inner seeds (Osiejuk Citation1994, Citation1998, Kędra & Mazgajski Citation2001, Dylewski & Myczko Citation2019). Great Spotted Woodpeckers can feed on as many as 50 cones a day (Winkler & Christie Citation2002) and considering that coniferous trees produce as many as 650 cones annually, the species may be one of the major consumers of cones (Myczko & Benkman Citation2011). One study reported that coniferous trees may enable range expansion and colonization of new areas by the Great Spotted Woodpecker (La Mantia et al. Citation2002). In contrast, our findings suggest that the occurrence of the Grey-headed Woodpecker may not be related to forest type. Previous studies have reported that the Grey-headed Woodpecker inhabits mixed forests (Koskimies Citation1989), has a preference for deciduous over coniferous forests (Rassati Citation2014), and its abundance is unaffected by the proportion of coniferous trees in a forest (Pakkala et al. Citation2020).
Canopy cover is an important determinant of the composition and abundance of the forest bird community (Beşkardeş et al. Citation2018, Menon & Shahabuddin Citation2021). A high canopy cover offers the necessary microclimate for breeding (Şekercioḡlu et al. Citation2002) and lowers the probability of a nest being discovered by predators (Liebezeit & George Citation2002). Notably, canopy cover can also provide an indicator of the abundance of insects, which act as a food source for many forest birds (Kumar et al. Citation2011). However, in this study, canopy cover was not significantly correlated with the occurrence of Great Spotted Woodpeckers, which concurred with the results of previous studies (Beşkardeş et al. Citation2018; Beşkardeş Citation2020). In contrast, the occurrence of Grey-headed Woodpeckers was negatively correlated with canopy cover. Despite the previously described benefits, high canopy cover may reduce biodiversity on the forest floor (Lohr et al. Citation2002) and the Grey-headed Woodpecker has a characteristic preference for an open environment (Koskimies Citation1989, Gorman Citation2004, Alder & Marsden, Citation2010).
Forest area was not significantly correlated with the occurrence of either woodpecker species, lending support to the observation that even small forests can provide bird habitats (Winkler Citation2005); both species are commonly found using small farms or parks with small areas of forest (O'Connor & Shrubb Citation1986, Lee et al. Citation2020). Similarly, the occurrence of the Great Spotted Woodpecker and the Grey-headed Woodpecker in the Niraj Valley in Romania is reportedly not influenced by the forest area (Domokos & Cristea Citation2014).
Although the size of trees can be an important determinant of woodpecker habitat stability in general (Salvati et al. Citation2001, Bai Citation2005, Domokos & Cristea Citation2014), the occurrence of the two species in this study showed no significant correlation with forested areas containing more than 50% medium- to large-diameter trees. An increase in tree size is associated with an increase in the abundance of insects, and so the availability of food for insectivorous birds (Torgersen & Bull Citation1995, Rolstad et al. Citation1995, Lõhmus et al. Citation2010, Sukovata & Jaworski Citation2010, Menon & Shahabuddin Citation2021), and also an increase in potential nesting space (Rolstad et al. Citation1995, La Mantia et al. Citation2002). The Great Spotted Woodpecker, however, has been shown to prefer environments with relatively small trees, of less than 15 cm diameter at breast height (Lee Citation2013), and its population tends to decrease in forests that are mainly comprised of trees with large diameters (Hong et al. Citation2013). Notably, Great Spotted Woodpeckers prefer trees with smaller diameters as foraging sites (Hogstad Citation1971). The Grey-headed Woodpecker has a larger body size than the Great Spotted Woodpecker, and has a preference for forest containing large-diameter aspen Populus or birch Betula trees in Northern Europe. Indeed, the preference of the Grey-headed Woodpecker for larger trees has previously been correlated with nesting sites in boreal forests in Finland (Pakkala et al. Citation2020). Nevertheless, in Norway, the Grey-headed Woodpecker has been shown to inhabit coastal areas which lack large trees, which implies that the species can inhabit some regions despite limited nest site availability (Gjerde et al. Citation2005). Additionally, Grey-headed Woodpeckers might have a foraging advantage within forest stands comprised of one-year-old saplings or trees of small diameters (Spitznagel Citation1990).
The occurrence of the two woodpecker species was not correlated with the number of vegetation populations composed of trees in the forested area. Both species have also displayed flexibility enabling them to inhabit managed forests with a single vegetation population (Angelstam & Mikusiński Citation1994), which may help explain the results of the current study.
The diversity of the forest bird community is highly sensitive to variations in the forest environment and shows a strong positive correlation with woodpecker habitats (Mikusiński et al. Citation2001, Heikkinen et al. Citation2007, Camprodon et al. Citation2008, Drever et al. Citation2008, Cockle et al. Citation2011, Kumar et al. Citation2011, Segura et al. Citation2014, Menon & Shahabuddin Citation2021). Likewise, the species richness of forest birds was strongly and positively correlated with the occurrence of both the Great Spotted Woodpecker and the Grey-headed Woodpecker in this study. This is possibly because excavation by woodpeckers creates foraging and nesting sites for other forest birds (Drever et al. Citation2008). Indeed, Angelstam & Mikusiński (Citation1994) stated that specialized woodpeckers, such as the White-backed Woodpecker Dendrocopos leucostos and the Eurasian Three-toed Woodpecker Picoides tridactylus, were powerful predictors of the diversity of the forest bird community. The results of this study suggest that generalist species, such as Great Spotted and Grey-headed Woodpeckers, may have similar predictive value.
Despite strong correlations with the species richness of forest birds for both woodpecker species, the Great Spotted and Grey-headed Woodpeckers differed with respect to correlations with the species richness of forest insects belonging to various orders, which reflects their main food sources. In particular, the occurrence of the Great Spotted Woodpecker was positively correlated with the species richness of coleopteran insects while that of the Grey-headed Woodpecker was positively correlated with the species richness of hymenopteran and dipteran insects. While the Great Spotted Woodpecker shows substantial variation in its selection of food sources among regions and populations (Hannsson Citation1992, Michalek & Miettinen Citation2003), it is generally known to be an important predator of tree boring insects, such as bark and cerambycid beetles (Iwata et al. Citation1998, Jiao et al. Citation2008, Hu et al. Citation2009). Indeed, the presence of the Great Spotted Woodpecker was shown to reduce the survival rate of tree borers by 45–98% (Crockett & Hanoley Citation1978). In Sicily, plantation forestry of Eucalyptus trees has grown rapidly but while this tree provides a poor environment for most forest birds, the density of Great Spotted Woodpeckers tends to be elevated in such plantations (La Mantia et al. Citation2002). In fact, the importation of Eucalyptus trees was accompanied by the spread of the tree borer beetle Phoracantha semipunctata, which is used as a food source by the Great Spotted Woodpecker and contributed substantially to the expansion of the woodpecker population (La Mantia et al. Citation2002). Interestingly, coniferous trees, which were significantly correlated with the occurrence of the Great Spotted Woodpecker in this study, are also closely associated with tree boring beetles. Among 214 species of bark beetles inhabiting regions in Canada, approximately 73% (156/214 species) inhabit only coniferous trees (Bright Citation1976). Additionally, the main habitat of cerambycid beetles in Japan is coniferous trees (Iwata et al. Citation1998) and certain tree borer populations show less abundance, suggesting the tendency of woodpeckers to feed primarily on Coleoptera species (Iwata et al. Citation1997). Thus, our work adds to the growing evidence that supports a strong link between the occurrence of Great Spotted Woodpeckers, coniferous trees and coleopteran insects. Conversely, the Grey-headed Woodpecker has been shown to rely almost entirely on ants for food in boreal and hemiboreal forests (Angelstam & Mikusiński, Citation1994). Indeed, egg clusters and adult ants comprised approximately 97% of the diet of Grey-headed Woodpeckers during the breeding season in South Korea (Koo & Won Citation1986). The previously described preference of the Grey-headed Woodpecker for an open environment can be explained by the abundance and high diversity of ants in these areas (Dauber et al. Citation2006). In the central and southern regions of Scandinavia, the Grey-headed Woodpecker switched its main food source to Camponotus ants and dipteran insects when the winter cold persisted or snow depth exceeded 15 cm (Rolstad & Rolstad Citation1995), even though the abundance of Formica ants was high throughout the year. These observations aligned with the rapid decrease in the availability of Formica ants during this time, indicating that the Grey-headed Woodpeckers were using food sources that were hibernating under thin tree bark but which were relatively easy to obtain. In addition, analysis of the gastric contents of 16 dead Grey-headed Woodpeckers in South Korea revealed that approximately 94% of the insects were Hymenoptera species (with ants accounting for 87%) while the remaining 6% were Diptera species (Fennel Citation1965).
This study had some limitations. Data for dead trees, which are critical determinants of woodpecker distribution, were lacking (Bütler et al. Citation2004, Vergara & Schlatter Citation2004, Czeszczewik & Walankiewicz Citation2006, Smith Citation2007, Roberge et al. Citation2008, Garcia-del-Rey et al. Citation2009; Drever & Martin Citation2010, Cockle et al. Citation2011, Stański et al. Citation2020). The large-scale public data used in this study were collected by multiple investigators, including us, leading to some heterogeneity due to observer differences (Kwon et al. Citation2020). Additionally, the collected data were purely qualitative and noted only the presence/absence of target species (Nam et al. Citation2019), thus only species richness could be determined for forest bird and insect communities. Nevertheless, this study supports the results of previous studies dealing with the relationship between the Great Spotted Woodpecker and conifer trees and Coleoptera, and the Grey-headed Woodpecker and ants. Although Prendergast et al. (Citation1993), Berg & Tjernberg (Citation1996), and Pärt & Söderström (Citation1999) performed their surveys at a different scale to the 1:25,000 scale used in this study, we suggest that a metacommunity approach at various scales can provide important insights in understanding the occurrence patterns of species (Leibold et al. Citation2004).
Although woodpeckers are sedentary, resident birds, their occurrence and determining factors vary during the breeding and non-breeding seasons (Cahill & Matthysen Citation2007, Kumar et al. Citation2011). Our results suggest that the shared habitats of the two species can be explained by low competition resulting from differences in preferred forest characteristics and food sources.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alder, D. & Marsden, S. 2010. Characteristics of feeding-site selection by breeding Green Woodpeckers Picus viridis in a UK agricultural landscape. Bird Study 57: 100–107.
- Andradas, M.X., Arizaga, J. & Rodríguez-Pérez, J. 2019. Species co-occurrence and environmental factors and their effect on the distribution of forest birds in mature forests. Forestry 92: 568–576.
- Angelstam, P. & Mikusiński, G. 1994. Woodpecker assemblages in natural and managed boreal and hemiboreal forest—a review. Ann. Zool. Fenn 31: 157–172.
- Araújo, M.B. & Luoto, M. 2007. The importance of biotic interactions for modelling species distributions under climate change. Glob. Ecol. Biogeogr 16: 743–753.
- Arlettaz, R., Godat, S. & Meyer, H. 2000. Competition for food by expanding pipistrelle bat populations (Pipistrellus pipistrellus) might contribute to the decline of lesser horseshoe bats (Rhinolophus hipposideros). Biol. Conserv 93: 55–60.
- Arslangundogdu, Z. 2010. Presence of insectivorous birds in the forest area of Istanbul university, Turkey. J. Environ. Biol 31: 197–206.
- Atkinson, P.W., Fuller, R.J., Vickery, J.A., Conway, G.J., Tallowin, J.R.B., Smith, R.E.N., Haysom, K.A., Ings, T.C., Asteraki, E.J. & Brown, V.K. 2005. Influence of agricultural management, sward structure and food resources on grassland field use by birds in lowland England. J. Appl. Ecol 42: 932–942.
- Bai, M.L. 2005. Tree cavity abundance and nest site selection of cavity nesting birds in a natural boreal forest of West Khentey, Mongolia. Ph.D. Thesis, Georg-August-Universität zu Göttingen, Göttingen.
- Bastolla, U., Lässig, M., Manrubia, S.C. & Valleriani, A. 2005. Biodiversity in model ecosystems, I: coexistence conditions for competing species. J. Theor. Biol 235: 521–530.
- Berg, Å & Tiernberg, M. 1996. Common and rare Swedish vertebrates: distribution and habitat preferences. Biodivers. Conserv 5: 101–128.
- Beşkardes, V. 2020. Habitat selection of insectivorous birds in western Black Sea region of Turkey. Appl. Ecol. Environ. Res 18: 7551–7562.
- Beşkardeş, V., Keten, A., Kumbaşlı, M., Pekin, B., Yılmaz, E., Makineci, E. & Ozdemir, E. 2018. Bird composition and diversity in oak stands under variable coppice management in Northwestern Turkey. iForest 11: 58–63.
- BrightJrD.E. 1976. The Insects and Arachnids of Canada Part 2: The Bark Beetles of Canada and Alaska. Canada Department of Agriculture, Ottawa.
- Buckingham, D.L. & Peach, W.J. 2005. The influence of livestock management on habitat quality for farmland birds. Anim. Sci 81: 199–203.
- Bütler, R., Angelstam, P., Ekelund, P. & Schlaepfer, R. 2004. Dead wood threshold values for the three-toed woodpecker presence in boreal and sub-Alpine forest. Biol. Conserv 119: 305–318.
- Cahill, J.R. & Matthysen, E. 2007. Habitat use by two specialist birds in high-Andean Polylepis forests. Biol. Conserv 140: 62–69.
- Camprodon, J., Salvanyà, J. & Soler-Zurita, J. 2008. The abundance and suitability of tree cavities and their impact on hole-nesting bird populations in beech forests of NE Iberian Peninsula. Acta Ornithol 43: 17–31.
- Carvalho, J.C. & Gomes, P. 2003. Habitat suitability model for European wild rabbit (Oryctolagus cuniculus) with implications for restocking. Game Wildl. Sci 20: 287–301.
- Chesson, P. 2000. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst 31: 343–366.
- Cockle, K.L., Martin, K. & Wesołowski, T. 2011. Woodpeckers, decay, and the future of cavity-nesting vertebrate communities worldwide. Front Ecol. Environ 9: 377–382.
- Crockett, A.B. & Hanoley, P.L. 1978. Apparent response of picoides woodpeckers to outbreaks of the pine bark beetle. West. Birds 9: 67–70.
- Czeszczewik, D. & Walankiewicz, W. 2006. Logging affects the white-backed woodpecker Dendrocopos leucotos distribution in the Białowieża Forest. Ann. Zool. Fenn 43: 221–227.
- Dauber, J., Bengtsson, J. & Lenoir, L. 2006. Evaluating effects of habitat loss and land-use continuity on ant species richness in seminatural grassland remnants. Conserv. Biol 20: 1150–1160.
- de Araújo, C.B., Marcondes-Machado, L.O. & Costa, G.C. 2014. The importance of biotic interactions in species distribution models: a test of the Eltonian noise hypothesis using parrots. J. Biogeogr 41: 513–523.
- Domokos, E. & Cristea, V. 2014. Effects of managed forests structure on woodpeckers (Picidae) in the Niraj valley (Romania): Woodpecker populations in managed forests. North-West J. Zool 10: 110–117.
- Drever, M.C., Aitken, K.E.H., Norris, A.R. & Martin, K. 2008. Woodpeckers as reliable indicators of bird richness, forest health and harvest. Biol. Conserv 141: 624–634.
- Drever, M.C. & Martin, K. 2010. Response of woodpeckers to changes in forest health and harvest: implications for conservation of avian biodiversity. For. Ecol. Manag 259: 958–966.
- Dylewski, Ł & Myczko, Ł. 2019. Great spotted woodpecker (Dendrocopos major) and red squirrel (Sciurus vulgaris) prefer different cone features of European larch (Larix decidua). Biologia 74: 515–519.
- Fennell, C.M. 1965. Stomach analyses of Korean birds. J. Yamashina Inst. Ornithol 4: 172–183.
- Freeman, B.G. & Mason, N.A. 2015. The geographic distribution of a tropical montane bird is limited by a tree: Acorn Woodpeckers (Melanerpes formicivorus) and Colombian oaks (Quercus humboldtii) in the Northern Andes. PLoS One 10: e0128675.
- Garcia-del-Rey, E., Fernández-Palacios, J.M. & Muñoz, P.G. 2009. Intra-annual variation in habitat choice by an endemic woodpecker: implications for forest management and conservation. Acta Oecol. 35: 685–690.
- Giannini, T.C., Chapman, D.S., Saraiva, A.M., Alves-dos-Santos, I. & Biesmeijer, J.C. 2013. Improving species distribution models using biotic interactions: a case study of parasites, pollinators and plants. Ecography 36: 933–945.
- Gjerde, I., Sætersdal, M. & Nilsen, T. 2005. Abundance of two threatened woodpecker species in relation to the proportion of spruce plantations in native pine forests of western Norway. Biodiv. Conserv 14: 377–393.
- Gorman, G. 2004. Woodpeckers of Europe: a study of the European picidae. Bruce Coleman Books, London.
- Gorman, G. 2014. Woodpeckers of the World: the complete guide. Bloomsbury Publishing, London.
- Guisan, A. & Zimmermann, N.E. 2000. Predictive habitat distribution models in ecology. Ecol. Modell 135: 147–186.
- Haila, Y., Nicholls, A.O., Hanski, I. & Raivo, S. 1996. Stochasticity in bird habitat selection: year-to-year changes in territory locations in a boreal forest bird assemblage. Oikos 76: 536–552.
- Hannsson, L. 1992. Requirements by the great spotted woodpecker Dendrocopos major for a suburban life. Ornis Svec 2: 1–6.
- Hawkins, B.A., Field, R., Cornell, H.V., Currie, D.J., Guegan, J.F., Kaufman, D.M., Kerr, J.T., Mittelbach, G.G., Oberdorff, T., O’Brien, E.M., Porter, E.E. & Turner, J.R.G. 2003. Energy, water, and broad-scale geographic patterns of species richness. Ecology 84: 3105–3117.
- Heikkinen, R.K., Luoto, M., Virkkala, R., Pearson, R.G. & Körber, J.H. 2007. Biotic interactions improve prediction of boreal bird distributions at macro-scales. Global Ecol. Biogeogr 16: 754–763.
- Hilszczański, J. 2008. Bark of dead infested spruce trees as an overwintering site of insect predators associated with bark and wood boring beetles. For. Res. Pap 69: 15–19. (in Polish with English abstract).
- Hof, A.R., Jansson, R. & Nilsson, C. 2012. How biotic interactions may alter future predictions of species distributions: future threats to the persistence of the arctic fox in Fennoscandia. Divers. Distrib 18: 554–562.
- Hogstad, O. 1971. Stratification in winter feeding of the Great Spotted Woodpecker Dendrocopos major and the Three-toed Woodpecker Picoides tridactylus. Ornis Scand 2: 143–146.
- Hogstad, O. 1978. Sexual dimorphism in relation to winter foraging and territorial behaviour of the Three-toed Woodpecker Picoides tridactylus and three Dendrocopos species. Ibis 120: 198–203.
- Holbrook, S.J. & Schmitt, R.J. 1988. The combined effects of predation risk and food reward on patch selection. Ecology 69: 125–134.
- Hong, S.H., Kim, J.S., Ki, K.S., Park, S.G. & Kurosawa, K. 2013. Woodpeckers appearance and forest vegetation type in urban forests of Seoul area, Korea. J. Fac. Agric. Kyushu Univ 58: 253–258.
- Hosmer, D.W. & Lemeshow, S. 2000. Applied Logistic Regression. 2nd edn. John Wiley and Sons, New York.
- Hu, J., Angeli, S., Schuetz, S., Luo, Y. & Hajek, A.E. 2009. Ecology and management of exotic and endemic Aian longhorned beetle Anoplophora glabripennis. Agric. For. Entomol 11: 359–375.
- Iwata, R., Sakakibara, Y. & Yamada, F. 1998. Boring activity on coniferous tree branches by Xylotrechus villioni (Villard) larvae (Coleoptera: Cerambycidae). J. For. Res 3: 247–249.
- Iwata, R., Yamada, F., Katô, H., Makihara, H., Araya, K., Ashida, H. & Takeda, M. 1997. Nature of galleries, durability of boring scars, and density of Xylotrechus villioni (Villard) larvae (Coleoptera: Cerambycidae), on coniferous tree trunks. Pan-Pac. Entomol 73: 213–224.
- Jiao, Z.B., Wan, T., Wen, J.B., Hu, J.F., Luo, Y.Q., Fu, L.J. & Zhang, L.S. 2008. Seasonal diet of the Great Spotted Woodpecker (Picoides major) in shelterwood plantations of Wulate Qianqi County, Inner Mongolia. For. Stud. China 10: 119–124.
- Kędra, A.H. & Mazgajski, T.D. 2001. Factors affecting anvils utilization by Great Spotted Woodpecker Dendrocopos major. Pol. J. Ecol 49: 79–86.
- KMA. 2023. Climate of South Korea. Korea Meteorological Administraion, Seoul. https://devweather.kma.go.kr/weather/climate/average_south.jsp.
- KOFPY. 2017. The Production of Forest Type Map actualizing development of manual for DB V3.1. Korea Forestry Promotion Institute, Seoul (in Korean).
- Koo, T.H. & Won, P.O. 1986. The reproductive success of the Grey-headed green woodpecker, Picus canus griseoviridis (Clark). Bull. Korea Inst. Ornithol 1: 57–67.
- Koskimies, P. 1989. Distribution and Numbers of Finnish Breeding Birds: Appendix to Suomen Lintuatlas. Lintutieto, Helsinki.
- Kruszyk, R. 2003. Population density and foraging habits of the Middle Spotted Woodpecker Dendrocopus medius and Great Spotted Woodpecker D. major in the Odra valley near Wrocław. Notatki Ornitologiczne 44: 75–88. (in Polish with English abstract).
- Kumar, R., Shahabuddin, G. & Kumar, A. 2011. How good are managed forests at conserving native woodpecker communities? A study in sub-Himalayan dipterocarp forests of northwest India. Biol. Conserv 144: 1876–1884.
- Kusch, J. & Schmitz, A. 2013. Environmental factors affecting the differential use of foraging habitat by three sympatric species of Pipistrellus. Acta Chiropterol 15: 57–67.
- Kwon, H.S., Lee, Y.K., Yoo, S.H., Kim, D.W. & Kim, J.S. 2020. Distribution patterns of biodiversity hotspot using birds data from the 3rd National Ecosystem Survey in South Korea. J. Korea Soc. Environ. Restor. Technol 23: 81–89. (in Korean with English abstract).
- La Mantia, T., Spoto, M. & Massa, B. 2002. The colonisation of the Great Spotted Woodpecker (Picoides major L.) in Eucalypt woods and popular cultivations in Sicily. Ecol. Mediterr 28: 65–73.
- Lee, K.M. 2013. The effect of urban green space characteristics and landscape structure on Dendrocopos major and Dendrocopos kizuki distribution. Master’s Thesis, Seoul National University, Seoul (in Korean).
- Lee, K. 2015. Classification of forested vegetation and successional development in the Central-Eastern Korean Peninsula. Ph.D. Thesis, Kangwon National University, Chuncheon (in Korean).
- Lee, W.S., Koo, T.H. & Park, J.Y. 2020. A Field Guide to the Birds of Korea. 2nd edn. LG Foundation, Seoul (in Korean).
- Leibold, M.A. 1995. The niche concept revisited: mechanistic models and community context. Ecology 76: 1371–1382.
- Leibold, M.A., Holyoak, M., Mouquet, N., Amarasekare, P., Chase, J.M., Hoopes, M.F., Holt, R.D., Shurin, J.B., Law, R., Tilman, D., Loreau, M. & Gonzalez, A. 2004. The metacommunity concept: a framework for multi-scale community ecology. Ecol. Lett. 7: 601–613.
- Leibold, M.A. & McPeek, M.A. 2006. Coexistance of the niche and neutral perspectives in community ecology. Ecology 87: 1399–1410.
- Liebezeit, J.R. & George, T.L. 2002. Nest predators, nest-site selection, and nesting success of the Dusky Flycatcher in a managed ponderosa pine forest. Condor 104: 507–517.
- Lima, S.L. & Dill, L.M. 1990. Behavioral decisions made under the risk of predation: A review and prospectus. Can. J. Zool 68: 619–640.
- Lõhmus, A., Kinks, R. & Soon, M. 2010. The importance of dead-wood supply for woodpeckers in Estonia. Balt. For 16: 76–86.
- Lohr, S.M., Gauthreaux, S.A. & Kilgo, J.C. 2002. Importance of coarse woody debris to avian communities in loblolly pine forests. Conserv. Biol 16: 767–777.
- Luck, G.W. 2002. The habitat requirement of the Rufous Treecreeper (Climacteris rufa). 1. Preferential habitat use demonstrated at multiple spatial scales. Biol. Conserv 105: 383–394.
- MacArthur, R.H. & MacArthur, J. 1961. On bird species diversity. Ecology 42: 594–598.
- Mardiastuti, A. 2019. Bird community changes across gradient of habitat quality in the urban green open space. IOP Conf. Ser.: Earth Environ. Sci 407: 012012.
- Matthews, S.N., Iverson, L.R., Prasad, A.M. & Peters, M.P. 2011. Changes in potential habitat of 147 North American breeding bird species in response to redistribution of trees and climate following predicted climate change. Ecography 34: 933–945.
- Menon, T. & Shahabuddin, G. 2021. Assessing woodpeckers as indicators of bird diversity and habitat structure in managed forests. Biodiv. Conserv 30: 1689–1704.
- Michalek, K.G. & Miettinen, J. 2003. Dendrocopos major Great Spotted Woodpecker. Birds West. Palearct5: 101–184.
- Mikusiński, G. & Angelstam, P. 1997. European woodpeckers and anthropogenic habitat change. Die Vogelwelt 118: 277–283.
- Mikusiński, G., Gromadzki, M. & Chylarecki, P. 2001. Woodpeckers as indicators of forest bird diversity. Conserv. Biol 15: 208–217.
- Myczko, Ł & Benkman, C.W. 2011. Great spotted woodpeckers Dendrocopos major exert multiple forms of phenotypic selection on Scots pine Pinus sylvestris. J. Avian. Biol 42: 429–433.
- Nam, H.Y., Choi, C.Y., Park, J.Y. & Hur, W.H. 2019. Comparison of population monitoring methods for breeding forest birds in Korean temperate mixed forests. J. Korean For. Soc 108: 663–674. (in Korean with English abstract).
- NIBR. 2022. 2021 National Species List of Korea. National Institute of Biological Resources, Incheon. https://www.kbr.go.kr/stat/ktsnfiledown.
- NIE. 2019. The Fifth National Ecosystem Survey Guideline. National Institute of Ecology, Seocheon (in Korean).
- Nie, Y., Zhou, W., Gao, K., Swaisgood, R.R. & Wei, F. 2019. Seasonal competition between sympatric species for a key resource: implications for conservation management. Biol. Conserv 234: 1–6.
- NIER. 2012. The Fourth National Ecosystem Survey Guideline. National Institute of Environmental Research, Incheon (in Korean).
- O’Connor, R.J. & Shrubb, M. 1986. Farming and Birds. Cambridge University Press, Cambridge.
- Oppel, S., Schaefer, H.M., Schmidt, V. & Schröder, B. 2004. Habitat selection by the pale-headed brush-finch (Atlapetes pallidiceps) in southern Ecuador: implications for conservation. Biol. Conserv 118: 33–40.
- Osiejuk, T.S. 1994. Sexual dimorphism in foraging behaviour of the Great Spotted Woodpecker Dendrocopos major during winters with rich crops of Scotch pine cones. Ornis Fenn. 71: 144–150.
- Osiejuk, T.S. 1998. Study on the intersexual differentiation of foraging niche in relation to abundance of winter food in Great Spotted Woodpecker Dendrocopos major. Acta Ornithol 33: 135–141.
- Pakkala, T., Tiainen, J., Pakkala, H., Piha, M. & Kouki, J. 2020. Nest tree characteristics of Grey-headed Woodpeckers (Picus canus) in boreal forests. Ornis Fenn. 97: 89–100.
- Park, J.G. 2014. Identification Guide to Birds of Korea. Econature, Seoul (in Korean).
- Park, J.M., Do, M.R., Sim, W.D. & Lee, J.S. 2019. A study on the improvement of guideline in digital forest type map. J. Korean Ass. Geogr. Inf. Studies 22: 168–182. (in Korean).
- Pärt, T. & Söderström, B. 1999. Conservation value of semi-natural pastures in Sweden: contrasting botanical and avian measures. Conserv. Biol 13: 755–765.
- Pianka, E.R. 1976. Competition and niche theory. In May, R.M. (ed) Theoretical Ecology, 114–121. Blackwell Scientific Publications, Oxford.
- Porej, D., Micacchion, M. & Hetherington, T.E. 2004. Core terrestrial habitat for conservation of local populations of salamanders and wood frogs in agricultural landscapes. Biol. Conserv 120: 399–409.
- Prendergast, J.R., Quinn, R.M., Lawton, J.H., Eyersham, B.C. & Gibbons, D.W. 1993. Rare species, the coincidence of diversity hotspots and conservation strategies. Nature 365: 335–337.
- Rahbek, C. & Graves, G.R. 2001. Multiscale assessment of patterns of avian species richness. Proc. Natl. Acad. Sci. USA 98: 4534–4539.
- Rassati, G. 2014. Responsiveness to acoustic stimulation, distribution and habitat preferences of the Grey-headed Woodpecker, Picus canus, and the Three-toed Woodpecker, Picoides tridactylus, in Friuli-Venezia Giulia (North-eastern Italy). Riv. Ital. Ornitol 84: 41–52.
- Roberge, J.M., Angelstam, P. & Villard, M.A. 2008. Specialised woodpeckers and naturalness in hemiboreal forests-deriving quantitative targets for conservation planning. Biol. Conserv 141: 997–1012.
- Rolstad, J. & Rolstad, E. 1995. Seasonal patterns in home range and habitat use of the grey-headed woodpecker Picus canus as influenced by the availability of food. Ornis Fenn. 72: 1–13.
- Rolstad, J., Rolstad, E. & Stokke, P.K. 1995. Feeding habitat and nest-site selection of breeding Great Spotted Woodpeckers Dendrocopos major. Ornis Fenn. 72: 62–71.
- Salvati, L., Manganaro, A. & Ranazzi, L. 2001. Wood occupation and area requirement of the Great Spotted Woodpecker Picoides major in Rome (Central Italy). Acta Ornithol 36: 19–23.
- Sarhangzadeh, J., Yavari, A.R., Hemami, M.R., Jafari, H.R. & Shams-Esfandabad, B. 2013. Habitat suitability modeling for wild goat (Capra aegagrus) in a mountainous arid area, central Iran. Casp. J. Environ. Sci 11: 41–51.
- Şekercioḡlu, ÇH, Ehrlich, P.R., Daily, G.C., Aygen, D., Goehring, D. & Sandí, R.F. 2002. Disappearance of insectivorous birds from tropical forest fragments. Proc. Natl. Acad. Sci. USA 99: 263–267.
- Segura, A., Castaño-Santamaría, J., Laiolo, P. & Obeso, J.R. 2014. Divergent responses of flagship, keystone and resource-limited bio-indicators to forest structure. Ecol. Res 29: 925–936.
- Sexton, J.P., McIntyre, P.J., Angert, A.L. & Rice, K.J. 2009. Evolution and ecology of species range limits. Ann. Rev. Ecol. Evol. Syst 40: 415–436.
- Smith, K.W. 2007. The utilization of dead wood resources by woodpeckers in Britain. Ibis 149: 183–192.
- Spitznagel, A. 1990. The influence of forest management on woodpecker density and habitat use in floodplain forests of the upper Rhine Valley. In Carlson, A. & Aulén, G. (ed) Conservation and Management of Woodpecker Populations, Report 17, 147–164. Department of Wildlife Ecology, Swedish University of Agricultural Sciences, Uppsala.
- Stański, T., Czeszczewik, D., Stańska, M. & Walankiewicz, W. 2020. Foraging behaviour of the Great Spotted Woodpecker Dendrocopos major in relation to sex in primeval stands of the Białowieża National Park. Acta Ornithol 55: 120–128.
- Sukovata, L. & Jaworski, T. 2010. The abundance of the nun moth and lappet moth larvae on trees of different trunk thickness in Scots pine stands in the Notec forest complex. For. Res. Pap 71: 231–237. (in Polish with English abstract).
- Torgersen, T.R. & Bull, E.L. 1995. Down logs as habitat for forest-dwelling ants- the primary prey of pileated woodpeckers in northeastern Oregon. Northwest. Sci. 69: 294–303.
- Török, J. 1990. Resource partitioning among three woodpecker species Dendrocopos spp. during the breeding season. Ecography 13: 257–264.
- Van Buskirk, J. 2005. Local and landscape influence on amphibian occurrence and abundance. Ecology 86: 1936–1947.
- Vergara, P. & Schlatter, R.P. 2004. Magellanic Woodpecker (Campephilus magellanicus) abundance and foraging in Tierra del Fuego, Chile. J. Ornithol 145: 343–351.
- Vickery, J.A., Tallowin, J.R., Feber, R.E., Asteraki, E.J., Atkinson, P.W., Fuller, R.J. & Brown, V.K. 2001. The management of lowland neutral grasslands in Britain: effects of agricultural practices on birds and their food resources. J. Appl. Ecol 38: 647–664.
- Virkkala, R. 2006. Why study woodpeckers? The significance of woodpeckers in forest ecosystems. Ann. Zool. Fenn 43: 82–85.
- Winkler, D. 2005. Ecological succession of breeding bird communities in deciduous and coniferous forest in the Sopron Mountains. Acta Silv. Lignaria Hung 1: 49–58.
- Winkler, H. & Christie, D.A. 2002. Family Picidae (woodpeckers). In Hoyo, J.d., Elliott, A. & Sargatal, J. (eds) Handbook of the Birds of the World, Jacamars to Woodpeckers, Vol. 7, 296–558. Jacamars to woodpeckers. Lynx Edicions, Barcelona.
- Wisz, M.S., Pottier, J., Kissling, W.D., Pellissier, L., Lenoir, J., Damgaard, C.F., Dormann, C.F., Forchhammer, M.C., Grytnes, J., Guisan, A., Heikkinen, R.K., Høye, T.T., Kühn, I., Luoto, M., Maiorano, L., Nilsson, M., Normand, S., Öckinger, E., Schmidt, N.M., Termansen, M., Timmermann, A., Wardle, D.A., Aastrup, P. & Svenning, J.C. 2013. The role of biotic interactions in shaping distributions and realised assemblages of species: implications for species distribution modelling. Biol. Rev 88: 15–30.
- Zar, J.H. 1999. Biostatistical Analysis. Prentice Hall, New Jersey.
