ABSTRACT
Capsule:
Red-necked Falcons Falco chicquera shift their trophic niche, from eating birds to bats, during the rainy monsoon season.
Aims:
To explore seasonal changes in prey weight, diet composition, and mobbing interactions of the globally Near Threatened Red-necked Falcon in Bangladesh.
Methods:
We analysed long-term direct observations of 19 adult pairs feeding on prey between 2002 and 2019, exploring changes in mean prey weight, prey group, and mobbing interactions throughout the cool dry winter, hot pre-monsoon, and rainy monsoon seasons.
Results:
The probability of bats being present within the diet increased over time, from 16% in the cool dry winter and 11% in the pre-monsoon hot season, to 48% in the rainy monsoon season. This coincided with a significant decline in mean prey weight and biomass consumed during the rainy monsoon season. Probabilities of falcons being mobbed during feeding varied throughout the seasons, depending on mobbing species.
Conclusion:
Red-necked Falcons appeared to track changing environments by shifting their trophic niche during the rainy monsoon season. This may be due to: (a) an optimal foraging response to bat population fluxes, itself a response to weather-induced increases in flying insect abundances, (b) opportunistic hunting of bats during periods of crepuscular activity peaks, and/or (c) the energetic demands of fledglings during the rainy monsoon season.
Studying animal diets is fundamental to the full understanding of a species’ ecology and resource requirements throughout time and space (Neilsen et al. Citation2018). Knowledge of how a species’ trophic niche may change over time, in response to changes within its environment, can improve our understanding of species responses to environmental change (Descombes et al. Citation2015). Such information is essential for effective conservation management and is important for species that maintain high positions within trophic networks, such as raptors (Speziale & Lambertucci Citation2013, Sullivan et al. Citation2015, Buechley et al. Citation2019, Panter et al. Citation2022).
Methods used to study raptor diets vary depending on the study species and resources available to the researcher. Traditional methods include collecting prey remains (McPherson et al. Citation2016, Suri et al. Citation2017) and/or pellets (Oro & Tella Citation1995, Šalek et al. Citation2010, Sumasgutner et al. Citation2013), radio-telemetry (Kenward Citation1982, Rutz Citation2003, Citation2004), cameras or hides (Newton Citation1978, Murgatroyd et al. Citation2016, Harrison et al. Citation2019), and, more recently, satellite-telemetry (Arkumarev et al. Citation2021), stable isotope analyses (Catry et al. Citation2016, Johnson et al. Citation2020, Jones et al. Citation2023), DNA metabarcoding (Bourbour et al. Citation2019, Citation2021), and web-sourced photography (Naude et al. Citation2019, Berryman & Kirwan Citation2021, Panter & Amar Citation2021, Citation2022). Long-term direct field observations, by experienced observers, allow for repeated visits to known territories over time (Snyder & Wiley Citation1976, Collopy Citation1983) and are a suitable method for studying species whose pellets do not provide accurate representations of their diet (Marti Citation1987, Redpath et al. Citation2001). Consequently, this approach remains a popular method to study raptor diets as it provides the most accurate measure of diet (Redpath et al. Citation2001, Lewis et al. Citation2010, Margalida et al. Citation2010). Despite the fact that long-term field observations may be labour and time intensive (Lewis et al. Citation2010), the approach can provide high-resolution data on the diets of individuals or at the local-population level. Long-term field observations can be an effective tool when studying diets of raptor species with declining population trends, such as the Red-necked Falcon Falco chicquera.
The Red-necked Falcon is widely distributed across Africa and the Indian subcontinent where two disjunct populations occur (del Hoyo et al. Citation2020). Within these two populations, three sub-species have been described. The African populations form the two sub-species Falco chicquera ruficollis and F. c. horsbrughi; some authorities treat these as species separately from the Asian populations (Wink & Sauer-Gürth Citation2000, Bhatt Citation2022). Asian birds, F. c. chicquera, are the smallest in size of the three taxa and occur from south-east Iran through Pakistan, India, Nepal, and Bangladesh (del Hoyo et al. Citation2020). In Bangladesh, the Red-necked Falcon is considered a rare, localized breeder (Naoroji Citation2007) and is difficult to observe in the field due to its small size (adult males 160 g, adult females 250 g; Tobias et al. Citation2022), crepuscular behaviour and preference for perching in foliage (Naoroji Citation2007). It frequents open habitat interspersed with tree groves, cultivated land, and villages, and avoids dense forest and coastlines (Dharmakumarsinhji Citation1954, Ali & Ripley Citation1978, Cade & Digby Citation1982, Naoroji Citation2007, Bhatt Citation2022). Globally, the species is experiencing moderately rapid population declines due to habitat degradation (Rasmussen & Anderton Citation2005, Naoroji Citation2007) and the number of mature individuals currently remains unknown (BirdLife International Citation2023). Subsequently, the species is listed as Near Threatened on the International Union for the Conservation of Nature’s Red List of Threatened Species (BirdLife International Citation2023).
The Red-necked Falcon is one of the lesser-known species within the genus Falco and has received relatively little research attention, as is often the case with tropical Asian raptors (Buechley et al. Citation2019). Despite this, there have been some attempts to quantify foraging-related information (Ali & Ripley, Citation1978, Khan, Citation1978, Naoroji, Citation2007, Citation2011, Foysal Citation2015) and reproductive rates for the species (Bhatt Citation2022). A paper by Subramayana (Citation1985) focused on hunting and feeding behaviour of Red-necked Falcons but there is a paucity of research on its diet, especially outside the breeding season, and existing information is based on small sample sizes. Consequently, it remains unknown how this species might respond to environmental change, but such information could aid effective on-the-ground conservation across parts of its range.
In this study, we quantify long-term direct field observations of Red-necked Falcons from eight districts across Bangladesh. We explore seasonal changes in mean prey weight and prey groups, and provide data on inter- and intraspecific mobbing interactions during feeding. As resource availability is known to vary across seasons, for example, due to changes in tropical insect abundances (Janzen & Schoener Citation1968, Kishimoto-Yamada & Itioka Citation2015), we predict that Red-necked Falcons respond to these changes by shifting their trophic niche through the year, for example by tracking population increases in other trophic groups, such as bats (Chiroptera spp.). In addition, interspecific competition for resources and anti-predatory responses from prey species can limit hunting efficiency and prey supply. For example, a recent study found that reduced mobbing by more dominant species resulted in higher reproductive success in Northern Goshawks Accipiter gentilis (Rebollo et al. Citation2022). Therefore, we provide an overview of inter- and intraspecific mobbing events during feeding, and explore variation in the probabilities of Red-necked Falcons being mobbed across the three seasons (the cool dry winter, pre-monsoon hot and rainy monsoon seasons) in Bangladesh. We predict an increase in mobbing frequency during the rainy monsoon season, coinciding with the breeding season of most subtropical birds, as an anti-predatory response during this critical period of the year.
Methods
Study area
Bangladesh is a subtropical country in south Asia, spanning 20°34ʹ to 26°33ʹ N and 88°01ʹ to 92°41ʹ E. It is one of the most densely populated countries in the world, with a human population of approximately 170 million people (United Nations Citation2022). Zoogeographically, it falls within the Oriental region at the confluence of two major biotic subregions: the Indo-Himalayas and Indo-China (Khan Citation2008). Climatically, Bangladesh is a tropical monsoon with marked seasonal variations. The ‘rainy monsoon’ season occurs during June to October, followed by the ‘cool dry winter’ through November to February and a ‘pre-monsoon hot’ season between March and May. Daytime temperatures range from 11°C to 29°C in the cool dry winter and 21–34°C during the pre-monsoon hot season. Mean annual rainfall varies from 1100 mm in the extreme west to 5700 mm in the north-east of Bangladesh, with about 70–80% falling during the rainy monsoon season (Khan Citation2008).
Field observations
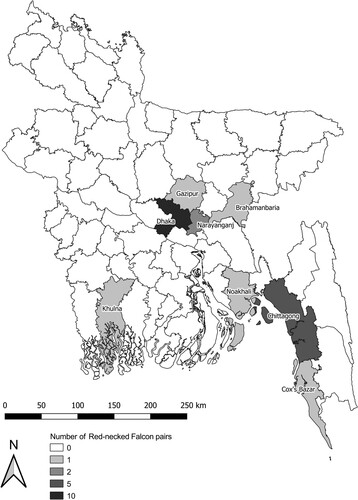
Between 2002 and 2019, direct field observations of 19 adult Red-necked Falcon pairs were conducted across eight districts in Bangladesh, including urban, suburban, and rural locations (). Falcons were observed in the districts of Brahamanbaria, Chattogram, Cox’s Bazar, Dhaka, Gazipur, Khulna, Narayanganj, and Noakhali (). Red-necked Falcons are a crepuscular species (Khan Citation1978, Ferguson-Lees & Christie Citation2001, Naoroji Citation2007); therefore, observations were made predominantly during morning and evening hours. Field observations began as early as 04:35 h and typically ended at 10:00 h, while later observations lasted from mid-day up to 19:30 h, approximately 30 min after sunset. Observations were conducted from the ground or a suitable rooftop using a Kowa TSN-664 spotting scope 20–60x and 10 × 42 binoculars. The following data were collected during each field observation period: (1) sex, (2) prey species identified to the lowest taxonomic level possible, (3) time and date, and (4) mobbing species (if applicable).
Ageing and sexing Red-necked Falcons
When possible, adult Red-necked Falcons were sexed based on the descriptions provided by Ferguson-Lees & Christie (Citation2001) and del Hoyo et al. (Citation2020). The sex of adults, i.e. those older than their first calendar year (1 cy), was distinguished predominantly by size, as females averaged approximately 10% larger than males. The tarsi of adults are especially rich yellow in colour, whereas juveniles (<1 cy) have a duller leg colour. Additionally, the plumage of adults tends to have a brownish wash above, appearing darker and more strongly marked, especially on the flanks, in comparison to juveniles, which have a brown crown and nape, buff patches on the lower hindneck with distinctive rufous-washed barred underparts (Ferguson-Lees & Christie Citation2001). Where reliable sex and age identifications could not be made in the field, bird sex, and/or age was classified as ‘unknown’.
Prey taxonomies and mean weights
All avian prey taxonomies were standardized to follow the taxonomic backbone of BirdLife International which uses the taxonomy published in the two volumes of the Handbook of the Birds of the World and the BirdLife International Illustrated Checklist of the Birds of the World (Handbook of the Birds of the World and BirdLife International Citation2020). Mean prey weights were extracted from the AVONET Database for each prey species (Tobias et al. Citation2022). For prey species that could not be identified to species-level, we averaged the adult weights of birds under 35 g, classifying these as ‘unidentified small bird spp.’ in line with Panter & Amar (Citation2021, Citation2022). For mammalian prey, i.e. bats, we sourced mean prey weights from Wilmen et al. (Citation2014) and Faurby et al. (Citation2018). For small bats that could not be identified to species-level, but were recorded as ‘Pipistrellus spp.’, we averaged the weights of the four Pipistrellus species occurring in Bangladesh using Wilmen et al. (Citation2014) and Faurby et al. (Citation2018) including the Indian Pipistrelle P. coromandra (4.6 g), Least Pipistrelle P. tenuis (4.7 g), Javan Pipistrelle P. javanicus (5.3 g), and Dormer’s Pipistrelle Scotozous dormeri (6.8 g), resulting in an estimated mean prey weight for ‘Pipistrellus spp.’ of 5.4 g. We repeated the same process for prey recorded as ‘Sturnidae sp.’ (N = 1) and ‘Hirundinidae sp.’ (N = 11), averaging weights for all starling and swallow species occurring in Bangladesh from the AVONET Database, respectively (online Table S1).
Statistical analyses
All statistical analyses were conducted in R (R Core Team Citation2022). We omitted invertebrate prey data from the statistical analyses due to low sample sizes (N = 3 observations) but present it alongside the diet summary. We used a linear mixed model to explore changes in prey weight throughout the seasons, with ‘mean prey weight’ fitted as the response variable and ‘season’ fitted as the explanatory variable. To account for non-independence between the diet of individual pairs, we fitted ‘pair ID’ as a random effect. The ‘emmeans’ package (Lenth Citation2022) was used to calculate prey weight means for each season and to undertake additional post hoc contrasts. To examine seasonal changes in the probability of bats within the diet, we created a binary response variable ‘is.bat’, scoring each feeding event as either 1 or 0 depending on the presence or absence of bats as prey items for each feeding event recorded. We used a generalized linear mixed model (GLMM), from the ‘lme4’ package (Bates et al. Citation2015) and fitted the ‘is.bat’ binary variable as a response variable and ‘season’ as an explanatory variable with ‘pair ID’ fitted as a random effect. We repeated this model for birds, instead creating a binary ‘is.bird’ response variable and ‘season’ fitted as the explanatory variable with ‘pair ID’ fitted as a random effect to explore seasonal changes in the probability of birds being in the diet. Both bat and bird GLMMs were run using binomial distributions with ‘logit’ link functions. Finally, to examine seasonal differences in the probabilities of mobbing by species with at least 30 observations, i.e. the most common mobbing species, we used the MULTINOM function to produce a multinomial log-linear model, within the ‘nnet’ package (Venables & Ripley Citation2002), with the categorical variable ‘most common mobbing species’ fitted as the response variable, and ‘season’ fitted as the explanatory variable. Seasonal probabilities, per mobbing species, and post hoc seasonal contrasts were extracted using the ‘emmeans’ package.
Results
Diet composition and prey biomass
Between 2002 and 2019, we observed 1614 Red-necked Falcon hunting events (). Across all seasons, birds comprised nearly 75% of falcon diet (N = 1,205 hunting events), with bats totalling approximately 25% (N = 406) and insects forming less than 1% of prey (N = 3) (). Birds constituted 93% of prey biomass (online Table S2). House Sparrows Passer domesticus dominated the diet (N = 933; 58% of prey items; 75% of total prey biomass), followed by Pipistrellus bats (N = 404; 25% of prey items; 7% of total prey biomass) and unidentified small birds (N = 236; 15% of total prey items) (, online Table S2). We were able to identify 59% of prey items to species-level (), with the majority of feeding events being observed during the pre-monsoon hot season (N = 818; 51%), followed by the rainy monsoon season (N = 436; 27%) and the cool dry winter (N = 360; 22%).
Table 1. Red-necked Falcon Falco chicquera prey species, derived from long-term field observations between 2002 and 2019, with mean prey weights (g) in Bangladesh. N = number.
Seasonal prey weight differences and trophic niche shifts
During the rainy monsoon season, there was a significant decrease in mean weight of prey items (F2,1602 = 132.06, P < 0.0001; , online Table S3). Furthermore, the probability of Red-necked Falcons preying on bats increased significantly from 16% in the cool dry winter and 11% in the pre-monsoon hot season, to 48% in the rainy monsoon season (, ), indicating a trophic niche shift from birds to bats during the rainy monsoon season. Consequently, there was a significant decline in the probability of birds being preyed upon by Red-necked Falcons during the rainy monsoon season (, online Table S4).
Figure 2. Seasonal changes in mean prey weight (g) and the proportion of birds (white bars) and bats (grey bars) in the diet of Red-necked Falcons Falco chicquera in Bangladesh between 2002 and 2019. Error bars = 95% confidence intervals, N = sample sizes.
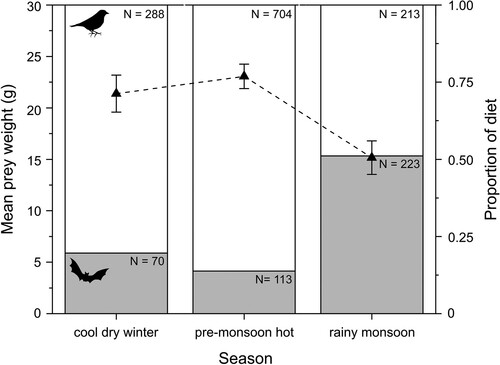
Table 2. GLMM parameters and contrasts exploring seasonal changes in the probability of bats (Chiroptera spp.) in the diet of Red-necked Falcons (Falco chicquera) in Bangladesh between 2002 and 2019. SE = standard error and df = degrees of freedom.
Inter- and intraspecific mobbing
Throughout the study period, we observed 738 mobbing events during periods of feeding (). Red-necked Falcons were most commonly mobbed by House Crows Corvus splendens (N = 358), Black Kites Milvus migrans (N = 170), other Red-necked Falcons (N = 84), Black Drongos Dicrurus macrocercus (N = 33), and Large-billed Crows C. macrorhynchos (N = 31) (). There were significant seasonal differences in the probabilities of some species mobbing Red-necked Falcons during feeding (). For example, House Crows were more likely to mob during the rainy monsoon season relative to the cool dry winter (t1,12 = 2.80, P = 0.040; a, online Table S5). Black Kites were more likely to mob Red-necked Falcons during the pre-monsoon hot season compared to the cool dry winter and rainy monsoon seasons (cool dry winter–pre-monsoon hot: t1,12 = −2.98, P = 0.028; pre-monsoon hot–rainy monsoon: t1,12 = 5.05, P < 0.001; b; online Table S5). Intraspecific mobbing by other falcons was more likely during the cool dry winter relative to the pre-monsoon hot season (t1,12 = 3.16, P = 0.021) and there was a significantly lower probability during the pre-monsoon season when compared to the rainy monsoon season (t1,12 = −3.60, P < 0.01); however, there was no significant difference between the cool dry winter and rainy monsoon seasons (t1,12 = −0.62, P = 0.813; c, Table S5). Black Drongos were more likely to mob Red-necked Falcons in the rainy monsoon season when compared to the other two seasons (cool dry winter–rainy monsoon: t1,12 = −4.91, P < 0.01; pre-monsoon hot–rainy monsoon: t1,12 = −4.11, P < 0.01; d, online Table S5). There were no seasonal differences in the probabilities of falcons being mobbed by Large-billed Crows (e, online Table S5).
Figure 3. Seasonal changes in the probabilities of Red-necked Falcons Falco chicquera being mobbed while feeding by the most common species, i.e. ≥ 30 observations: (a) House Crows Corvus splendens (N = 358), (b) Black Kites Milvus migrans (N = 170), (c) Red-necked Falcons (N = 84), (d) Black Drongos Dicrurus macrocercus (N = 33), and (e) Large-billed Crows Corvus macrorhynchos (N = 31) in Bangladesh between 2002 and 2019.
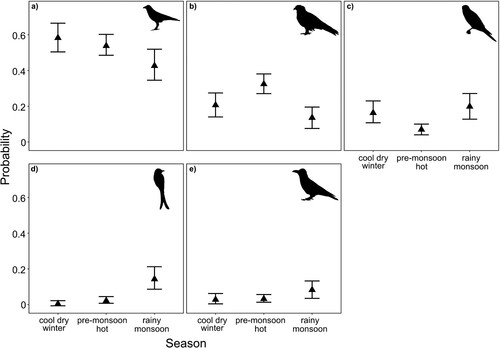
Table 3. Observed inter- and intraspecific mobbing events during Red-necked Falcon Falco chicquera feeding activities in Bangladesh between 2002 and 2019. Species presented above the dotted line represent the most common mobbing species, i.e. those with ≥ 30 observations, and were used in statistical analyses.
Discussion
Our findings suggest that Red-necked Falcons track changing environments by shifting their trophic niche during the rainy monsoon season. We found significant declines in the mean weight of prey items and a significant increase in the probability of bats within the diet of Red-necked Falcons during the rainy monsoon season, and there were significant seasonal differences in the probabilities of inter- and intraspecific mobbing events during feeding.
Seasonal changes in diet
Across all seasons, birds comprised approximately 75% of the diet of Red-necked Falcons with bats forming the majority of the remaining 25%. Our findings are in line with those of Pande et al. (Citation2018) who explored the diet composition of six raptor species in India, including the Red-necked Falcon. In their study, small mammals (presumably bats) made up approximately 25% of the diet of Red-necked Falcons, with roughly 50% of total diet comprising of birds (Pande et al. Citation2018). Mahmood & Hussain (Citation2015) analysed pellets and prey remains from Red-necked Falcon nests located on the Potohar Plateau in Pakistan and found that birds and small mammals comprised the main prey species, with insects forming a very small proportion of the diet (<0.1%). However, they also frequently reported larger birds, such as Collared Doves Streptopelia decaocto and Rock Doves Columba livia, within the diet whereas we did not. Despite this, their findings are broadly similar to ours, whereby House Sparrows formed a large proportion of avian prey (Mahmood & Hussain Citation2015). In contrast, Bhatt (Citation2022) also used long-term field observations to quantify the breeding season diet of Red-necked Falcons in Gujarat, India, and found that the entire breeding season diet comprised of birds. However, it remains unclear at what point during the day these observations were recorded, which may reflect an over abundance of birds in the diet of the Indian population. Red-necked Falcons are a crepuscular species (Khan Citation1978, Ferguson-Lees & Christie Citation2001, Naoroji Citation2007), hunting preferably during twilight hours. Opportunistic hunting is well-documented in small- to medium-sized raptors, especially those that are specialist mid-air hunters (Newton Citation1979), and may explain why we found that approximately 25% of prey items were bats during our field surveys which spanned early mornings and late evenings. Predation of bats during twilight hours has also been observed for other small falcons, such as the Eurasian Hobby Falco subbuteo (Stanton Citation2016).
Across the year, there was a distinct increase in bat consumption during the rainy monsoon season (June to October). This may be due to an increase in bat populations in response to weather-induced increases in flying insect abundances during periods of high rainfall in the tropics (Janzen & Schoener Citation1968, Kishimoto-Yamada & Itioka Citation2015). However, this is speculative and future research should explore the effects of weather on hunting behaviour of Red-necked Falcons.
In Bangladesh, the Red-necked Falcon breeding season begins during the cool dry winter (December/January) with young fledging towards the end of March and April (Foysal Citation2014). Therefore, adults may shift their trophic niche during the rainy monsoon season and focus hunting efforts on smaller prey such as bats, which only comprised 7% of total dietary biomass (vs. 93% biomass from birds), to maximize hunting efficiency during critical periods of the year when they are raising young. For example, breeding pairs may deliver smaller but more frequent prey into the nest to meet the energetic demands of growing fledglings (Steen et al. Citation2012). Somewhat contrary to this, our direct observations suggest that adults may only supplement their diet with bats if they are unsuccessful in hunting birds. Once the crop is full from successful hunts on birds during the day, then they did not appear to preferentially hunt bats following their emergence in the evening. This suggests that bird-hunting attempts may be lower during the rainy monsoon season relative to the rest of the year. To explore this further, continued research conducted across seasons using sufficient sample sizes is needed from elsewhere in the species’ geographic range in Asia.
Seasonal changes in mobbing frequency
Using long-term direct observation data allowed us to quantify inter- and intraspecific mobbing events and compare these across seasons. Of the five most common mobbing species, two species increased their mobbing frequency during the rainy monsoon season: other Red-necked Falcons and Black Drongos. Intraspecific mobbing by other Red-necked Falcons during the rainy monsoon season may be affected by between-pair competition for heterogeneous food resources during periods when breeding pairs maintain smaller breeding season ranges (Peery Citation2000). Aggression towards larger birds is well-documented in Black Drongos (Milstead & Myers Citation1972). Furthermore, drongos are insectivores (Asokan et al. Citation2009, Narayana et al. Citation2014) and may come into indirect competition with Red-necked Falcons over flying insects during periods of high rainfall. Previous research has suggested that Black Drongos may increase the frequency and intensity of mobbing behaviours during the breeding season when rearing young; however, due to year-round residency of drongos it may also be an evolutionary adaptive strategy to avoid predation (Nijman Citation2004).
Limitations
Insects constituted a relatively small proportion of the diet of Red-necked Falcons in our study (about 0.2%); however, our estimates are likely to under-represent true consumption of invertebrate prey due to difficulties associated with observing such events in the field. Other techniques for studying raptor diets, such as stable isotope analysis (Catry et al. Citation2016, Johnson et al. Citation2020, Jones et al. Citation2023) or DNA metabarcoding (Bourbour et al. Citation2019, Citation2021), may be more appropriate when quantifying the proportion of insect prey within the diet. Despite this, our diet estimates for prey from higher trophic levels (birds and bats) closely align with those from other studies (Mahmood & Hussain Citation2015, Pande et al. Citation2018). In addition, our ability to identify prey to species-level is dependent on visibility and there may be a bias towards more common prey and/or those that are easier to identify in the field.
Conclusions
Long-term direct field observations of Red-necked Falcons feeding in Bangladesh, across seasons, revealed notable declines in mean weight of prey items during the rainy monsoon season. In addition, the birds may shift their trophic niche during this period to track changing environments during periods of prolonged, high rainfall. Due to these weather-induced environmental changes, food resources available to falcons likely change and the birds may alter their hunting behaviour to maximize foraging efficiency, especially during periods when they are raising young. Furthermore, opportunistic hunting of bats during crepuscular activity peaks may allow Red-necked Falcons to optimize hunting efforts during the rainy monsoon season. Our data suggest that hunting success on birds may decline during the rainy monsoon season; however, further research on prey population dynamics is required across multiple trophic levels throughout the year to improve our understanding of how predators such as Red-necked Falcons track changing environments. Such information will allow us to better understand species’ responses to environmental change in a warming world.
Supplemental Material
Download MS Word (30.6 KB)ACKNOWLEDGEMENTS
Ian Newton and Pratik Gupte made comments on earlier drafts. The authors are grateful to those who permitted use of their rooftops to study Red-necked Falcons in the field and for additional assistance in the field. The spotting telescope was provided by the Bangladesh Spoon-billed Sandpiper Conservation Project (BSCP). Further thanks to Nadim Parvees and Ashraf Ul Hasan for informative discussion on bats.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Ali, S. & Ripley, S.D. 1978. Handbook of the Birds of India and Pakistan, Vol. 1 & 2nd edn. Oxford University Press, Delhi.
- Arkumarev, V., Dobrev, D., Stamenov, A., Terziev, N., Delchev, A. & Stoychev, S. 2021. Seasonal dynamics in the exploitation of natural carcasses and supplementary feeding stations by a top avian scavenger. J. Ornithol. 162: 723–735.
- Asokan, S., Ali, A.M.S. & Manikannan, R. 2009. Diet of three insectivorous birds in Nagapattinam district, Tamil Nadu, India – a preliminary study. JoTT 1: 327–330.
- Bates, D., Machler, M., Bolker, B. & Walker, S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67: 1–48.
- Berryman, A.J. & Kirwan, G.M. 2021. Is the Tiny Hawk (Accipiter superciliosus) really a specialized predator on hummingbirds? Using citizen science data to elucidate dietary preferences of a little-known Neotropical raptor. J. Raptor Res. 55: 276–280.
- Bhatt, N. 2022. Reproductive rate of the Red-headed Falcon (Falco chicquera) in Surendranagar district, Gujarat, India. J. Raptor Res. 57: 75–80.
- BirdLife International. 2023. Species factsheet: Falco chicquera. http://datazone.birdlife.org/species/factsheet/red-headed-falcon-falco-chicquera on 04/07/2023.
- Bourbour, R.P., Aylward, C.M., Tyson, C.W., Martinico, B.L., Goodbla, A.M., Ely, T.E., Fish, A.M., Hull, A.C. & Hull, J.M. 2021. Falcon fuel: metabarcoding reveals songbird prey species in the diet of juvenile Merlins (Falco columbarius) migrating along the Pacific Coast of western North America. Ibis 163: 1282–1293.
- Bourbour, R.P., Martinico, B.L., Crane, M.M., Hull, A.C. & Hull, J.M. 2019. Messy eaters: swabbing prey DNA from the exterior of inconspicuous predators when foraging cannot be observed. Ecol. Evol. 9: 1452–1457.
- Buechley, E.R., Santangeli, A., Girardello, M., Neate-Clegg, M.H.C., Oleyar, D., McClure, C.J.W. & Şekercioğlu, ÇH. 2019. Global raptor research and conservation priorities: tropical raptors fall prey to knowledge gaps. Divers. Distrib. 25: 856–869.
- Cade, T.J. & Digby, D.R. 1982. The Falcons of the World. Cornell University Press, Ithaca, New York.
- Catry, I., Catry, T., Alho, M., Franco, A.M.A. & Moreira, F. 2016. Sexual and parent-offspring dietary segregation in a colonial raptor as revealed by stable isotopes. J. Zool. 299: 58–67.
- Collopy, M.W. 1983. A comparison of direct observations and collections of prey remains in determining the diet of Golden Eagles. J. Wildl. Manag. 47: 360–368.
- del Hoyo, J., Kemp, A.C., Kirwan, G.M., Collar, N. & Marks, J.S. 2020. Red-necked Falcon (Falco chicquera), version 1.0. In Billerman, S.M., Keeney, B.K., Rodewald, P.G. & Schulenberg, T.S. (ed) Birds of the World. Cornell Lab of Ornithology, Ithaca, NY. https://birdsoftheworld.org/bow/species/renfal1/cur/introduction.
- Descombes, P., Pradervand, J.-N., Golay, J., Guisan, A. & Pellissier, L. 2015. Simulated shifts in trophic niche breath modulate range loss of alpine butterflies under climate change. Ecography 39: 796–804.
- Dharmakumarsinhji, K.S. 1954. Birds of Saurashtra. Dharmakumarsinhji, Dil Bahar.
- Faurby, S., Davis, M., Pedersen, RØ, Schowanek, S.D., Antonelli, A. & Svenning, J.-C. 2018. PHYLACINE 1.2: the phylogenetic atlas of mammal macroecology. Ecology 99: 2626.
- Ferguson-Lees, J. & Christie, D.A. 2001. Raptors of the World. Christopher Helm, London.
- Foysal, M. 2014. Status and breeding ecology of the Red-headed Falcon in Bangladesh. Falco 44: 4–6.
- Foysal, M. 2015. Observations of Red-headed Falcon Falco chicquera (Aves: Falconiformes: Falconidae) nest at Keraniganj, Dhaka, Bangladesh, with a focus on post-fledging behavior. JoTT 7: 7138–7145.
- Handbook of the Birds of the World and BirdLife International. 2020. Handbook of the Birds of the World and BirdLife International Digital Checklist of the Birds of The world. Version 5. http://datazone.birdlife.org/userfiles/file/Species/Taxonomy/HBW-BirdLife_Checklist_v5_Dec20.zip.
- Harrison, J.T., Kochert, M.N., Pauli, B.P. & Heath, J.A. 2019. Using motion-activated trail cameras to study diet and productivity of cliff-nesting Golden Eagles. J. Raptor Res. 53: 26–37.
- Janzen, D.H. & Schoener, T.W. 1968. Differences in insect abundance and diversity between wetter and drier sites during a tropical dry season. Ecology 49: 96–110.
- Johnson, D.L., Henderson, M.T., Anderson, D.L., Booms, T.L. & Williams, C.T. 2020. Bayesian stable isotope mixing models effectively characterize the diet of an Arctic raptor. J. Anim. Ecol. 89: 2972–2985.
- Jones, G.C.A., Woods, D., Broom, C.M., Panter, C.T., Sutton, L.J., Drewitt, E.J.A. & Fathers, J. 2023. Fine scale spatial variation in Eurasian Kestrel (Falco tinnunuclus) diet in southern England revealed from indirect prey sampling and direct stable isotope analysis. Ardea.
- Kenward, R.E. 1982. Goshawk hunting behaviour, and range size as a function of food and habitat availability. J. Anim. Ecol. 51: 69–80.
- Khan, M.A.R. 1978. Notes on the ecology and behavior of the Red-headed Merlin Falco chicquera chicquera Daudin from Bangladesh. J. Asiat. Soc. Bangladesh Sci. 4: 9–14.
- Khan, M.A.R. 2008. Protected areas of Bangladesh – a guide to wildlife. Nishorgo Program, Bangladesh Forest Department, Dhaka.
- Kishimoto-Yamada, K. & Itioka, T. 2015. How much have we learned about seasonality in tropical insect abundance since Wolda (1988)? . Entomol. Sci. 18: 407–419.
- Lenth, R. 2022. emmeans: estimated marginal means, aka least-squares means. R Package version 1.8.2, https://CRAN.R-project.org/package=emmeans.
- Lewis, S.B., Fuller, M.R. & Titus, K. 2010. A comparison of three methods for assessing raptor diet during the breeding season. Wildl. Soc. Bull. 32: 373–385.
- Mahmood, T. & Hussain, R. 2015. A note on nest characteristics and diet of the Red-headed Merlin Falco chicquera, Common Kestrel Falco tinnunuclus and Saker Falcon Falco cherrug in Chakwal district, Potohar Plateau, Pakistan. Podoces 10: 15–20.
- Margalida, A., Manosa, S., Bertran, J. & García, D. 2010. Biases in studying the diet of the Bearded Vulture. J. Wildl. Manag. 71: 1621–1625.
- Marti, C.D. 1987. Raptor food habit studies. In Giron Pendleton, B.A., Millsap, B.A., Cline, K.W. & Bird, D.M. (ed) Raptor Management Techniques Manual, 67–80. National Wildlife Federation, Washington, DC.
- McPherson, S.C., Brown, M. & Downs, C.T. 2016. Diet of the Crowned Eagle (Stephanoaetus coronatus) in an urban landscape: potential for human-wildlife conflict? Urban Ecosyst. 19: 383–396.
- Milstead, W.W. & Myers, R.F. 1972. Intergeneric competition among three Bangladeshian birds. Am. Midl. Nat. 87: 536–538.
- Murgatroyd, M., Avery, G., Underhill, L.G. & Amar, A. 2016. Adaptability of a specialist predator: the effects of land use on diet diversification and breeding performance of Verreaux’s Eagles. J. Avian Biol. 47: 834–845.
- Naoroji, R. 2007. Birds of Prey of the Indian Subcontinent. Om Books International, New Delhi.
- Naoroji, R. 2011. Breeding of the Red-headed Falcon Falco chicquera in Saurashtra, Gujarat, India. Forktail 27: 1–6.
- Narayana, B.L., Rao, V.V. & Venkateswara, R. 2014. Foraging behaviour of Black Drongo (Dicrurus macrocercus) in Nalgonda district of Andhra Pradesh, India. The Bioscan 9: 467–471.
- Naude, V.N., Smyth, L.K., Weideman, E.A., Krochuk, B.A. & Amar, A. 2019. Using web-sourced photography to explore the diet of a declining African raptor, the Martial Eagle (Polemaetus bellicosus). Condor Ornithol. Appl. 121: duy015.
- Neilsen, J.M., Clare, E.L., Hayden, B., Brett, M.T. & Kratina, P. 2018. Diet tracing in ecology: method comparison and selection. Methods Ecol. Evol. 9: 278–291.
- Newton, I. 1978. Feeding and development of Sparrowhawk Accipiter nisus nestlings. J. Zool. 184: 465–487.
- Newton, I. 1979. Population Ecology of Raptors. T. & A.D. Poyser, Berkhamsted, UK.
- Nijman, V. 2004. Seasonal variation in naturally occurring mobbing behaviour of drongos (Dicruridae) towards two avian predators. Ethol. Ecol. Evol. 16: 25–32.
- Oro, D. & Tella, J.L. 1995. A comparison of two methods for studying the diet of the Peregrine Falcon. J. Raptor Res. 29: 210–213.
- Pande, S., Yosef, R., Morelli, F., Pawar, R. & Mone, R. 2018. Diet and habitat affinities in six raptor species in India. Avian Res. 9: 36.
- Panter, C.T., Allen, S., Backhouse, N., Mullineaux, E., Rose, C.-A. & Amar, A. 2022. Causes, temporal trends, and the effects of urbanization on admissions of wild raptors to rehabilitation centers in England and Wales. Ecol. Evol. 12: e8856.
- Panter, C.T. & Amar, A. 2021. Sex and age differences in the diet of the Eurasian Sparrowhawk (Accipiter nisus) using web-sourced photographs: exploring the feasibility of a new citizen science approach. Ibis 163: 928–947.
- Panter, C.T. & Amar, A. 2022. Using web-sourced photographs to examine temporal patterns in sex-specific diet of a highly sexually dimorphic raptor. R. Soc. Open Sci. 9: 220779.
- Peery, Z.M. 2000. Factors affecting interspecies variation in home-range size of raptors. Auk 117: 511–517.
- Rasmussen, P. & Anderton, J. 2005. Birds of South Asia: the Ripley guide, Lynx edn. Lynx Edicions, Barcelona.
- R Core Team. 2022. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
- Rebollo, S., Pérez-Camacho, L., Martínez-Hesterkamp, S., Tapia, L., Fernández-Pereira, J.M. & Morales-Castilla, I. 2022. Anything for a quiet life: shelter from mobbers drives reproductive success in a top-level avian predator. J. Avian Biol. 2023: e03060.
- Redpath, S.M., Clarke, R., Madders, M. & Thirgood, S.J. 2001. Assessing raptor diet: comparing pellets, prey remains and observational diet at Hen Harrier nests. Condor 103: 184–188.
- Rutz, C. 2003. Assessing the breeding season diet of Goshawks Accipiter gentilis: biases of plucking analysis quantified by means of continuous radio-monitoring. J. Zool. 259: 209–217.
- Rutz, C. 2004. Breeding season diet of Northern Goshawks Accipiter gentilis in the city of Hamburg, Germany. Corax 19: 311–322.
- Šalek, M., Riegert, J. & Křivan, V. 2010. The impact of vegetation characteristics and prey availability on breeding habitat use and diet of Little Owls Athene noctua in central European farmland. Bird Study 57: 495–503.
- Snyder, N.F.R. & Wiley, J.W. 1976. Sexual size dimorphism in hawks and owls of North America. A.O.U. Monogr. 20: 1–95.
- Speziale, K.L. & Lambertucci, S.A. 2013. The effect of introduced species on raptors. J. Raptor Res. 47: 133–144.
- Stanton, D.J. 2016. Predation of dawn-swarming bats by Eurasian Hobby (Falco subbuteo). J. Raptor Res. 50: 317–319.
- Steen, R., Sonerud, G.A. & Slagsvold, T. 2012. Parents adjust feeding effort in relation to nestling age in the Eurasian Kestrel (Falco tinnuculus). J. Ornithol. 153: 1087–1099.
- Subramayana, S. 1985. Hunting and feeding habits of the Red-headed Merlin Falco chicquera. Newsletter for Birdwatchers 25: 4–8.
- Sullivan, A.R., Flaspohler, D.J., Froese, R.E. & Ford, D. 2015. Climate variability and the timing of spring raptor migration in eastern North America. J. Avian Biol. 47: 208–218.
- Sumasgutner, P., Krenn, H., Düesberg, J., Gaspar, T. & Gamauf, A. 2013. Diet specialisation and breeding success along an urban gradient: the Kestrel (Falco tinnunuclus) in Vienna, Austria. Beitr. Jagd-Wildforsch. 38: 385–397.
- Suri, J., Sumasgutner, P., Hellard, E., Koeslag, A. & Amar, A. 2017. Stability in prey abundance may buffer Black Sparrowhawks Accipiter melanoleucus from health impacts of urbanization. Ibis 159: 38–54.
- Tobias, J.A., Sheard, C., Pigot, A.L., Devenish, A.J.M., Yang, J., Sayol, F., Neate-Clegg, M.H.C., Alioravainen, N., Weeks, T.L., Barber, R.A., Walkden, P.A., MacGregor, H.E.A., Jones, S.E.I., Vincent, C., Phillips, A.G., Marples, N.M., Montaño-Centellas, F.A., Leandro-Silva, V., Claramunt, S., Darski, B., Freeman, B.G., Bregman, T.P., Cooney, C.R., Hughes, E.C., Capp, E.J.R., Varley, Z.K., Friedman, N.R., Korntheuer, H., Corrales-Vargas, A., Trisos, C.H., Weeks, B.C., Hanz, D.M., Töpfer, T., Bravo, G.A., Remeš, V., Nowak, L., Carneiro, L.S., Moncada R., A.J., Matysioková, B., Baldassarre, D.T., Martínez-Salinas, A., Wolfe, J.D., Chapman, P.M., Daly, B.G., Sorensen, M.C., Neu, A., Ford, M.A., Mayhew, R.J., Silveira, L.F., Kelly, D.J., Annorbah, N.N.D., Pollock, H.S., Grabowska-Zhang, A.M., McEntee, J.P., Gonzalez, J.C.T., Meneses, C.G., Muñoz, M.C., Powell, L.L., Jamie, G.A., Matthews, T.J., Johnson, O., Brito, G.R.R., Zyskowski, K., Crates, R., Harvey, M.G., Zevallos, M.J., Hosner, P.A., Bradfer-Lawrence, T., Maley, J.M., Stiles, F.G., Lima, H.S., Provost, K.L., Chibesa, M., Mashao, M., Howard, J.T., Mlamba, E., Chua, M.A.H., Bicheng, L., Gómez, M.I., García, N.C., Päckert, M., Fuchs, J., Ali, J.R., Derryberry, E.P., Carlson, M.L., Urriza, R.C., Brzeski, K.E., Prawiradilaga, D.M., Rayner, M.J., Miller, E.T., Bowie, R.C.K., Lafontaine, R-M., Scofield, R.P., Lou, Y., Somarathna, L., Lepage, D., Illif, M., Neuschulz, E.L., Templin, M., Dehling, D.M., Cooper, J.C., Pauwels, O.S.G., Analuddin, K., Fjeldså, J., Seddon, N., Sweet, P.R., DeClerck, F.A.J., Naka, L.N., Brawn, J.D., Aleixo, A., Böhning-Gaese, K., Rahbek, C., Fritz, S.A., Thomas, G.H. & Schleuning, M. 2022. AVONET: morphological, ecological and geographical data for all birds. Ecol. Lett. 25: 581–597. Doi: 10.1111/ele.13898.
- United Nations. 2022. World Population Prospects 2022. Department of Economic and Social Affairs Population Division, United Nations. https://population.un.org/wpp/.
- Venables, W.N. & Ripley, B.D. 2002. Modern Applied Statistics with S, 4th edn. Springer, New York.
- Wilmen, H., Belmaker, J., Simpson, J., de la Rosa, C., Rivadeneira, M.M. & Jetz, W. 2014. Eltontraits 1.0: species-level foraging attributes of the world’s birds and mammals. Ecology 95: 2027–2027.
- Wink, M. & Sauer-Gürth, H. 2000. Advances in the molecular systematics of African raptors. In Chancellor, R.D. & Meyburg, B.-U. (ed) Raptors at Risk, 135–147. WWGBP/Hancock House, Surrey.