ABSTRACT
The diet of the European Shag Gulosus aristotelis was assessed at one of their most northerly roosts in the UK; Bluemull Sound, Shetland. One pellet and 40 faecal samples were collected during the non-breeding season. The most frequent prey was Velvet Swimming Crab Necora puber, while the highest number of otoliths were from Saithe Pollachius virens and estimated mean (±sd) fish length was 143.9 ± 66.9 mm (range 81.4–223.6 mm).
The European Shag Gulosus aristotelis (hereafter Shag) is a Bird of Conservation Concern (BoCC) in the UK, added to the Red List due to steep declines in breeding populations (BoCC, Eaton et al. Citation2015). It is presumed that high winter mortality of adults and declines in prey (especially Lesser Sandeel Ammodytes marinus) availability and/or quality are some of the driving mechanisms behind this decline (Frederiksen et al. Citation2008, Heubeck et al. Citation2015). Therefore, studying the diet composition of Shags specifically during the non-breeding season is important if we are to increase our understanding of these mechanisms. Due to logistical constraints, however, most diet studies of Shags, like those of many seabirds, are conducted during the breeding season (Barrett et al. Citation2007). Such studies most often make use of regurgitated pellets that are examined for fish otoliths and other prey items (Johnstone et al. Citation1990, Howells et al. Citation2018). Fish otoliths can often be identified down to species level and the age class of the fish determined from the length of the otolith (Härkönen Citation1986). While less commonly used in diet studies than pellets, faecal matter (also called excreta) can also be examined for prey remains (Lumsden & Haddow Citation1946, Barrett et al. Citation2007, Radhakrishnan et al. Citation2010).
Although long-term diet studies have been conducted (e.g. on the Isle of May; Howells et al. Citation2018), Shag diet may vary considerably between locations and across seasons, rendering such studies unlikely to be representative of other geographical regions (Michelot et al. Citation2017, Nascimento et al. Citation2021). It is, therefore, paramount to capture information on diet from multiple locations, including opportunistically in remote areas where longitudinal studies are challenging. This study reports on the diet composition of Shags at one of their most northerly roosts in the UK.
Bluemull Sound (60° 41′19.1″N 0° 58'51.5″W) is a coastal channel with strong tidal currents located between the islands of Yell and Unst, on the northernmost edge of the North Sea, Shetland, UK. The sound lies near (<8 km) the Hermaness, Saxa Vord, and Valla Field Species Protection Area (SPA) that supports internationally important numbers of breeding seabirds, including Shags (NatureScot Citation2009). Underwater tidal turbines have been operating in Bluemull Sound since 2016, with three active at the time of this study (2019–2020). Throughout the channel, there are roosting sites of Shags, including the selected roost of about 100 individuals at Hoga Ness near Belmont (60°41′16.5″N 0°58′51.4″W) (Langlois Lopez et al. Citation2022).
Fresh pellet, otolith, and faecal samples were opportunistically collected or scraped from the rocky and grassy substrate at the roost while performing trail camera maintenance as part of another study (Isaksson Citation2022). Samples were, therefore, collected on 6 and 9 September 2019 (N = 17 and 10), 12 November 2019 (N = 10), and 1 September 2020 (N = 4). All samples were frozen upon collection, then thawed prior to analysis, washed using water and a 60 µm mesh sieve, and subsequently dried (Kubetzki & Garthe Citation2003). Fish otoliths were paired based on matching appearance, wear, width, and length. Pairs were subsequently identified down to species, where possible, using a reference collection (C. Angus, University of the Highlands and Islands, Shetland) and an otolith identification guide (Härkönen Citation1986). Lengths were measured using a binocular microscope and callipers, and these were used to calculate fish length and body mass according to the equations provided in Härkönen (Citation1986). Anything other than fish otoliths (e.g. crustaceans) was identified as far as possible using an identification guide for marine fauna (Hayward & Ryland Citation2017), a reference collection (D. Riley, University of the Highlands and Islands, Shetland) and expert opinion (D. Riley, pers. comm.). Finally, the frequency of occurrence of different prey items was calculated.
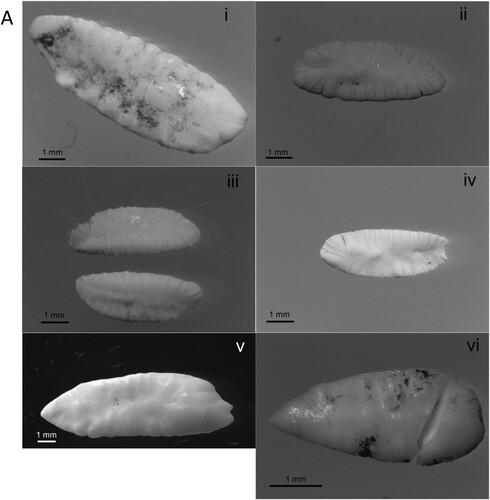
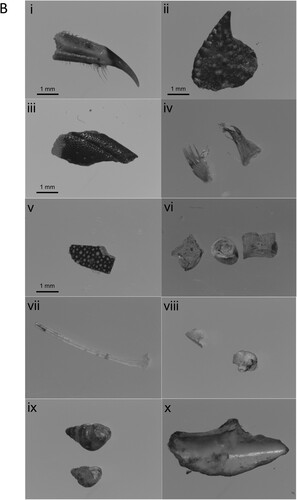
A total of 41 samples were recovered from the roost site. Almost all samples were faecal matter (N = 40). The singular pellet contained six otoliths: from three Saithe Pollachius virens, one Atlantic Cod Gadus morhua, one Norway Pout Trisopterus esmarkii, and one unidentified gadoid. A solitary Haddock Melanogrammus aeglefinus otolith was also found at the site ( and , (a)). The majority of faecal samples contained crustaceans, predominantly from Velvet Swimming Crabs Necora puber and Edible Crabs Cancer pagurus and unidentified lobster (most likely Rugose Squat Lobster Munida rugosa, D. Riley pers. comm.; , (b)). Additionally, 14 samples contained fish remains, either bones, plates, scales, or vertebrae ((b), ).
Figure 1. Identified fish otoliths (a) and examples of prey (b) from European Shag roost diet samples: (a) (i) Atlantic Cod, (ii)–(iv) Saithe, (v) Haddock, (vi) Norway Pout; (b) (i) Edible Crab Cancer pagurus, (ii) Velvet Swimming Crab Necora puber, (iii) Risso’s Crab Xantho pilipes (iv) unidentified lobster, likely Munida rugosa, (v) Common Sea Urchin Echinus esculentus, (vi)–-(vii) unidentified fish bones, (viii) fish scales, (ix) molluscs, likely Cingula trifasciata (top) and Spiralinella spiralis (bottom), and (x) unidentified bivalve.
Table 1. Frequency of occurrence of prey species in diet samples (pellet N = 1, faecal N = 40) from European Shags roosting at Bluemull Sound, 2019–2020.
Table 2. Details on otoliths collected, including length, mass, fish length, and weight calculated from equations in Härkönen (Citation1986), and estimated age class as per Hillersøy & Lorentsen (Citation2012).
This study reports on Shag prey during the non-breeding season at one of the most northerly roost sites in the UK. The high frequency of occurrence of crustacean species, especially Velvet Swimming Crabs, suggests that such species are an important component of Shag diet at this site ((a), ). This finding is consistent with fish trap studies within Bluemull Sound and close to our study site where Edible and Velvet Swimming Crabs were among the most frequent species caught (Fraser & Waggitt Citation2022). As these crabs are epibenthic (bottom-dwelling), the high occurrence of these species in Shag diet at the roost suggests that Shags, as elsewhere, are primarily foraging at the seabed (Neal & Wilson Citation2008, Watanuki et al. Citation2008, Dehnhard et al. Citation2022). However, as most samples were from faecal matter it is possible that remains from larger prey (i.e. otoliths) that would be expelled in pellet form are underrepresented here.
The fish otoliths that were identifiable within this study were mostly from gadoid species, and Saithe especially (). While Lesser Sandeels are the more typical prey in the North Sea (Howells et al. Citation2018), Shag diet is known to be more varied in other areas of Scotland (Swann et al. Citation2008) and neighbouring seas (Hillersøy & Lorentsen Citation2012). Notably, Saithe have been recorded on cameras mounted to tidal turbines within Bluemull Sound (Hutchison et al. Citation2020). This is likely to be linked to the predominant habitat type in the area, in turn, influenced by the strong tidal currents unique to this geographic region.
It is important to acknowledge the small sample size (N = 41) and bias of the samples towards faecal matter, which likely mean that the full diet of Shags at this site was not captured in this study. As the Shag roost is active year-round and accessible, we recommend conducting further studies. Collecting year-round information on Shag diet could help assess vulnerability to extreme weather events and marine renewable energy development. As the channel contains tidal stream turbines, determining the extent to which Shag diet is composed of species that may be affected by current energy extraction and/or underwater infrastructure is highly relevant. Tagging Shags there especially would allow for important habitats to be identified and for foraging behaviour to be characterized, which, combined with information on diet, could greatly aid in conservation efforts directed towards this vulnerable diving seabird.
Acknowledgements
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Research Ethics Committee of University of the Highlands and Islands (OL - ETH SHE – 1230, approved on 21/02/2019). Thanks to Martha Devine and Glen Tyler for assistance with roost selection, Chevonne Angus, David Riley, and Saro Saravanan for assistance during laboratory work and Derek Jamieson for granting permission to use the site. Thanks to Ben Wilson and Maria Bogdanova for extensive comments on the PhD thesis chapter draft of this study and to Nina Dehnhard and editors for review of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Barrett, R.T., Camphuysen, C.J., Anker-Nilssen, T., Chardine, J.W., Furness, R.W., Garthe, S., Hüppop, O., Leopold, M.F., Montevecchi, W.A. & Veit, R.R. 2007. Diet studies of seabirds: a review and recommendations. ICES JMS 64: 1675–1691.
- Dehnhard, N., Mattisson, J., Tarroux, A. & Anker-Nilssen, T. 2022. Predicting foraging habitat of European Shags – a multi-year and multi-colony tracking approach to identify important areas for marine conservation. Front. Mar. Sci. 9: 1–15.
- Eaton, M.A., Brown, A.F., Hearn, R., Noble, D.G., Musgrove, A.J., Lock, L., Stroud, D. & Gregory, R.D. 2015. Birds of conservation concern 4: the population status of birds in the United Kingdom, Channel Islands and Isle of Man. Br. Birds 108: 708–746.
- Fraser, S. & Waggitt, J.J. 2022. Practical approaches for providing empirical data on seabird behavior and prey assemblages in tidal channels. Front. Mar. Sci. 9: 1–17.
- Frederiksen, M., Daunt, F., Harris, M.P. & Wanless, S. 2008. The demographic impact of extreme events: stochastic weather drives survival and population dynamics in a long-lived seabird. J. Anim. Ecol. 77: 1020–1029.
- Härkönen, T. 1986. Guide to the Otoliths of the Bony Fishes of the Northeast Atlantic. Danbiu ApS., Hellerup, Denmark.
- Hayward, P.J. & Ryland, J.S. 2017. Handbook of the Marine Fauna of North-West Europe. Oxford University Press, Oxford.
- Heubeck, M., Mellor, R.M., Gear, S. & Miles, W.T.S. 2015. Population and breeding dynamics of European Shags Phalacrocorax aristotelis at three major colonies in Shetland, 2001-15. Seabird 28: 55–77.
- Hillersøy, G. & Lorentsen, S.H. 2012. Annual variation in the diet of breeding European Shag (Phalacrocorax aristotelis) in Central Norway. Waterbirds 35: 420–429.
- Howells, R.J., Burthe, S.J., Green, J.A., Harris, M.P., Newell, M.A., Butler, A., Wanless, S. & Daunt, F. 2018. Pronounced long-term trends in year-round diet composition of the European Shag Phalacrocorax aristotelis. Mar. Biol. 165: 1–15.
- Hutchison, I., Morgan, P., Sheehy, J. & Tait, C. 2020. Review of underwater video data collected around operating tidal turbines. NatureScot Research Report No. 1225.
- Isaksson, N. 2022. Seabird use of tidal stream environments and potential interactions with tidal energy devices. PhD Thesis, University of the Highlands and Islands.
- Johnstone, G., Harris, M.P., Wanless, S. & Graves, J.A. 1990. The usefulness of pellets for assessing the diet of adult Shags Phalacrocorax aristotelis. Bird Study 37: 5–11.
- Kubetzki, U. & Garthe, S. 2003. Distribution, diet and habitat selection by four sympatrically breeding gull species in the south-eastern North Sea. Mar. Biol. 143: 199–207.
- Langlois Lopez, S., Isaksson, N., Fraser, S. & Masden, E.A. 2022. Successful trial of mist nets and whoosh nets to catch non-breeding European Shags Gulosus aristotelis at a daytime roost. Ring. Migr. 37: 45–50.
- Lumsden, W.H.R. & Haddow, A.J. 1946. The food of the Shag (Phalacrocorax aristotelis) in the Clyde Sea Area. J. Anim. Ecol. 15: 35–42.
- Michelot, C., Pinaud, D., Fortin, M., Maes, P., Callard, B., Leicher, M. & Barbraud, C. 2017. Seasonal variation in coastal marine habitat use by the European Shag: insights from fine scale habitat selection modeling and diet. Deep Sea Res. Part II: Top. Stud. Oceanogr. 141: 224–236.
- Nascimento, T., Oliveira, N. & Luís, A. 2021. Hey, that’s my fish – overlap in prey composition between European Shag and local fisheries in Portugal. Ardea 109: 77–98.
- NatureScot. 2009. Citation for Special Protection Area (SPA): Hermaness, Saxa Vord and Valla Field (UK9002011).
- Neal, K.J. & Wilson, E. 2008. Cancer pagurus. Edible Crab. In Tyler-Walters, H. & Hiscock, K. (ed) Marine Life Information Network: biology and sensitivity key information reviews. Marine Life Information Network (MarLIN), Plymouth. Accessed online: https://www.marlin.ac.uk/species/detail/1179.
- Radhakrishnan, K.V., Liu, M., He, W., Murphy, B.R. & Xie, S. 2010. Otolith retrieval from faeces and reconstruction of prey-fish size for Great Cormorant (Phalacrocorax carbo) wintering at the East Dongting Lake National Nature Reserve, China. Environ. Biol. Fishes 89: 505–512.
- Swann, R.L., Harris, M.P. & Aiton, D.G. 2008. The diet of European Shag Phalacrocorax aristotelis, Black-legged Kittiwake Rissa tridactyla and Common Guillemot Uria aalge on Canna during the chick-rearing period 1981–2007. Seabird 21: 44–54.
- Watanuki, Y., Daunt, F., Takahashi, A., Newell, M., Wanless, S., Sato, K. & Miyazaki, N. 2008. Microhabitat use and prey capture of a bottom-feeding top predator, the European Shag, shown by camera loggers. Mar. Ecol. Prog. Ser. 356: 283–293.