Abstract
1. The gene expression of carbonic anhydrase, a key enzyme for the production of sub-embryonic fluid (SEF), was assessed in turned and unturned eggs of the Japanese quail. The plasma membrane-associated isoforms CA IV, CA IX, CA XII, CA XIV, and the cytoplasmic isoform CA II, were investigated in the extra-embryonic tissue of the blastoderm and in embryonic blood.
2. Eggs were incubated at 37·6°C, c.60% RH, and turned hourly (90°) or left unturned. From 48 to 96 h of incubation mRNA was extracted from blastoderm tissue, reverse-transcribed to cDNA and quantified by real-time qPCR using gene-specific primers. Blood collected at 96 h was processed identically.
3. Blastoderm CA IV gene expression increased with the period of incubation only in turned eggs, with maxima at 84 and 96 h of incubation. Only very low levels were found in blood.
4. Blastoderm CA II gene expression was greatest at 48 and 54 h of incubation, subsequently declining to much lower levels and unaffected by turning. Blood CA II gene expression was about 25-fold greater than in the blastoderm.
5. The expression of CA IX in the blastoderm was the highest of all isoforms, yet unaffected by turning. CA XII did not amplify and CA XIV was present at unquantifiable low levels.
6. It is concluded that only gene expression for CA IV is sensitive to egg turning, and that increased CA IV gene expression could account for the additional SEF mass found at 84 to 96 h of incubation in embryos of turned eggs.
Introduction
During incubation of the avian egg the water produced by metabolism matches evaporative losses through the shell; in consequence the correct water content of the hatchling is ensured (Ar, Citation1991 a, Citation b ; Rahn, Citation1991). However, at lay, most of the available water is located in the albumen outside the fertilised ovum (Baggott, Citation2001). In order that embryonic tissue can access this water, it is transferred from the albumen to the yolk sac in the early part of incubation, a process resulting in the production of sub-embryonic fluid (SEF) by the blastoderm (Baggott et al., Citation2002). In the Japanese quail the bulk production of SEF started at 48 h of incubation, with a peak in mass from 84 h (Babiker and Baggott, Citation1992). In both the domestic fowl and Japanese quail, static incubation reduced the mass of SEF during the period of its formation (Deeming, Citation1989 c; Babiker and Baggott, Citation1992; Baggott et al., Citation2002).
The production of SEF by the quail blastoderm is dependent on the availability of albumen sodium (Babiker and Baggott, Citation1995; Latter and Baggott, Citation2002), the movement of which from albumen to SEF can be inhibited by amiloride (sodium/proton carrier-mediated exchange) and ouabain (active sodium transport) (Latter and Baggott, Citation2002). The production is also dependent upon the enzyme carbonic anhydrase (CA): its inhibition results in a dose-dependent reduction in SEF volume, with pharmacological and histochemical evidence indicating that this enzyme is associated with the plasma membrane (Latter and Baggott, Citation2002). In turkey also, carbonic anhydrase was localised histochemically to the plasma membrane (Bakst and Holm, Citation2003). Based on this evidence Latter and Baggott (Citation2002) proposed a key role for carbonic anhydrase: located on the yolk-facing, basolateral, plasma membrane of the endodermal cell it catalyses the hydration of carbon dioxide to form bicarbonate and hydrogen ions. The protons are exchanged passively for sodium ions at the surface of the cell facing the albumen. Sodium ions move from albumen to cell as a sodium ATPase, located on the basolateral membrane, pumps sodium into the lateral intercellular spaces of the endoderm. This creates a local osmotic gradient that promotes water movement from albumen to yolk sac.
Carbonic anhydrase enzymes are encoded by three independent gene families: α-CA found in eukaryotes, β-CA in prokaryotes and γ-CA in plants (Chegwidden and Carter, Citation2000). The α-CA are mainly cytosolic or membrane-associated isoforms. Those currently characterised as plasma membrane associated are CA IV, CA IX, CA XII and CA XIV: the isoform CA IV has been identified as a major participant in CO2 transport, and the latter three are all associated with tumours (Sly, Citation2000). CA IV participates in ion transport ‘metabolons’ with either the transporter NCB1 (Alvarez et al., Citation2003) or with the
anion exchanger (Sterling et al., Citation2002). CA IX has also been shown to enhance transport by the
anion exchanger (Morgan et al., Citation2007). CA II is the commonest cytosolic isoform being almost universally expressed in some cell types of all major mammalian tissues (Chegwidden and Carter, Citation2000). It is the CA isoform found in chicken erythrocytes, both adult (Bernstein and Schraer, Citation1972) and embryonic (Dragon and Baumann, Citation2001).
The mechanism by which static incubation diminishes SEF secretion remains partly unresolved. Latter and Baggott (Citation1996) found that unincubated eggs had a low sodium concentration in the albumen adjacent to the vitelline membranes. Egg turning increased the sodium concentration at this location by the same magnitude throughout the period of SEF production, whereas unturned eggs retained this ‘depleted layer’. Baggott et al. (Citation2002) suggested that in unturned eggs this ‘depleted layer’ would reduce the sodium available for ion transport by endodermal cells and thus decrease SEF formation. Alternatively, or in addition, it was suggested that the lower rate of expansion of the area vasculosa present in unturned eggs (Deeming, Citation1989 a) might also reduce SEF mass. However, neither hypothesis provides a complete explanation of the occurrence in turned eggs of additional fluid at the time of maximum SEF mass (Babiker and Baggott, Citation1992). An increase in ion transport specifically at this time would be a potential mechanism.
The objective of this study was, then, to quantify the gene expression of the key enzyme, carbonic anhydrase, in the extra-embryonic blastoderm and blood of turned and unturned eggs during the period of SEF production. Quantification of all the main plasma membrane-associated carbonic anhydrases, CA IV, CA IX, CA XII and CA XIV, was attempted with a subsidiary aim of identifying which isoforms are likely participants in the SEF secretion process. In addition, the gene expression of the cytoplasmic carbonic anhydrase, CA II, was quantified; this enzyme is known to be present in avian erythrocytes (Bernstein and Schraer, Citation1972; Dragon and Baumann, Citation2001).
Methods
Tissue sampling
Freshly collected eggs incubated at 37·6 ± 0·1°C, c.60% RH, in a still-air incubator (Brinsea Ltd, Sandford, North Somerset, UK) were rotated hourly 90° around their long axis or left unturned in the same incubator. After 48, 54, 60, 72, 84 or 96 h of incubation 6 embryos from turned eggs and 6 unturned, were removed from the shell. One hemisphere of the extra-embryonic blastoderm (abutting the embryo dorsal side) was removed to Pannett and Compton Ringer (Citation1924) at 4°C, and then washed twice in cold Ringer. A tissue piece (c.5 mm × 5 mm) was excised from the anterior quadrant, transferred to 200 µl of RNAlater and incubated at 4°C for 24 h before storing at −80°C until used. In 10 different embryos from turned eggs at 96 h, blood was sampled into microcapillaries and placed immediately in buffer RLT for storage until used.
RNA extraction
The blastoderm tissue was transferred to 2 ml Eppendorf tubes containing a sterile 5 mm steel bead and homogenised in RLT lysis buffer at 20 Hz for one minute in a TissueLyser (Qiagen, Crawley, West Sussex, UK). Blood (50 to 100 µl) was mixed 1 : 1 with buffer RLT, vortexed and centrifuged at 9000 g for 2 min to pellet debris. Blastoderm lysates and blood supernatants were transferred to RNeasy spin columns and RNA was extracted according to the manufacturer's instructions (Qiagen) which included an on-column DNase digest digestion step. RNA purity and concentration were determined using a NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). For all samples RNA 260/280 nm absorbance ratio was ≥2·0. RNA integrity was assessed by formaldehyde–agarose gel electrophoresis and densitometry of 28S and 18S rRNA bands, which yielded ratios ≥1·9.
Reverse transcription
RNA (450 ng) was reverse-transcribed to cDNA according to the manufacturer's instructions, using a Qiagen Reverse-Transcriptase PCR kit containing a mix of random and oligoDT primers. The protocol included a second genomic DNA elimination step, prior to reverse transcription. Complementary DNA was diluted 5-fold with nuclease-free water containing tRNA (50 ng/ml; Sturzenbaum, Citation1999) and stored at −80°C until used.
Primer design
Messenger RNA sequences for carbonic anhydrases II, IV, IX, XII and XIV were obtained for G. gallus, M. musculus, R. norvegicus, B. taurus, H. sapiens and, where available, D. rerio. For G. gallus, the actual mRNA sequence was available only for CA II and other CA sequences were those predicted in the NCBI database (http://www.ncbi.nlm.nih.gov/genome/guide/build.html). These nucleotide sequences were aligned using the online version of MAFFT v5 (http://align.bmr.kyushu-u.ac.jp/mafft/online/server/) and regions of high similarity were identified. The carbonic anhydrase sequences of G. gallus were aligned using MAFFT and regions of low similarity between isoforms were identified. Primers were designed within regions of both high interspecific similarity and low similarity between enzyme isoforms, and with melting temperatures of approximately 61 to 62°C. The primer sequences used and accession numbers of the CA genes are reported in . These chicken primers were then tested using quail blastoderm cDNA and the PCR conditions given below to verify amplification. With all primers, except those for CA XII, a single PCR product was amplified judged from agarose gel electrophoresis and ethidium bromide staining and melting analysis on the Rotor-Gene 6000. The size of each CA PCR product was similar to that anticipated on the basis of the position of the primers in the G. gallus CA genes.
Table 1. G. gallus primer sequences for carbonic anhydrases and M. musculus reference gene sequences that were used to prime C. coturnix cDNA. Sequences are written 5′-3′
Mouse reference gene primer sequences designed using Universal Probe Library (Roche Applied Science, Burgess Hill, West Sussex, UK) were aligned against the equivalent G. gallus mRNA sequences and 5 genes most similar to G. gallus were selected: beta actin (Actb), beta 2-microglobulin (B2m), phospholipase A2 (Pla2), succinate dehydrogenase subunit A (Sdha), TATA box binding protein (Tbp). Quantitative PCR assays for each of these were established and the three most stable were identified using geNorm (Vandesompele et al., Citation2002; ).
Quantitative real-time PCR
Two microlitres of cDNA from blastoderm or blood were amplified by PCR using a Sensimix NoRef DNA kit and SYBR green (Quantace, London, UK) on a Rotor-Gene 6000 (Corbett Research, St Neots, Cambs., UK). PCR standards (107 to 101 copies/µl), amplified and purified from blastoderm cDNA using a QiaQuick gel extraction kit (Qiagen), were included in each PCR run, as was a control reaction containing no template. PCR conditions were as follows: 95°C for 10 min followed by 40 cycles of 95°C for 15 s, 57°C for 20 s and 72°C for 10 s.
Sequencing and alignment
PCR products for CA II, CA IV and CA IX were sequenced in both directions with a 3130 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) using the dye termination method. Using EMBOSS (http://www.ebi.ac.uk/emboss/align/index.html) the amplicon for each pair of primers, excluding the primer sequences, was aligned against the G. gallus sequence located between the forward and reverse primers.
Data analyses
PCR efficiencies were determined from the standard curves and were ≥95% for all genes. All standard curves were linear (r 2 > 0·997) from 107 to 101 copies. For each reference and CA gene, the sample threshold cycle, cT, and hence copy number/µl cDNA, was determined from the standard curve using the Rotor-Gene 6000 series software v1.7 (Corbett Research). The ratio of the copy number/µl cDNA and the normalisation factor of the reference genes (obtained from geNorm) was determined for each sample. This measure was analysed by a two-way factorial ANOVA using a General Linear Model (GLM) in Minitab v14.2 (Minitab Inc., State College, PA, USA) and means were compared using Tukey's pairwise test. Where data-sets did not meet the assumptions for GLM, values were log-transformed before the analysis. All reported values are mean±standard error and the level of significance was set at P < 0·05.
Results
The CA IV gene expression in blastoderm samples increased with the period of incubation (two-way ANOVA, F 1,5 = 7·05, P < 0·001), whereas turning alone did not change gene expression (F 1,60 = 3·48, P = 0·067), although there was a significant interaction (F 5,60 = 2·56, P = 0·036). Thus, by 84 and 96 h of incubation CA IV gene expression was markedly greater in turned eggs than at earlier times (48 to 60 h), whereas gene expression in unturned eggs had not changed (). There was no effect of turning on CA II gene expression (F 1,59 = 0·18, P = 0·67) in blastoderm samples and no interaction (F 5,59 = 0·61, P = 0·69). However, overall CA II gene expression diminished substantially over the period of incubation investigated (F 1,5 = 10·18, P < 0·001), levels from 60 to 96 h being about half those at 48 and 54 h of incubation (). Similarly, there was no effect of turning on CA IX gene expression (F 1,60 = 0·24, P = 0·62) in blastoderm samples and no interaction (F 5,60 = 1·54, P = 0·19). The period of incubation did affect gene expression (F 5,60 = 2·70, P = 0·03) but the effect was discernable only as levels at 84 h exceeding those at 48 h of incubation (). Although an amplicon was obtained for CA XIV, it was below the quantifiable range (data not presented). No amplification of CA XII was observed.
Figure 1. The expression (mean ± SEM, n = 6) of carbonic anhydrase IV mRNA by the blastoderm of the Japanese quail in turned and unturned eggs from 48 to 96 h of incubation. For turned eggs, means sharing lower case superscripts differ (P < 0·05); for unturned eggs means sharing upper case superscripts differ (P < 0·05).
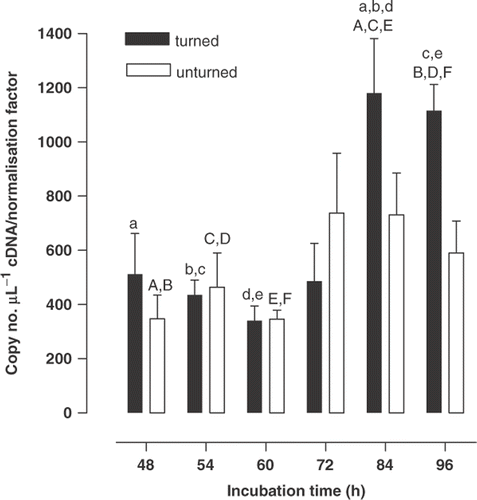
Figure 2. The expression (mean ± SEM, n = 6) of carbonic anhydrase II mRNA by the blastoderm of the Japanese quail in turned and unturned eggs from 48 to 96 h of incubation. For turned and unturned eggs combined, and for each period of incubation, means sharing the same superscript differ (P < 0·05).
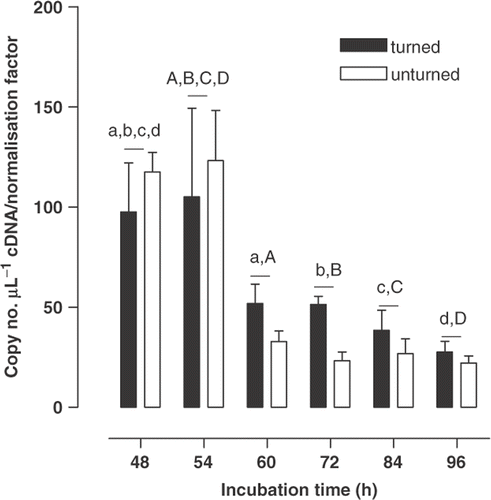
Figure 3. The expression (mean ± SEM, n = 6) of carbonic anhydrase IX mRNA by the blastoderm of the Japanese quail in turned and unturned eggs from 48 to 96 h of incubation. For turned and unturned eggs combined, and for each period of incubation, means sharing the same superscript differ (P < 0·05).
CA II exhibited the lowest levels of gene expression in blastoderm samples: for example, in turned eggs at 48 to 56 h CA II was approximately 4-fold lower than the levels for CA IV and reached a c.40-fold difference at 84 and 96 h of incubation. CA IX had the highest levels of gene expression in the blastoderm ranging from approximately 400 to 2000-fold greater than for CA II and 50 to 100-fold for CA IV. In blood, CA II levels of gene expression were almost 100-fold greater than that for CA IV (744·2 ± 211·1 copies/µl cDNA/normalisation factor for CA II and 8·5 ± 1·5 copies/µl cDNA/normalisation factor for CA IV; n = 10). Compared to CA II, CA IX was also expressed at a low level in blood (13·8 ± 4·5 copies/µl cDNA/normalisation factor; n = 10). As for the blastoderm samples, CA XIV was present in blood but unquantifiable and CA XII did not amplify.
The quail amplicons for CA II and CA IX (excluding primers) exhibited high % similarity compared with G. gallus, 100% in the case of CA II (). The missing nucleotides for the quail CA IV amplicon reduced the similarity to a lower figure, even though the remaining 26 known nucleotides included only two mismatches. Reliable sequence data could not be obtained for CA XIV due to low amounts of amplicon.
Table 2. Sequences of C. coturnix (Cot) PCR products (excluding primers) aligned against G. gallus (Gal) sequences. Sequences are written 5′-3′. Mismatches between two sequences are underlined. For CA IV, two nucleotides represented by ‘n’ could not be determined as the signal was too low
Discussion
Of the 5 carbonic anhydrase isoforms investigated, quantitative data were obtained for only three: CA II, CA IV and CA IX. As anticipated, CA II gene expression was greater (c.25-fold) in blood than in blastoderm tissue from turned eggs at the equivalent time period. Blood expressed much lower levels of CA IV than CA II, as has been reported for human erythrocytes (Wistrand et al., Citation1999), providing further evidence that these primers were indeed isoform-specific. Although amplification was achieved for CA XIV in tissue and blood, the amount of amplicon was below the quantifiable range; hence, this isoform appears to be either amplified inefficiently with the primers used or is present, but in very low amounts at the times investigated. As no amplification of CA XII was observed either the primers used were unsuitable for quail, or this isoform was not present in quail blastoderm or blood. As all the other primer pairs amplified quail cDNA with high efficiency, suggesting that the quail and chicken mRNA sequences are sufficiently similar to be primed with identical primer pairs, we suggest that CA XII was indeed not present in the tissues used.
The isoform CA IX was expressed at uniformly high levels in the extra-embryonic blastoderm tissue with a maximum at 84 h of incubation. CA IX has been well characterised for a variety of tumours and is a marker of tissue hypoxia during vascular growth (Beasley et al., Citation2001). Indeed, at this time during incubation oxygen transport is diffusion-limited in the fowl (Meuer and Baumann, Citation1987) suggesting that the high level of gene expression for CA IX may reflect a hypoxia associated with the extensive vasculogenesis and angiogenesis proceeding in the blastoderm at this time (Vico et al., Citation1998; Baggott, Citation2001). In contrast gene expression for blastoderm CA II was greatest at 48 and 54 h of incubation, decreasing later to lower levels. Whilst quantification of CA IV and CA IX within erythrocytes resident within the blastoderm tissue should be of negligible magnitude due to the low expression in blood, this was likely not the case for CA II. The relatively high levels of CA II gene expression in blood could, we suggest, contribute substantially to CA II expression quantified in blastoderm samples. This would, indeed, be most evident at 48 and 54 h of incubation, as in the fowl the percentage of blood in extra-embryonic tissues is highest at the beginning of the period of SEF formation, having decreased by a third after 4 d (Romanoff, Citation1967). Thus, the levels found from 60 to 96 h of incubation are most probably those representative of blastodermal CA II gene expression.
In the domestic fowl static incubation of eggs during the critical period of 3 to 7 d of incubation (0·14 to 0·33 as fraction of incubation period) reduces hatchability and embryonic growth even if eggs are turned at other times (Deeming, Citation1989 b). SEF mass is also reduced if eggs are unturned during this critical period (Deeming et al., Citation1987; Deeming, Citation1989 c; Baggott et al., Citation2002). In the blastoderm samples only gene expression for CA IV was affected by egg turning, with maxima at 84 and 96 h of incubation (0·20 and 0·24 of the incubation period), times located approximately in the middle of the critical period. This is also the time of peak SEF mass in the Japanese quail (Babiker and Baggott, Citation1992). If, as seems probable, enhanced CA IV gene expression results in increased enzyme activity and endodermal cell ion transport, these changes could account for the additional fluid mass observed in turned eggs at 84 and 96 h of incubation (Babiker and Baggott, Citation1992). In unturned eggs gene expression for CA IV at these times did not differ from earlier sampling times: presumably permitting continued SEF formation yet without forming the additional fluid mass produced with egg turning (Deeming, Citation1989 c; Babiker and Baggott, Citation1992). Carbonic anhydrase localised to the endodermal cells has also been reported for the turkey blastoderm (Bakst and Holm, Citation2003). In this species, cold storage of eggs for 21 d prior to incubation did not alter CA activity, assessed histochemically, in eggs incubated for up to 72 h (Bakst and Holm, Citation2003). However, in view of the time-specific increase in CA IV gene expression reported here an assessment for specific isoforms later in incubation might repay investigation; particularly as egg storage produces lower growth rates, as well as higher embryonic mortality (Fasenko, Citation2007).
Recent evidence for transporting epithelia indicates that CA II, CA IV and CA IX can enhance ion transport rates. CA II has been shown to increase Na+/H+ exchanger activity (NHE1; Li et al., Citation2002, Citation2006), an exchanger known to participate in SEF secretion (Latter and Baggott, Citation2002), and to enhance the transport capacity of the Na+/bicarbonate co-transporter (NBCe1; Becker and Deitmer, Citation2007). CA IV has also been found to increase NBC1 activity in conjunction with CA II (Alvarez et al., Citation2003). Similarly, interactions with anion exchangers can require both CA II and CA IV (Sterling et al., Citation2001, Citation2002). And additionally, CA IX has also been demonstrated to enhance
anion exchange (AE1, AE2 and AE3), binding AE2 to the catalytic domain and being co-localised with AE2 in human gastric mucosa (Morgan et al., Citation2007). In those studies where a membrane-associated CA participated in ion transport activity, CA II was shown to be necessary for maximal effect (except, so far, for CA IX). However, in blastoderm tissue CA II gene expression was reduced by the time of maximal CA IV expression in turned eggs, a consequence, we suggest, of quantifying both endodermal and erythrocyte CA II expression in blastoderm tissue samples. Also, although Babiker and Baggott (Citation1995), using inhibitors, found no evidence for participation of
anion exchange in the formation of SEF, secretion of this fluid is accompanied by increased fluid bicarbonate due to a metabolic alkalosis (Babiker and Baggott, Citation1991).
On balance, the effect of turning in stimulating fluid transport and CA IV gene expression in combination with the types of ion transporters known to be essential for SEF secretion, argues strongly for a blastoderm ‘transport metabolon’ involving CA IV and an ion exchanger. Whether CA II participates in such a ‘metabolon’ first requires a clearer demonstration of the presence, and quantities, of CA II in the extra-embryonic tissues, excluding blood. Conceivably, CA IV and CA IX could contribute to separate ion exchange ‘metabolons’: for example, one incorporating CA IV and sensitive to egg turning, possibly using an exchanger other than AEs or NBC1; another for anion transport incorporating CA IX with one of these exchangers. Evidently, the role of anion exchangers in SEF formation by blastoderm requires further clarification, particularly with regard to NBC1, a co-transporter not investigated in previous studies.
In summary, CA II, CA IV and CA IX gene expression were quantifiable in both extra-embryonic blastoderm tissue and blood up to 96 h of incubation. Of these three isoforms only CA IV expression was stimulated by egg turning at a time coincident with additional SEF formation in turned eggs. This is, we believe, the first demonstration of a change in gene expression in the extra-embryonic tissues induced by an alteration of a factor—in this case egg turning—essential to successful incubation.
References
- Alvarez , BV , Loiselle , FB , Supuran , CT , Schwartz , GJ and Casey , JR . 2003 . Direct extracellular interaction between carbonic anhydrase IV and the human NBC1 sodium/bicarbonate co-transporter . Biochemistry , 42 : 12321 – 12329 .
- Ar , A . 1991a . “ Egg water movements during incubation ” . In Avian Incubation, Poultry Science Symposium , Edited by: Tullett , SG . Vol. 22 , 157 – 173 . Cambridge : Butterworth-Heinemann .
- Ar , A . 1991b . “ Roles of water in avian eggs ” . In Egg Incubation: Its Effects on Embryonic Development in Birds and Reptiles , Edited by: Deeming , DC and Ferguson , MJ . 229 – 243 . Cambridge : Cambridge University Press .
- Babiker , EM and Baggott , GK . 1991 . “ The acid–base status of the sub-embryonic fluid of the Japanese quail Coturnix coturnix japonica ” . In Avian Incubation, Poultry Science Symposium , Edited by: Tullett , SG . Vol. 22 , 326 Cambridge : Butterworth-Heinemann .
- Babiker , EM and Baggott , GK . 1992 . Effect of turning upon the sub-embryonic fluid and albumen of the egg of the Japanese quail . British Poultry Science , 35 : 973 – 991 .
- Babiker , EM and Baggott , GK . 1995 . The role of ion transport in the formation of sub-embryonic fluid by the embryo of the Japanese quail . British Poultry Science , 36 : 371 – 383 .
- Baggott , GK . 2001 . “ Development of extra-embryonic membranes and fluid compartments ” . In Perspectives in Fertilisation and Embryonic Development in Poultry , 23 – 29 . Lincoln : Ratite Conference Books .
- Baggott , GK , Deeming , DC and Latter , GV . 2002 . Electrolyte and water balance of the early avian embryo: Effects of egg turning . Avian and Poultry Biology Reviews , 13 : 105 – 119 .
- Bakst , MR and Holm , L . 2003 . Impact of egg storage on carbonic anhydrase activity during early embryogenesis in the turkey . Poultry Science , 82 : 1193 – 1197 .
- Beasley , NJP , Wykoff , CC , Watson , PH , Leek , R , Turley , H , Gatter , K , Pastorek , J , Cox , GJ , Ratcliffe , P and Harris , AL . 2001 . Carbonic anhydrase IX, an endogenous hypoxia marker, expression in head and neck squamous cell carcinoma and its relationship to hypoxia, necrosis, and microvessel density . Cancer Research , 61 : 5262 – 5267 .
- Becker , HM and Deitmer , JW . 2007 . Carbonic anhydrase II increases the activity of the human electrogenic . Journal of Biological Chemistry , 282 : 13508 – 13521 .
- Bernstein , RS and Schraer , R . 1972 . Purification and properties of an avian carbonic anhydrase from the erythrocytes of Gallus domesticus . Journal of Biological Chemistry , 247 : 1306 – 1322 .
- Chegwidden , WR and Carter , ND . 2000 . “ Introduction to the carbonic anhydrases ” . In The Carbonic Anhydrases: New Horizons , Edited by: Chegwidden , WR . 13 – 28 . Basel : Birkhauser .
- Deeming , DC . 1989a . Failure to turn eggs during incubation: Development of the area vasculosa and embryo growth . Journal of Morphology , 201 : 179 – 185 .
- Deeming , DC . 1989b . Characteristics of unturned eggs. A critical period, retarded embryonic growth and poor albumen utilisation . British Poultry Science , 30 : 239 – 249 .
- Deeming , DC . 1989c . Importance of sub-embryonic fluid and albumen in the embryo's response to turning of the egg during incubation . British Poultry Science , 30 : 591 – 606 .
- Deeming , DC , Rowlett , K and Simkiss , K . 1987 . Physical influences on embryo development . Journal of Experimental Zoology Supplement , 1 : 341 – 345 .
- Dragon , S and Baumann , R . 2001 . Erythroid carbonic anhydrase and hsp70 expression in chick embryonic development: Role of cAMP and hypoxia . American Journal of Physiology Regulatory, Integrative Comparative Physiology , 280 : R870 – R878 .
- Fasenko , GM . 2007 . Egg storage and the embryo . Poultry Science , 86 : 1020 – 1024 .
- Latter , GV and Baggott , GK . 1996 . Effect of egg turning and fertility upon the sodium concentration of albumen of the Japanese quail . British Poultry Science , 37 : 301 – 308 .
- Latter , GV and Baggott , GK . 2002 . Role of carbon dioxide and ion transport in the formation of sub-embryonic fluid by the blastoderm of the Japanese quail . British Poultry Science , 43 : 104 – 116 .
- Li , X , Alvarez , B , Casey , JR , Reinhart , RAF and Fliegel , L . 2002 . Carbonic anhydrase II binds to and enhances activity of the Na+/H+ exchanger . Journal of Biological Chemistry , 277 : 36085 – 36091 .
- Li , X , Liu , YS , Alvarez , BV , Casey , JR and Fliegel , L . 2006 . A novel carbonic anhydrase II binding site regulates NHE1 activity . Biochemistry , 45 : 2414 – 2424 .
- Meuer , H-J and Baumann , R . 1987 . Oxygen supply of early chick embryo in normoxia and hypoxia . Journal of Experimental Zoology Supplement , 1 : 203 – 207 .
- Morgan , PE , Pastoreková , S , Stuart-Tilley , AK , Alper , SL and Casey , JR . 2007 . Interactions of transmembrane carbonic anhydrase, CAIX, with bicarbonate transporters . American Journal of Physiology, Cell Physiology , 293 : C738 – C748 .
- Pannett , CA and Compton , A . 1924 . The cultivation of tissues in saline embryonic juice . Lancet , 1 : 381 – 384 .
- Rahn , H . 1991 . Energy budget and gas exchange of avian eggs Avian Incubation, Poultry Science Symposium Edited by: Tullett , SG . Vol. 22 , 175 – 193 . Cambridge : Butterworth-Heinemann .
- Romanoff , AL . 1967 . Biochemistry of the Avian Embryo , New York : Wiley .
- Sly , WS . 2000 . “ The membrane carbonic anhydrases: From CO2 transport to tumor markers ” . In The Carbonic Anhydrases: New Horizons , Edited by: Chegwidden , WR . 13 – 28 . Basel : Birkhauser .
- Sterling , D , Reinhart , RAF and Casey , JR . 2001 . A transport metabolon: Functional interaction of carbonic anhydrase II and chloride/bicarbonate exchangers . Journal of Biological Chemistry , 276 : 47886 – 47894 .
- Sterling , D , Alvarez , BV and Casey , JR . 2002 . The extracellular component of a transport metabolon . Journal of Biological Chemistry , 277 : 25239 – 25246 .
- Sturzenbaum , SR . 1999 . Transfer RNA reduces the formation of primer artifacts during quantitative PCR . BioTechniques , 27 : 50 – 52 .
- Vandesompele , J , De Preter , K , Pattyn , F , Poppe , B , Van Roy , N , De Paepe , A and Speleman , F . 2002 . Accurate normalisation of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes . Genome Biology , 3 : 0034.1 – 0034.11 .
- Vico , PG , Kyriacos , S , Heymans , O , Louryan , S and Cartilier , L . 1998 . Dynamic study of the extraembryonic vascular network of the chick embryo by fractal analysis . Journal of Theoretical Biology , 195 : 525 – 532 .
- Wistrand , PJ , Carter , ND , Conroy , CW and Mahieu , I . 1999 . Carbonic anhydrase IV activity is located on the exterior surface of human erythrocytes . Acta Scandinavica , 165 : 211 – 218 .