ABSTRACT
1. Infectious diseases have a large impact on poultry health and economics. Elucidating the pathogenesis of a certain disease is crucial to implement control strategies.
2. Multiplication of a pathogen and its characterisation in vitro are basic requirements to perform experimental studies. However, passaging of the pathogen in vitro can influence the pathogenicity, a process targeted for live vaccine development, but limits the reproduction of clinical signs.
3. Numerous factors can influence the outcome of experimental infections with some importance on the pathogen, application route and host as exemplarily outlined for Histomonas meleagridis, Gallibacterium anatis and fowl aviadenoviruses (FAdVs).
4. In future, more comprehensive and detailed settings are needed to obtain as much information as possible from animal experiments. Processing of samples with modern diagnostic tools provides the option to closely monitor the host–pathogen interaction.
Introduction
Robert Koch (1843–1910) was a physician and microbiologist who made eminent contributions to understand important bacterial diseases in humans like tuberculosis, cholera and anthrax. In his innovative discoveries, he focused on the characterisation of the microorganisms in vitro and in vivo. These observations led to the formulation of postulates which describe the basic requirements for a microorganism and its link to certain causalities (Gradmann, Citation2014). They are regarded as the gold standard to summarise principle investigations ranging from the isolation of a pathogen from a diseased host and its pure cultivation until re-isolation following induction of the disease in susceptible hosts. For his achievements, he was awarded with the Nobel Prize in Physiology and Medicine in 1905.Since their discovery, the knowledge about microorganisms and their interaction with the host extended substantially, arguing for a revision and modification of the original postulates. Specifically, endemic microorganisms that induce disease only under certain conditions hardly ever comply with the postulates. Rigorousness and exclusive application of the postulates were already questioned by Robert Koch himself, because he noticed that some infectious bacteria can appear as a commensal but the context between pathogen–host and environment were not the main focus at that time.
Good health is considered as a major principle to sustain animal welfare in addition to good housing and feeding, together with appropriate behaviour (European Food Saftey Authority (EFSA), Citation2012). This is also of high importance to support the prosperity and sustainability of production. Robert Fraser Gordon published different articles about the importance of diseases for the economy in poultry production and he already claimed that not only mortality needs to be considered in this context (Gordon, Citation1967, Citation1971, Citation1973). According to R.F. Gordon, “adverse effects on production and reproduction, food conversion and body weight and the costs for disease control and prevention” contribute to economic losses. Research is mandatory to address these challenges and as a consequence he set up the Houghton Poultry Research Station which was solely focused on poultry research (Gordon, Citation1973). Ever since, infectious diseases in poultry have had a high impact on health and production and the number of pathogens known to influence health has increased constantly (Swayne et al., Citation2013). However, for some microorganisms, the link between pathogenicity and being a sole commensal is not that clear. This is also supported by controversial results of animal experiments reported in the literature. Some imponderabilities, like the inoculum, the route of application and the host are discussed in this article based upon infections of chickens with Histomonas meleagridis, Gallibacterium anatis and fowl aviadenoviruses (FAdVs). The main aim of this paper is to highlight influences which contribute to the pathogenicity in the field, and the necessity to address and consider such features in experimental settings, in order to strengthen the association with causation. Considering the wide range of pathogens – from a parasite to a virus – it remains obvious that not all necessary references can be given for each individual subject in this review, which should not prevent the truth of the main conclusions becoming clear.
Histomonas meleagridis
Taxonomy
H. meleagridis was originally regarded as an amoeba and named Amoeba meleagridis (Smith, Citation1895). Extensive and detailed morphological investigations revealed a close relationship but not identity to trichomonads and, therefore, Tyzzer (Citation1920) suggested a reclassification as H. meleagridis. Later on, Dwyer (Citation1972) did a series of studies in which he investigated the antigenic relationship between H. meleagridis, Entamoeba histolytica, Entamoeba invadens, Dientamoeba fragilis and Trichomonas gallinae by fluorescent antibodies, gel diffusion and immunoelectrophoresis. As an outcome of this research, a somewhat close relationship between H. meleagridis and D. fragilis was noticed with much fewer antigens in common with the Entamoeba species. The close genetic relationship between D. fragilis and H. meleagridis was also confirmed by genetic data via sequencing of the small subunit rRNA sequence (Gerbod et al., Citation2001). This was further elaborated by applying a multilocus sequence approach considering three genes with different levels of sequence identity and isolates from different geographic regions, altogether identifying two genotypes (Bilic et al., Citation2014). Consequently, considering ultrastructural and molecular–phylogenetic studies, it was concluded that H. meleagridis was a member of the family Dientamoebidae, order Tritrichomonadida, class Tritrichomonadea (Cepicka et al., Citation2010).
Field reports with focus on chickens
Histomonosis caused by H. meleagridis, was first described in turkeys where it can be a fastidious disease (Cushman, Citation1893). In addition to turkeys, various other galliformes can also be infected but the following sections will mainly focus on infections and the appearance of histomonosis in chicken, as the primary pathogenicity of H. meleagridis in these birds is not clear. Instead, chickens are often considered as reservoir to spread the parasite. Additional and more general aspects of the parasite and its biology can be found in recently published reviews (McDougald, Citation2005; Hess et al., Citation2015). Furthermore, the reviews of Lund (Citation1969) and Reid (Citation1967) are highly recommended to obtain a detailed overview on the earlier literature and the debate about the aetiology of blackhead. The fact that hardly any research was carried out after those reviews were published until the twenty-first century makes them even more valuable.
Soon after the first description in turkeys, histomonosis was described in chickens (Chester and Robin, Citation1900) and Bayon and Bishop (Citation1937) reported the appearance in various laying chicken flocks in England without presenting further clinical data. Exceptionally high mortality of 50% was reported in broilers, reflecting very much the disease in turkeys (Eveleth, Citation1943). Following those initial reports, the disease in chickens disappeared due to an increase of biosecurity with the exception of a few case reports in pullets with mortalities up to 10% in cases of co-infection with Eimeria (Schulze, Citation1975; Müller, Citation1990; Homer and Butcher, Citation1991). The availability of effective drugs, mainly arsenicals, nitrofurans and imidazols, was helpful to prevent or minimise losses (Liebhart et al., Citation2016). Furthermore, the introduction of cage systems for layers contributed to the disappearance of the disease. This changed with the recent introduction of new legislation in Europe banning the major anti-parasitic drugs and an increasing number of layers kept in alternative housing systems (Hafez, Citation2001; Kaufmann-Bart and Hoop, Citation2009; Stokholm et al., Citation2010). Low biosecurity is also reported as a reason for the occurrence of histomonosis in broilers (Ganapathy et al., Citation2000; Cortes et al., Citation2004; Popp et al., Citation2011). In addition to such single case reports, the development of enzyme-linked immunosorbent assay (ELISA) systems for monitoring antibodies offered the chance to perform more comprehensive epidemiological studies. Whereas Grafl et al. (Citation2011) were able to demonstrate a link between the level of biosecurity and the prevalence in Austrian layers such a connection was not confirmed by Van Der Heijden and Landman (Citation2011) who found a high infection rate in Dutch layer chickens independent of the housing system. The DNA of the parasite was detected by polymerase chain reaction (PCR) in 42% of organ or environmental samples taken from turkey, chicken or peacock flocks in Germany with a higher prevalence during the summer (Hauck et al., Citation2010). A much less pronounced seasonality was noticed in Vietnamese chicken samples with a slightly higher number of samples tested positive from dry areas in comparison with rainy ones (Nguyen et al., Citation2015). However, the study highlighted the differences between applied diagnostics as the presence of macroscopic lesions did not always coincide with the detection of parasitic DNA and vice versa.
In addition to caecal and liver lesions, an infection at the end of rearing can have an impact on egg production which reached standard targets only at 22 weeks of life (Gerth et al., Citation1985). An influence on laying performance was also reported by Esquenet et al. (Citation2003) who noticed a drop in egg production of 9% between weeks 57 and 59 and an increase in weekly mortality of 0.8% resulting in a total loss of 6% due to histomonosis. The authors reported that pathomorphological lesions were mainly observed in the caeca with microscopic necrosis in livers. This is similar to other studies in chickens (Homer and Butcher, Citation1991; Stokholm et al., Citation2010) and helps to explain the somewhat lower mortality in comparison with turkeys. Co-infections with Escherichia coli have also been described (Müller, Citation1990; Stokholm et al., Citation2010) which can complicate the clinical outcome and indicate that lesions in the caeca due to H. meleagridis might pave the way for E. coli. However, multilocus sequence typing and plasmid profile analysis of E. coli isolates indicated that colibacillosis appearing in combination with histomonosis should be regarded as a primary disease (Olsen et al., Citation2011).
Interestingly, histomonosis was recently also reported in broiler breeders during rearing (weeks 16 and 19) and production (weeks 25–44) (Dolka et al., Citation2015). In rearing birds various clinical signs were noticed with an increase of mortality reaching 5% for 2 weeks following a total mortality in the flock of 1.05% until onset of the disease. The described cases indicate that the disease can also occur in more bio-secure breeding stock and it contradicts, together with the above-mentioned reports, the assumptions that chickens are solely a reservoir for the pathogen.
Experimental studies focusing on the pathogen
Of all three organisms described in this review, H. meleagridis is by far the most complicated one, with consequence on the clinical picture and the reproduction of lesions in experimental studies. This is mainly due to the complex life cycle of the parasite and a so far unresolved interaction of the parasite and bacteria. The frequent appearance of other protozoan parasites in the gut of chickens and turkeys is of relevance for the preparation of a defined inoculum as well as for the application of diagnostic procedures.
Due to the fact that the disease is most severe in turkeys, the majority of experimental studies were performed in these birds in order (i) to investigate the pathogenesis, (ii) to determine the value of intervention strategies and (iii) to apply different diagnostic techniques. A detailed review on experimental studies with H. meleagridis was recently published by Hauck and Hafez (Citation2013). The authors compiled data about the various routes of infection applied to reproduce the disease in turkeys and chickens, from contaminated litter to eggs or other stages of Heterakis gallinarum towards in vitro grown parasites. Applying eggs harbouring H. meleagridis goes back to the initial observation that the ceacal worm H. gallinarum is an important intermediate host for the protozoan parasite (Graybill and Smith, Citation1920). However, the fact that lesions induced by H. meleagridis established a less favourable environment for the caecal worm together with the observation that certain feed components can influence the interaction between both parasites in vivo indicates a complicated relationship (Lund, Citation1958; Daş et al., Citation2011). If unprotected parasites are used, the rectal route is the most reliable one due to the low tenacity of the parasite, already noticed in the early studies of Tyzzer (Citation1934). Oral infection of chickens with cultures of H. meleagridis is possible but most effective if birds have been starved for a certain time and an alkali is given to neutralise the negative consequences of a low pH in the alimentary tract (Horton-Smith and Long, Citation1956).
The necessity of live bacteria to successfully infect chickens and turkeys is a further complication to studying the host–pathogen interaction. Doll and Franker (Citation1963) described that only 1 out of 12 gnotobiotic turkeys was successfully infected with bacteria-free heterakis eggs harbouring H. meleagridis, whereas nearly all conventional turkeys died. The authors hypothesised that a heat-labile factor released from the bacteria is necessary for the growth of the parasite and to establish an infection in the host. Bradley and Reid (Citation1966) noticed that E. coli on its own was helpful to support the infection of the turkeys, whereas killed bacteria or bacterial filtrate were insufficient. The situation in chickens seems even more complex as a more diverse range of bacteria is needed in order to reproduce the disease compared with turkeys (Springer et al., Citation1970). Based on surgically ex vivo inoculated caeca, the authors demonstrated that the infection of chickens was less consistent than in turkeys and the authors even speculated that some non-cultivable bacteria might be needed to induce the disease. Their conclusion that bacteria are more essential to complete the development of H. gallinarum is a further indication for the complexity of the infection process. The fact that H. meleagridis could only be isolated from liver lesions in the presence of bacteria, as summarised by Goedbloed and Bool (Citation1962), illustrates further this unresolved and complex interaction.
Interactions between H. meleagridis and caecal bacteria are also very relevant for in vitro cultivation, a prerequisite to obtain a standardised inoculum for infection experiments. Bishop (Citation1938) already regarded the cultivation of histomonads free of bacteria to induce the disease as a crucial test to unambiguously prove the aetiology, except a virus might be symbiotically present in the culture. Adding antibiotics to the culture medium, Delappe (Citation1953) noticed the difficult balance between bacteria and parasite in vitro. Increasing the antibiotics might kill the bacteria with negative consequences on growth of the parasite, similar to overgrowth of bacteria, necessitating changes in serum and glucose levels (Devolt, Citation1950). An axenic culture was so far only once reported by Lesser (Citation1961), achieved by replacing caecal bacteria with hamster liver and a mix of certain metals, altogether a rather complicated substrate. All other cultures used for in vivo studies consisted of H. meleagridis and a non-defined bacterial mixture, a so-called xenic culture. Frequent co-infection of birds with other protozoa can further complicate the establishment of a defined inoculum. In this context, Tetratrichomonas gallinarum, Parahistomonas wenrichi and Blastocystis spp. can be mentioned. Although they resemble certain morphological similarities, PCRs combined with sequencing can be used for precise discrimination from H. meleagridis and to elucidate the phylogenetic relationship (Bilic et al., Citation2014).
Summarising the reports above, it is very obvious that various factors – in addition to the primary pathogen – can contribute to the pathogenicity and the outcome of in vivo trials. This does not exclude that general observations and conclusions can be drawn from individual studies but it limits the comparison. Considering the difficulties of demonstrating an unambiguous host–pathogen interaction, it is not surprising that the true aetiology of blackhead remained under debate for a long time, due to the difficulties and inability of fulfilling Koch’s Postulates, despite the fact that numerous experimental studies were available indicating the importance of H. meleagridis as an etiological agent of histomonosis. However, in 1967 – following half a century of intense research – Reid (Citation1967) summarised all possible theories and highlighted the importance of improved laboratory methods to confirm the true aetiology of noticed lesions.
In order to obtain a more defined and standardised inoculum for experimental trials, we applied micromanipulation with which defined cultures of different protozoan parasites were established. Multiplication by binary fission enabled us to grow up a whole stock from a single cell. Using such a xenic clonal culture, numerous infection studies were performed and various diagnostic procedures were developed (reviewed by Hess et al., Citation2015). In addition, re-isolation of live parasites from cloacal swabs was implemented in all trials, enabling the investigation of the transmission dynamics. Consequently, it could be demonstrated that sentinel turkeys or chickens already excreted live parasites 2 d postinfection which questions cloacal drinking as the sole route of lateral transmission in the absence of any vector (Hess et al., Citation2006). Sequential culling of animals revealed new data about the pathogenesis of histomonosis in turkeys and chickens. The development of a monoxenic clonal culture not only confirmed earlier studies about the importance of E. coli for the in vitro growth but also demonstrated that pathogenicity itself is solely triggered by the parasite as long as successful infection can be established (Ganas et al., Citation2012). Beside this, the main aim of our studies was to test the consequences of in vitro passaging on the pathogenicity of H. meleagridis and the development of a possible live vaccine. It was not only possible to demonstrate that H. meleagrids could be attenuated in vitro, something questioned by Lund et al. (Citation1966), the efficacy of these attenuated parasites to protect against a severe challenge in turkeys and chickens could also be shown (reviewed by Liebhart et al., Citation2016). Finally, the availability of monoxenic clonal cultures with different levels of pathogenicity offers certain potential to enter into the “omics” era of histomonas research.
Gallibacterium anatis
Taxonomy
The genus Gallibacterium is classified within the family Pasteurellaceae Pohl 1981. It contains 4 species namely G. anatis, Gallibacterium melopsittaci, Gallibacterium trehalosifermentans and Gallibacterium salpingitidis, three genomospecies 1, 2 and 3 and an unknown taxon (group V) (Christensen et al., Citation2003; Bisgaard et al., Citation2009). Prior to this assignment as a separate entity, G. anatis was formerly known as avian Pasteurella haemolytica, Actinobacillus salpingitidis or Pasteurella anatis. Consequently, changes in terminology need to be considered when performing literature searches.
Field reports
First field reports date back to 1950 when Kjos-Hanssen (Citation1950) reported the isolation of a Pasteurella-like organism from the oviduct of hens with egg peritonitis, which was named “cloacal bacteria”. Salpingitis, peritonitis, degeneration of the ovary and oviduct, haemorrhagic follicles and a drop in egg production with varying mortality were also mentioned in later reports from the field (Hacking and Pettit, Citation1974; Jones and Owen, Citation1981; Mirle et al., Citation1991; Neubauer et al., Citation2009; Jones et al., Citation2013). In these reports, the bacterium was often isolated together with E. coli, although pure cultures were also noticed, especially from the reproductive tract, in combination with macroscopic lesions.
Various field studies described the presence of G. anatis in the upper respiratory tract. In a survey performed in Israel, 97% of 322 healthy chickens from 23 flocks carried the organism in the tracheal flora, whereas a much lower prevalence was noticed in turkeys, geese, ducks and wild birds (Mushin et al., Citation1980). Jones et al. (Citation2013) considered G. anatis as a primary pathogen of the respiratory tract with similar lesions as P. multocida. Performing extensive bacteriological investigation of 10 organs from 3–8 birds/flock, positive birds were detected in 30 flocks with only one flock recorded negative (Neubauer et al., Citation2009). Earlier on it was demonstrated that G. anatis was much more widespread in chicken flocks housed under lower biosecurity with broiler grandparents being negative and the vast majority of tracheal samples obtained from layers kept under organic conditions were found to be positive (Bojesen et al., Citation2003).
Beside lesions in the reproductive tract, necrotic and inflammatory changes in liver, intestine, heart and kidney were also noticed in connection with the isolation of G. anatis (Greenham and Hill, Citation1962; Harbourne, Citation1962; Harry, Citation1962; Hacking and Pettit, Citation1974; Addo and Mohan, Citation1985). In a recent case report, G. anatis was described in context with a hepatitis in layers, an unusual pathology attributed to the infestation of the free range birds with Ascaridia galli (Jung, Citation2012).
However, based on the fact that G. anatis was isolated from diseased and healthy chickens, different authors questioned the primary pathogenicity of the bacterium and suggested that it should be regarded rather as a commensal.
Experimental studies focusing on the infection route
First experimental studies with G. anatis were performed in 1-d-old chicks up to 15-week-old pullets (Matthes et al., Citation1969; Bisgaard, Citation1977; Gerlach, Citation1977; Mushin et al., Citation1980). The main focus was to elucidate whether the infection induces clinical signs, most likely respiratory symptoms, due to the frequent isolation of bacteria in the upper respiratory tract in field surveys. However, no clinical symptoms were noticed following intranasal or intratracheal infections. It was also noticed that G. anatis was unable to exacerbate clinical symptoms of infectious bronchitis virus in a co-infection model, although single infected groups were not reported (Bisgaard, Citation1977). Invasive (subcutaneous, intramuscular and intraperitoneal) and non-invasive (intranasal and intratracheal) infections were carried out in those earlier studies with very diverse outcomes. Most severe lesions were noticed in a study described by Matthes et al. (Citation1969) who reported high mortality following intravenous or intraperitoneal infection with prominent macroscopic lesions. Similarly, Gerlach (Citation1977) reported high mortality with haemorrhagic enteritis but attributed it to the young age of the birds as 1-d-old chicks were used. The studies had in common that no detailed laboratory investigations were performed. Following intratracheal infection, the bacteria did not obtain access to internal organs but no time point postinfection was mentioned and only three birds/group were used (Mushin et al., Citation1980). In contrast, re-isolation was successful from heart and liver after intranasal infection (Matthes et al., Citation1969). Overall, it was concluded in those earlier studies that G. anatis is part of the physiological tracheal flora in chickens and additional influences might be needed to induce any adverse effects. This was supported by frequent isolations from the upper respiratory tract in field studies, as mentioned above, but it neglected field reports that the bacteria were also isolated from reproductive tract lesions.
Following the initial studies, almost no experimental infections were reported until Bojesen et al. (Citation2004) performed a study with a detailed experimental design, including sequential killing of birds between 3 and 24 h postinfection, after intraperitoneal or intravenous infection. Intraperitoneal infection was applied by the same research group in other studies, in order to mimic a peritoneal infection due to ascending bacteria from the cloaca via the oviduct, a hypothesis well known from E. coli infections in layers (Bager et al., Citation2013; Pedersen et al., Citation2015; Pors et al., Citation2016). Testing certain deletion mutants, and/or the efficacy of recombinant proteins to be used as vaccine candidates, fibrinous and purulent peritonitis were noticed, similar to previous studies and expected due to the route of infection. As sexually mature birds were used, oophoritis and salpingitis were also recorded with G. anatis isolated from the reproductive tract. However, in these studies commercial birds were used that were already carrying G. anatis prior to infection, which complicates further conclusions on primary aetiologies, although it might be close to a clinical situation considering the widespread distribution of the bacteria with an increasing age of the birds.
First studies in 12-week-old specific pathogen-free (SPF) chickens were reported by Zepeda et al. (Citation2010), who demonstrated efficient intranasal infection. Although a wide dissemination of the bacteria in pullets and microscopic lesions was noticed, no samples were taken from the reproductive tract. In continuing studies, the same route of infection was used and with this it was possible to confirm that bacteria are widely disseminated in the organism with lesions in the reproductive tract of 4-week-old SPF chickens (Paudel et al., Citation2013). This research indicated that the intranasal route of infection was very suitable to mimic the clinical situation. Based on this study, two further separate experimental trials in mature SPF chickens, hens and cockerels, were performed (Paudel et al., Citation2014a, Citation2014b). Macroscopic and microscopic lesions in the reproductive tract were successfully induced and the infection resulted in a drop in egg production due to severe folliculitis in addition to egg peritonitis. Such conditions reflect very closely the field situation and question the view that infection of layers occurs via the oviduct. The intranasal infection of cockerels confirmed the predilection of G. anatis for the reproductive tract also for male birds, with consequences on semen quality. Histology and re-isolation of G. anatis completed the laboratory investigations and confirmed the pathogenic nature of the bacterium together with the potential of being vertically transmitted.
Despite the fact that clinical signs and macroscopic lesions could be successfully reproduced in SPF birds, mimicking a natural infection, numerous questions remain. Genetically different strains exist with major differences about the absence or presence of certain virulence factors and the consequences on pathogenicity are so far only established for some strains (Persson and Bojesen, Citation2015). Following infection by the nasal route, the bacterium has to pass the mucosal barrier in order to reach the ovaries/testes as G. anatis is less prevalent in internal organs, a phenomenon currently not understood. Furthermore, additional host and pathogen-driven influences for the recognised pathologies need to be considered. At least two studies reported the consequences of immunosuppression leading to a higher load of bacteria in internal organs (Bojesen et al., Citation2004; Paudel et al., Citation2015). However, as those treatments were introduced prior to infection, the impact of immunosuppression on latent carriers remains to be resolved.
Fowl aviadenoviruses (FAdVs)
Taxonomy
FAdVs are non-enveloped, double-strand DNA viruses which belong to the genus Aviadenovirus within the family Adenoviridae (Harrach et al., Citation2011). They are separated into 5 different species (FAdV-A to FAdV-E) with 12 different serotypes (Hess, Citation2000). The grouping into 5 different species was originally suggested by Zsak and Kisary (Citation1981) who digested the DNA of the 12 FAdV serotypes with restriction enzymes and obtained 5 different patterns, identical to the later species A–E.
In the older literature, the family Adenoviridae was split into two genera only, Mast- and Aviadenovirus, reflecting the origin of those viruses, either from mammals or birds. The latter ones were further separated into three different groups (I–III) to address genomic and biological differences between FAdVs, the haemorrhagic enteritis virus of turkeys (HEV) and the egg drop syndrome virus (EDSV). Although substantial differences between members of the three groups (e.g. host specificities, pathogenesis and antigenicity) were already known for some time, the complete sequence of the EDSV genome demonstrated a closer phylogenetic relationship to viruses isolated from mammals in comparison with FAdV questioning the existing taxonomy (Hess et al., Citation1997). In the actual taxonomy, characterised by genera containing viruses from different animal species, such similarities are considered and prioritised over the origin and host.
Field reports
Based on the existing field reports, FAdVs can induce three different diseases, adenoviral gizzard erosion (AGE), hydropericardium-hepatitis syndrome (HHS) and inclusion body hepatitis (IBH) (Hess, Citation2013). Comprehensive epidemiological studies in which genomic analyses were performed indicate that certain species/serotypes can be isolated from a specific disease (Schachner et al., Citation2016). Whereas AGE is mainly reported in connection to FAdV-1 (species A), virulent strains of FAdV-4 (species C) induce HHS and various serotypes of FAdV-D and FAdV-E are etiological agents of IBH. Although such epidemiological studies indicate a link between aviadenoviruses and a certain disease, it needs to be mentioned that FAdVs are also present in clinically healthy birds with mixed infections of different serotypes again confirmed in recent reports (Kaján et al., Citation2013; Niczyporuk, Citation2016). This underlines the importance of precise laboratory investigations in order to establish a robust link between detection of virus and disease. Various FAdVs can be isolated from a wide range of birds, ranging from poultry species to wild birds, indicating low host specificity. Like in chickens, FAdV infections in other bird species might occur without clinical signs but the appearance of virulent FAdV-4 in pigeons and ducks in coincidence with severe clinical signs and high mortality indicates the risk of such a spread (Naeem and Akram, Citation1995; Hess, Citation2013; Chen et al., Citation2016).
Clinical signs due to FAdV are mainly noticed in young birds up to 5 weeks of age. Since the first description by Yates and Fry (Citation1957), vertical transmission of FAdV from breeders to progeny is of special importance for spreading FAdV and the induction of disease in progenies (Toro et al., Citation2001; Grgic et al., Citation2006; Grafl et al., Citation2012). An impact on breeder performance with reduced egg production and hatchability was reported in cases of vertical transmission or due to infection with virulent FAdV-4 (Christensen and Saifuddin, Citation1989; Abe et al., Citation1998; Kim et al., Citation2008; Grafl et al., Citation2012). Introduction of FAdVs into a flock can also occur via horizontal transmission, but very often the source of entrance could not be resolved due to the fact that no accurate samples were available. Serum samples are of special importance to determine the infectious status of breeders and to prove vertical transmission in a serum neutralisation test using the virus from the diseased progenies.
Based on field data and experimental infections (see below), it was the general perception for a long time that a co-infection with an immunosuppressive virus (either infectious bursal disease virus (IBDV) or chicken anemia virus (CAV)) was needed for clinical manifestation of an FAdV-induced disease, mainly IBH and HHS. However, earlier reports about IBH in New Zealand had already indicated that IBH can be a primary disease which was confirmed in a series of more recent reports of IBH outbreaks in Canada, Australia and Japan (Christensen and Saifuddin, Citation1989; Gomis et al., Citation2006; Nakamura et al., Citation2011; Steer et al., Citation2011). In addition, the prevalence of FAdV does not coincide with the presence of IBDV or CAV (Eregae et al., Citation2014). However, as immunosuppression is an important trigger for IBH and HHS, special care should be taken to protect birds accordingly.
First reports about IBH date back to 1963 when Helmboldt and Frazier (Citation1963) described inclusion bodies in chicken livers with unknown significance. Later on, various studies aimed to isolate the relevant agent and FAdVs were isolated and typed by different research groups (reviewed by McFerran and Adair, Citation1977). A larger number of IBH outbreaks were reported from Canada (Pettit and Carlson, Citation1972) and Iraq (Al-Sheikhly and Mutalib, Citation1979) based on histological diagnosis until the disease was reported from New Zealand (Saifuddin et al., Citation1992) and Australia (Erny et al., Citation1991), predominantly caused by FAdV-8. Single case reports were published until about 10 years ago when the number of outbreaks in geographically different areas worldwide increased substantially (Gomis et al., Citation2006; Ojkic et al., Citation2008a, Citation2008b; Lim et al., Citation2011; Nakamura et al., Citation2011; Steer et al., Citation2011; Choi et al., Citation2012; Kaján et al., Citation2013; Maartens et al., Citation2015; Zhao et al., Citation2015; Niczyporuk, Citation2016; Schachner et al., Citation2016).
The clinical picture of IBH is characterised by sudden onset of mortality in 1–5-week-old birds. Mortality varies greatly between 1% and 30%, indicating that numerous factors might influence the disease. The majority of field cases were reported from broilers with severe hepatitis noticed during post-mortem investigations, sometimes accompanied with an atrophy of the bursa of Fabricius. Histological changes in the kidneys and inclusion bodies in the pancreas have also been noticed together with hypoglycaemia (Goodwin et al., Citation1993; Wilson et al., Citation2010).
In 1987, HHS was first described in Pakistan and later on in other Asian and Arabian countries and some parts of Middle and Latin America (Anjum et al., Citation1989; Hess et al., Citation1999). Clinical signs and pathomorphological lesions of HHS are similar to IBH, except that they are more severe. In addition, hydropericardium is noticeable characterised by a straw-coloured fluid in the pericardial sac. Exceptionally, clinical signs with 6.4% mortality in 35-week-old broiler breeders were reported (Abe et al., Citation1998). Several reviews are available describing the clinical signs together with pathomorphological and histological lesions (Ganesh and Raghavan, Citation2000; Balamurugan and Kataria, Citation2004; Asthana et al., Citation2013). Mixed pathologies, especially for HHS and IBH, are frequently reported but the presence of a hydropericardium in combination with a somewhat higher mortality is a strong indication for HHS. Circulating virulent FAdV-4 viruses inducing mixed pathologies of HHS and/or IBH have been reported from various Asian countries (Lim et al., Citation2011; Mittal et al., Citation2014; Zhao et al., Citation2015).
AGE is a rather new disease mainly noticed in broilers, although Grimes et al. (Citation1977) already reported AGE in the context of an FAdV infection during an IBH outbreak without further investigations. Later on, Tanimura et al. (Citation1993) noticed gizzard erosions with haemorrhages in the proventriculus of 10-week-old pullets. Inclusion bodies were demonstrated in the gizzard together with degeneration and desquamation of the keratinoid layer and necrosis of the epithelium. Clinically, AGE can already be differentiated from HHS and IBH due to the somewhat lower mortality. A slightly elevated mortality with higher selection rate was reported but the disease might go unnoticed, despite the fact that 50% of the birds can have lesions at the slaughterhouse leading to a higher condemnation rate (Ono et al., Citation2001, Citation2003; Grafl et al., Citation2012). The disease has so far been reported from Europe and Asia, with the majority of reports identifying FAdV-1 as etiological agent. As FAdV-1 is very widespread and as gizzard erosions can have various aetiologies (Gjevre et al., Citation2013), specific diagnostic methods should be applied to characterise FAdVs and to demonstrate the virus in the pathomorphological lesions. In addition, demonstration of specific antibodies is helpful to confirm the aetiology.
Experimental studies focusing on the type of bird
Although the field studies described above indicate a link between certain FAdVs and disease, the fact that FAdVs are also isolated from clinically healthy birds is a severe complication to fulfil Koch’s Postulates. The mechanism behind this is hardly understood and so far the genetic background of FAdVs in context of pathogenicity was only targeted in a single study highlighting the importance of the fibre protein for pathogenicity of FAdV-E viruses (Pallister et al., Citation1996). In the previous section, it was already outlined that IBH and HHS are somewhat similar with regard to the appearance in the field. Consequently, they share certain principles with regard to experimental trials, also mentioned in the different reviews cited above.
Soon after the first description of IBH in the field, Clemmer (Citation1965, Citation1972) reported an age resistance based upon virus excretion, following oral infection of chickens, at either 1-d-old or 45 d of age, with FAdV-1. The virus was mainly confined to the gastrointestinal tract and no lesions were noticed. Interestingly, he also noticed that birds infected at 12 d of age were refractory to a second infection 33 d later. Age resistance was confirmed later on by several authors who used different routes of infection, mainly intramuscular and subcutaneous at two different time points in birds up to 21 d of age, in order to reproduce IBH (Cook, Citation1974b; Nakamura et al., Citation2000; Lim et al., Citation2011; Dar et al., Citation2012). A very virulent FAdV-4 isolated from a broiler breeder hen suffering from HHS induced 70% mortality in 15-month-old SPF hens, the oldest birds ever used in experimental studies, with 100% mortality in 1-d-old birds independent of the infection route (Nakamura et al., Citation1999).
A different dose of the same virus was used in various studies to reproduce IBH (Erny et al., Citation1991; Mendelson et al., Citation1995; Nakamura et al., Citation1999; Dar et al., Citation2012). Differences became more significant as birds were getting older (> 1 week of age) indicating a negative correlation between age and a lower viral dose. However, virulent FAdV-E viruses given intranasally to 1-d-old birds at a low dose of 5 TCID50 were able to induce IBH (Erny et al., Citation1991). As an outcome of available studies, it can be concluded that after 1 week of age an invasive route, most often intramuscular, is needed to induce clinical signs characterised by apathy, huddling of birds and mortality. This was also demonstrated in the first study reproducing HHS in SPF chicks with plaque-purified FAdV (Mazaheri et al., Citation1998). Initial experiments to reproduce HHS were done in commercial broilers with a less well-characterised overall infectious status (Anjum, Citation1990; Chandra et al., Citation1997). The application of organ homogenates reflects the uncertainties at that time about the true aetiology of HHS, although an adenovirus was already demonstrated by electron microscopy.
Different to FAdV-1 mentioned above, other FAdV strains were more widespread in the organism following oral and intranasal infection of 1-d-old birds (Cook, Citation1983). Viruses could be recovered from the respiratory system, gonads, liver, spleen, bursa of Fabricius and some FAdVs were even recovered from the brain, although replication in the later tissue was questioned. Highest viral loads were noticed in different parts of the digestive tract, either proventriculus, caecum or ileum, which, together with the ceacal tonsils are the organs of virus persistence, something also noticed by measuring viral antigen by ELISA in numerous organs (Saifuddin and Wilks, Citation1991). Based upon detailed assessment of macroscopic and histological lesions, virulent IBH viruses induce most severe changes between 4 and 7 d postinfection (Steer et al., Citation2015).
Co-infection experiments with either IBDV or CAV indicated that reproduction of IBH depends on a compromised immune system (Fadly et al., Citation1976; Von Bülow et al., Citation1986). The importance of a fully functional immune system was also confirmed in an experimental study demonstrating significant differences between chemically immunosuppressed chickens versus untreated birds (Nakamura et al., Citation2003). However, infection of SPF birds with virulent FAdV-4 demonstrated a severe impact of FAdV itself on lymphocyte subpopulations in the thymus and bursa of Fabricius, although the true nature and mechanism still need to be elucidated (Schonewille et al., Citation2008). Furthermore, progenies investigated from intramuscularly infected serologically negative layer breeders developed microscopic lesions in the liver irrespective of a CAV co-infection confirming the primary pathogenicity of FAdV after vertical transmission (Toro et al., Citation2001).
In an earlier experimental study, Grimes et al. (1978) noticed a necrotising pancreatitis, something also reported from the field. In a recent study, detailed histological investigations of liver and pancreas together with an assessment of clinical chemistry analytes were combined, altogether correlating very well, which indicates that the pancreas is an important target organ in IBH pathogenesis (Matos et al., Citation2016b). As a consequence of this finding, we hypothesised that the type of bird is of importance for the outcome of an experimental infection. This was confirmed in another study in which much more severe clinical signs in SPF broilers in comparison with SPF layers, characterised by hypoglycaemia and severe pathological lesions in the pancreas, were noticed (Matos et al., Citation2016a). Such results might help to explain why IBH outbreaks in fast-growing broilers, who are physiologically very different from layers, are more frequently reported, as mentioned above. And it indicates that experiments performed decades ago, although different kinds of SPF birds were used (Cook, Citation1974a), are difficult to compare with current studies, considering the substantial recent progress in breeding and the accompanying change in bird metabolism.
The majority of experimental studies to reproduce AGE were performed in SPF White Leghorn layers (Okuda et al., Citation2001b, Citation2004; Nakamura et al., Citation2002; Ono et al., Citation2004, Citation2007; Domanska-Blicharz et al., Citation2011). Whereas mortality was only noticed in one study (Domanska-Blicharz et al., Citation2011) all the others did not report severe clinical signs. However, in all of those studies it was possible to induce lesions in the gizzard similar to those described from field outbreaks. Lesions were characterised by erosion of the koilin layer accompanied with an inflammation or ulceration of the gizzard mucosa, which peaked around 10 d postinfection. In all studies, such lesions were successfully reproduced by oral infection with field isolates characterised as FAdV-1, except in one study where a FAdV-8 strain was applied and lesions in the gizzard were less severe (Okuda et al., Citation2004). If intramuscular or intravenous application were used, more severe lesions were noticed including the induction of IBH with the FAdV-8 isolate.
Soon after the initial reproduction of AGE in SPF layers, Okuda et al. (Citation2001a) reproduced the disease in commercial broilers due to the assumption that layer-type chickens might be an inappropriate model. Despite the fact that the commercial birds had maternal antibodies, which were no longer present at 5 weeks of age, it was possible to induce severe lesions and, different to SPF layers, a drop in body weight gain. Such findings were confirmed by our own investigations in SPF broilers, which showed a more pronounced effect on body weight than commercial birds with maternal antibodies (Grafl et al., Citation2013). Applying real-time PCR, it could also be demonstrated that the viral load in the gizzard was higher than in the liver, emphasising a certain aberrant tissue tropism of FAdV-1. The finding that lesions can also be induced in older birds up to 53 d of age, completes the range of experimental data. Altogether, the animal experiments in combination with the applied laboratory methods, especially the confirmation of FAdV-1 virus in histological lesions from where it could be isolated, confirmed the aetiology of AGE and the fulfilment of Koch’s Postulates. Furthermore, the results indicate that the pathogenesis of AGE is somewhat different to IBH and HHS with consequences on protection. The absence of lesions following oral reinfection (Ono et al., Citation2004; Grafl et al., Citation2014) highlights the importance of a local immune response and closes the circle with the initial observation reported by Clemmer 50 years ago (Clemmer, Citation1965).
Conclusions and outlook
Based on the work of Robert Koch at the end of the nineteenth century, criteria were published which should be applied to determine the pathogenic potential of a certain microorganism, in order to define its status as a pathogen. The strength of such criteria is reflected by the fact that they are named “Postulates” and they still symbolise the basic principles of host–pathogen interaction and act as a gold standard in infection biology.
Since the first description of parasites, bacteria and viruses, laboratory techniques and methodologies have improved considerably. Genetic tools and the whole panel of techniques supplied by molecular biology and gene technology can nowadays be applied to characterise microorganisms and their life cycle more precisely, taken together these techniques are helpful to resolve and refine aetiologies. Despite this, the interaction of certain microorganisms with the host is poorly understood and studies to clarify functional aspects are still in their infancy. As a consequence, numerous questions remain to be addressed in order to resolve basic mechanisms influencing pathogenicity as summarised in this article and outlined in the for a parasite, bacterium and virus. H. meleagridis is a good example of how complex the host–pathogen interaction can be, considering that additional bacteria are needed to establish infection and to reproduce the disease. Resolving this interaction is also of vital importance for the field, considering the use of antimicrobial substances with (un-)targeted influence on the microbiota in vivo. As a consequence of this, more attention has to be paid in the future to include a broader range of parameters involved in pathogenicity, including microbiota (Byrd and Segre, Citation2016). Nevertheless, changes in husbandry and management procedures might influence the appearance of certain microorganisms and the severity of clinical signs, altogether not in the focus of Koch’s Postulates.
Figure. Parameters influencing experimental host–pathogen interaction of H. meleagridis, Gallibacterium anatis and fowl aviadenoviruses.
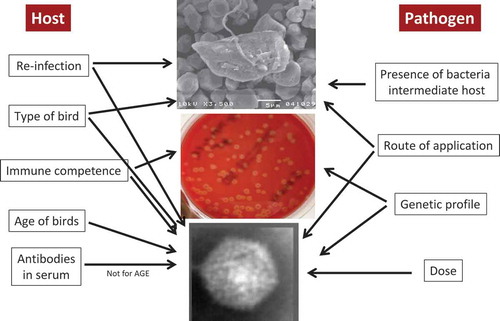
In recent years, legislation and regulations about animal experiments became stricter and ethical issues are of increasing importance with the focus on three Rs, refinement, replacement and reduction. Without doubt, certain animal experiments cited above would never pass an ethical committee today or would have been accepted for publication. Although regulations became much stricter in recent years, and that one out of two turkeys stolen in an initial animal experiment to reproduce histomonosis (Graybill and Smith, Citation1920) seems bizarre, animal research today – and in the future – will face constant challenges. Harmonisation and standardisation of animal experiments has a certain priority to compare results more accurately, keeping in mind that certain variables will always remain. Exchange of inocula and precise protocols would help to increase robustness – and acceptance – of findings. Publishing of “negative” results is not widely practiced due to the low acceptance in the scientific community but it would help to prevent duplication of animal experiments. Furthermore, the examples given here also highlight the need for animal experiments as the pathogen–host interaction cannot be substituted by in vitro technologies. However, in order to improve the outcome of animal experiments, the design and sampling schemes should be critically reviewed in order to include different disciplines. Combining expertise from different areas, infection biology, genetics, nutrition and husbandry will largely fulfil this demand. Altogether, it should help to elucidate aetiologies in a broader context, something not in the scope of Robert Koch in the nineteenth century. Despite this, numerous difficulties will remain to mimic the complex situation of a chicken house in an experimental setting.
Acknowledgements
The author would like to thank the Robert Fraser Gordon Memorial Trust and the Trustees for being nominated to present the Gordon Memorial Lecture in 2016. Special thanks to all coworkers and cooperation partners, their contribution was vital for the success of the referenced studies. The substantial contribution of various funding agencies was crucial to transfer certain ideas from theory into practice.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Abe, T., Nakamura, K., Tojo, H., Mase, M., Shibahara, T., Yamaguchi, S. & Yuasa, N. (1998) Histology, immunohistochemistry, and ultrastructure of hydropericardium syndrome in adult broiler breeders and broiler chicks. Avian Diseases, 42: 606–612. doi:10.2307/1592690
- Addo, P.B. & Mohan, K. (1985) Atypical Pasteurella haemolytica type A from poultry. Avian Diseases, 29: 214–217. doi:10.2307/1590710
- Al-Sheikhly, F. & Mutalib, A.A. (1979) Inclusion body hepatitis in broiler chickens in Iraq. Avian Diseases, 23: 763–767. doi:10.2307/1589755
- Anjum, A.D. (1990) Experimental transmission of hydropericardium syndrome and protection against it in commercial broiler chickens. Avian Pathology, 19: 655–660. doi:10.1080/03079459008418721
- Anjum, A.D., Sabri, M.A. & Iqbal, Z. (1989) Hydropericarditis syndrome in broiler chickens in Pakistan. Veterinary Record, 124: 247–248. doi:10.1136/vr.124.10.247
- Asthana, M., Chandra, R. & Kumar, R. (2013) Hydropericardium syndrome: current state and future developments. Archives of Virology, 158: 921–931. doi:10.1007/s00705-012-1570-x
- Bager, R.J., Nesta, B., Pors, S.E., Soriani, M., Serino, L., Boyce, J.D., Adler, B. & Bojesen, A.M. (2013) The fimbrial protein FlfA from Gallibacterium anatis is a virulence factor and vaccine candidate. Infection and Immunity, 81: 1964–1973. doi:10.1128/IAI.00059-13
- Balamurugan, V. & Kataria, J.M. (2004) The hydropericardium syndrome in poultry – a current scenario. Veterinary Research Communications, 28: 127–148. doi:10.1023/B:VERC.0000012115.86894.1e
- Bayon, H.P. & Bishop, A. (1937) Cultivation of Histomonas meleagridis from the liver lesions of a hen. Nature, 139: 370–371. doi:10.1038/139370b0
- Bilic, I., Jaskulska, B., Souillard, R., Liebhart, D. & Hess, M. (2014) Multi-locus typing of Histomonas meleagridis isolates demonstrates the existence of two different genotypes. PLoS One, 9: e92438. doi:10.1371/journal.pone.0092438
- Bisgaard, M. (1977) Incidence of Pasteurella haemolytica in the respiratory tract of apparently healthy chickens and chickens with infectious bronchitis. characterisation of 213 strains. Avian Pathology, 6: 285–292. doi:10.1080/03079457708418238
- Bisgaard, M., Korczak, B.M., Busse, H.-J., Kuhnert, P., Bojesen, A.M. & Christensen, H. (2009) Classification of the taxon 2 and taxon 3 complex of Bisgaard within Gallibacterium and description of Gallibacterium melopsittaci sp. nov., Gallibacterium trehalosifermentans sp. nov. and Gallibacterium salpingitidis sp. nov. International Journal of Systematic and Evolutionary Microbiology, 59: 735–744. doi:10.1099/ijs.0.005694-0
- Bishop, A. (1938) Histomonas meleagridis in domestic fowls (Gallus gallus) cultivation and experimental infection. Parasitology, 30: 181–194. doi:10.1017/S0031182000025749
- Bojesen, A.M., Nielsen, O.L., Christensen, J.P. & Bisgaard, M. (2004) In vivo studies of Gallibacterium anatis infection in chickens. Avian Pathology, 33: 145–152. doi:10.1080/03079450310001652059
- Bojesen, A.M., Nielsen, S.S. & Bisgaard, M. (2003) Prevalence and transmission of haemolytic Gallibacterium species in chicken production systems with different biosecurity levels. Avian Pathology, 32: 503–510. doi:10.1080/0307945031000154107
- Bradley, R.E. & Reid, W.M. (1966) Histomonas meleagridis and several bacteria as agents of infectious enterohepatitis in gnotobiotic turkeys. Experimental Parasitology, 19: 91–101. doi:10.1016/0014-4894(66)90057-9
- Byrd, A.L. & Segre, J.A. (2016) Infectious disease. Adapting Koch’s postulates. Science, 351: 224–226. doi:10.1126/science.aad6753
- Cepicka, I., Hampl, V. & Kulda, J. (2010) Critical taxonomic revision of Parabasalids with description of one new genus and three new species. Protist, 161: 400–433. doi:10.1016/j.protis.2009.11.005
- Chandra, R., Shukla, S.K., Kumar, M. & Garg, S.K. (1997) Electron microscopic demonstration of an adenovirus in the hepatocytes of birds experimentally infected with hydropericardium syndrome. Veterinary Record, 140: 70–71. doi:10.1136/vr.140.3.70-b
- Chen, H., Dou, Y., Zheng, X., Tang, Y., Zhang, M., Zhang, Y., Wang, Z. & Diao, Y. (2016) Hydropericardium hepatitis syndrome emerged in cherry valley ducks in China. Transboundary and Emerging Diseases. doi:10.1111/tbed.12500
- Chester, F.D. & Robin, M.D. (1900) Entero-hepatitis or blackhead of fowls, pp. 60–66 (Deleware College Agricultural Experimental Station).
- Choi, K.S., Kye, S.J., Kim, J.Y., Jeon, W.J., Lee, E.K., Park, K.Y. & Sung, H.W. (2012) Epidemiological investigation of outbreaks of fowl adenovirus infection in commercial chickens in Korea. Poultry Science, 91: 2502–2506. doi:10.3382/ps.2012-02296
- Christensen, H., Bisgaard, M., Bojesen, A.M., Mutters, R. & Olsen, J.E. (2003) Genetic relationships among avian isolates classified as Pasteurella haemolytica, ‘Actinobacillus salpingitidis’ or Pasteurella anatis with proposal of Gallibacterium anatis gen. nov., comb. nov. and description of additional genomospecies within Gallibacterium gen. nov. International Journal of Systemic and Evolutionary Microbiology, 53: 275–287.
- Christensen, N.H. & Saifuddin, M. (1989) A primary epidemic of inclusion body hepatitis in broilers. Avian Diseases, 33: 622–630. doi:10.2307/1591135
- Clemmer, D.I. (1972) Age-associated changes in fecal excretion patterns of strain 93 chick embryo lethal orphan virus in chicks. Infection and Immunity, 5: 60–64.
- Clemmer, D.L. (1965) Experimental enteric infection of chickens with an avian adeno-virus (strain 93). Proceedings of the Society for Experimental Biology and Medicine, 118: 943–948. doi:10.3181/00379727-118-30013
- Cook, J.K. (1974a) Pathogenicity of avian adenoviruses for day-old chicks. Journal of Comparative Pathology, 84: 505–515. doi:10.1016/0021-9975(74)90043-7
- Cook, J.K. (1974b) Spread of an avian adenovirus (CELO virus) to uninoculated fowls. Research in Veterinary Science, 16: 156–161.
- Cook, J.K. (1983) Fowl adenoviruses: studies on aspects of the pathogenicity of six strains for 1-day-old chicks. Avian Pathology, 12: 35–43. doi:10.1080/03079458308436147
- Cortes, P.L., Chin, R.P., Bland, M.C., Crespo, R. & Shivaprasad, H.L. (2004) Histomoniasis in the bursa of fabricius of chickens. Avian Diseases, 48: 711–715. doi:10.1637/7175-030404R
- Cushman, S. (1893) The production of turkeys. Rhode Island Agricultural Experiment Station Bulletin, 25: 89–123.
- Dar, A., Gomis, S., Shirley, I., Mutwiri, G., Brownlie, R., Potter, A., Gerdts, V. & Tikoo, S.K. (2012) Pathotypic and molecular characterization of a fowl adenovirus associated with inclusion body hepatitis in Saskatchewan chickens. Avian Diseases, 56: 73–81. doi:10.1637/9764-041911-Reg.1
- Daş, G., Abel, H., Humburg, J., Schwarz, A., Rautenschlein, S., Breves, G. & Gauly, M. (2011) Non-starch polysaccharides alter interactions between Heterakis gallinarum and Histomonas meleagridis. Veterinary Parasitology, 176: 208–216. doi:10.1016/j.vetpar.2010.11.004
- Delappe, I.P. (1953) Studies on Histomonas meleagridis. I. Use of antibiotics to facilitate in vitro isolation. Experimental Parasitology, 2: 79–86. doi:10.1016/0014-4894(53)90006-X
- Devolt, H.M. (1950) The different effect of artificially and naturally induced blackhead (infectious enterohepatitis) of turkeys on the prophylactic action of one quinoline derivative. Poultry Science, 29: 924–926. doi:10.3382/ps.0290924
- Dolka, B., Żbikowski, A., Dolka, I. & Szeleszczuk, P. (2015) Histomonosis – an existing problem in chicken flocks in Poland. Veterinary Research Communications, 39: 189–195. doi:10.1007/s11259-015-9637-2
- Doll, J.P. & Franker, C.K. (1963) Experimental histomoniasis in gnotobiotic turkeys. I. Infection and histopathology of the bacteria-free host. The Journal of Parasitology, 49: 411–414. doi:10.2307/3275809
- Domanska-Blicharz, K., Tomczyk, G., Smietanka, K., Kozaczynski, W. & Minta, Z. (2011) Molecular characterization of fowl adenoviruses isolated from chickens with gizzard erosions. Poultry Science, 90: 983–989. doi:10.3382/ps.2010-01214
- Dwyer, D.M. (1972) Analysis of the antigenic relationships among trichomonas, histomonas, dientamoeba, and entamoeba. I. Quantitative fluorescent antibody methods. The Journal of Protozoology, 19: 316–325. doi:10.1111/jeu.1972.19.issue-2
- Eregae, M.E., Dewey, C.E., Mcewen, S.A., Ouckama, R., Ojkić, D. & Guerin, M.T. (2014) Flock prevalence of exposure to avian adeno-associated virus, chicken anemia virus, fowl adenovirus, and infectious bursal disease virus among Ontario broiler chicken flocks. Avian Diseases, 58: 71–77. doi:10.1637/10612-071113-Reg.1
- Erny, K.M., Barr, D.A. & Fahey, K.J. (1991) Molecular characterization of highly virulent fowl adenoviruses associated with outbreaks of inclusion body hepatitis. Avian Pathology, 20: 597–606. doi:10.1080/03079459108418799
- Esquenet, C., De Herdt, P., De Bosschere, H., Ronsmans, S., Ducatelle, R. & Van Erum, J. (2003) An outbreak of histomoniasis in free-range layer hens. Avian Pathology, 32: 305–308. doi:10.1080/0307945031000097903
- EFSA. (2012) EFSA recommends use of animal-based measures when assessing welfare. Veterinary Record, 170: 112. doi:10.1136/vr.e776
- Eveleth, D.F. (1943) Histomoniasis in broilers. Veterinary Medicine, 38: 148–149.
- Fadly, A.M., Winterfield, R.W. & Olander, H.J. (1976) Role of the bursa of Fabricius in the pathogenicity of inclusion body hepatitis and infectious bursal disease viruses. Avian Diseases, 20: 467–477. doi:10.2307/1589379
- Ganapathy, K., Salamat, M.H., Lee, C.C. & Johara, M.Y. (2000) Concurrent occurrence of salmonellosis, colibaccillosis and histomoniasis in a broiler flock fed with antibiotic-free commercial feed. Avian Pathology, 29: 639–642. doi:10.1080/03079450020016000
- Ganas, P., Liebhart, D., Glösmann, M., Hess, C. & Hess, M. (2012) Escherichia coli strongly supports the growth of Histomonas meleagridis, in a monoxenic culture, without influence on its pathogenicity. International Journal for Parasitology, 42: 893–901. doi:10.1016/j.ijpara.2012.07.007
- Ganesh, K. & Raghavan, R. (2000) Hydropericardium hepatitis syndrome of broiler poultry: current status of research. Research in Veterinary Science, 68: 201–206. doi:10.1053/rvsc.1999.0365
- Gerbod, D., Edgcomb, V.P., Noel, C., Zenner, L., Wintjens, R., Delgado-Viscogliosi, P., Holder, M.E., Sogin, M.L. & Viscogliosi, E. (2001) Phylogenetic position of the trichomonad parasite of turkeys, Histomonas meleagridis (Smith) Tyzzer, inferred from small subunit rRNA sequence. The Journal of Eukaryotic Microbiology, 48: 498–504. doi:10.1111/jeu.2001.48.issue-4
- Gerlach, H. (1977) Significance of Pasteurella haemotytica in poultry. Der Praktische Tierarzt, 58: 324–328.
- Gerth, C., Rudiger-Boesch, B., Schmidt, U., Mumme, J. & Friedhoff, K.T. (1985) [Histomoniasis in pullet stock and its effect on later laying performance]. Tierärztliche Praxis, 13: 519–527.
- Gjevre, A.-G., Kaldhusdal, M. & Eriksen, G.S. (2013) Gizzard erosion and ulceration syndrome in chickens and turkeys: a review of causal or predisposing factors. Avian Pathology, 42: 297–303. doi:10.1080/03079457.2013.817665
- Goedbloed, E. & Bool, P.H. (1962) The protozoan etiology of blackhead. Avian Diseases, 6: 302–315. doi:10.2307/1587900
- Gomis, S., Goodhope, A.R., Ojkic, A.D. & Willson, P. (2006) Inclusion body hepatitis as a primary disease in broilers in Saskatchewan, Canada. Avian Diseases, 50: 550–555. doi:10.1637/7577-040106R.1
- Goodwin, M.A., Hill, D.L., Dekich, M.A. & Putnam, M.R. (1993) Multisystemic adenovirus infection in broiler chicks with hypoglycemia and spiking mortality. Avian Diseases, 37: 625–627. doi:10.2307/1591701
- Gordon, R.F. (1967) The economic effect of disease on the poultry industry. Veterinary Record, 80: 101–107. doi:10.1136/vr.80.3.101
- Gordon, R.F. (1971) The economic effect of ill health. Veterinary Record, 89: 496–500. doi:10.1136/vr.89.19.496
- Gordon, R.F. (1973) Houghton poultry research station. Nutrition & Food Science, 73: 15–18. doi:10.1108/eb058561
- Gradmann, C. (2014) A spirit of scientific rigour: Koch’s postulates in twentieth-century medicine. Microbes and Infection, 16: 885–892. doi:10.1016/j.micinf.2014.08.012
- Grafl, B., Aigner, F., Liebhart, D., Marek, A., Prokofieva, I., Bachmeier, J. & Hess, M. (2012) Vertical transmission and clinical signs in broiler breeders and broilers experiencing adenoviral gizzard erosion. Avian Pathology, 41: 599–604. doi:10.1080/03079457.2012.740614
- Grafl, B., Liebhart, D., Günes, A., Wernsdorf, P., Aigner, F., Bachmeier, J. & Hess, M. (2013) Quantity of virulent fowl adenovirus serotype 1 correlates with clinical signs, macroscopical and pathohistological lesions in gizzards following experimental induction of gizzard erosion in broilers. Veterinary Research, 44: 38. doi:10.1186/1297-9716-44-38
- Grafl, B., Liebhart, D., Windisch, M., Ibesich, C. & Hess, M. (2011) Seroprevalence of Histomonas meleagridis in pullets and laying hens determined by ELISA. Veterinary Record, 168: 160–164. doi:10.1136/vr.c6479
- Grafl, B., Prokofieva, I., Wernsdorf, P., Steinborn, R. & Hess, M. (2014) Infection with an apathogenic fowl adenovirus serotype-1 strain (CELO) prevents adenoviral gizzard erosion in broilers. Veterinary Microbiology, 172: 177–185. doi:10.1016/j.vetmic.2014.05.020
- Graybill, H.W. & Smith, T. (1920) Production of fatal blackhead in turkeys by feeding embryonated eggs of Heterakis papillosa. Journal of Experimental Medicine, 31: 647–655. doi:10.1084/jem.31.5.647
- Greenham, L.W. & Hill, T.J. (1962) Observation on an avian strain of Pasteurella haemolytica. Veterinary Record, 74: 861–863.
- Grgic, H., Philippe, C., Ojkic, D. & Nagy, E. (2006) Study of vertical transmission of fowl adenoviruses. Canadian Journal of Veterinary Research, 70: 230–233.
- Grimes, T.M., King, D.J., Kleven, S.H. & Fletcher, O.J. (1977) Involvement of a type-8 avian adenovirus in the etiology of inclusion body hepatitis. Avian Diseases, 21: 26–38. doi:10.2307/1589361
- Hacking, W.C. & Pettit, J.R. (1974) Pasteurella hemolytica in pullets and laying hens. Avian Diseases, 18: 483–486. doi:10.2307/1589119
- Hafez, H.M. (2001) Aktuelle Gefügelkrankheiten bei Legehennen im Zusammenhang mit alternativen Haltungssystemen. Tierärztliche Praxis, 29: 168–174.
- Harbourne, J.F. (1962) A haemolytic cocco-bacillus recovered from poultry. Veterinary Record, 74: 566–577.
- Harrach, B., Benkö, M., Both, G.W., Brown, M., Davison, A.J., Echavarria, M., Hess, M., Jones, M.S., Kajon, A., Lehmkuhl, H.D., Mautner, V., Mittal, S.K. & Wadell, G. (2011) Family adenoviridae, in: King, A.M.Q., Adams, M.J., Carstens, E.B. & Lefkowitz, E.J. (Eds) Virus taxonomy: classification and nomenclature of viruses. Ninth report of the international committee on taxonomy of viruses, pp. 95–111 (San Diego, CA, Elsevier).
- Harry, E.G. (1962) A haemolytic coccobacillus recovered from poultry. Veterinary Record, 74: 640.
- Hauck, R., Balczulat, S. & Hafez, H.M. (2010) Detection of DNA of Histomonas meleagridis and Tetratrichomonas gallinarum in German poultry flocks between 2004 and 2008. Avian Diseases, 54: 1021–1025. doi:10.1637/9261-012910-Reg.1
- Hauck, R. & Hafez, H.M. (2013) Experimental infections with the protozoan parasite Histomonas meleagridis: a review. Parasitology Research, 112: 19–34. doi:10.1007/s00436-012-3190-5
- Helmboldt, C.F. & Frazier, M.N. (1963) Avian hepatic inclusion bodies of unknown significance. Avian Diseases, 7: 446–450. doi:10.2307/1587881
- Hess, M. (2000) Detection and differentiation of avian adenoviruses: a review. Avian Pathology, 29: 195–206. doi:10.1080/03079450050045440
- Hess, M. (2013) Aviadenovirus infections, in: Glisson, J.R., McDougald, L.R., Nolan, L.K., Suarez, D.L., Swayne, D. & Nair, V. (Eds) Diseases of poultry, 13th edn., pp. 290–300 (Ames, Wiley-Blackwell). ISBN:978-0-470-95899-5.
- Hess, M., Blöcker, H. & Brandt, P. (1997) The complete nucleotide sequence of the egg drop syndrome virus: an intermediate between mastadenoviruses and aviadenoviruses. Virology, 238: 145–156. doi:10.1006/viro.1997.8815
- Hess, M., Grabensteiner, E. & Liebhart, D. (2006) Rapid transmission of the protozoan parasite Histomonas meleagridis in turkeys and specific pathogen free chickens following cloacal infection with a mono-eukaryotic culture. Avian Pathology, 35: 280–285. doi:10.1080/03079450600815507
- Hess, M., Liebhart, D., Bilic, I. & Ganas, P. (2015) Histomonas meleagridis–new insights into an old pathogen. Veterinary Parasitology, 208: 67–76. doi:10.1016/j.vetpar.2014.12.018
- Hess, M., Raue, R. & Prusas, C. (1999) Epidemiological studies on fowl adenoviruses isolated from cases of infectious hydropericardium. Avian Pathology, 28: 433–439. doi:10.1080/03079459994443
- Homer, B.L. & Butcher, G.D. (1991) Histomoniasis in Leghorn pullets on a Florida farm. Avian Diseases, 35: 621–624. doi:10.2307/1591231
- Horton-Smith, C. & Long, P.L. (1956) Studies in histomoniasis 1. The infection of chickens (Gallus gallus) with histomonad suspensions. Parasitology, 46: 79–90.
- Jones, H.G. & Owen, D.M. (1981) Reproductive tract lesions of the laying fowl with particular reference to bacterial infection. Veterinary Record, 108: 36–37. doi:10.1136/vr.108.2.36
- Jones, K.H., Thornton, J.K., Zhang, Y. & Mauel, M.J. (2013) A 5-year retrospective report of Gallibacterium anatis and Pasteurella multocida isolates from chickens in Mississippi. Poultry Science, 92: 3166–3171. doi:10.3382/ps.2013-03321
- Jung, A. (2012) Hepatitis bei Legehennen, verursacht durch gleichzeitige Infektion mit Gallibacterium anatis und Ascaridia galli – Fallbericht. Praktischer Tierarzt, 93: 246–250.
- Kaján, G.L., Kecskeméti, S., Harrach, B. & Benkő, M. (2013) Molecular typing of fowl adenoviruses, isolated in Hungary recently, reveals high diversity. Veterinary Microbiology, 167: 357–363. doi:10.1016/j.vetmic.2013.09.025
- Kaufmann-Bart, M. & Hoop, R.K. (2009) Diseases in chicks and laying hens during the first 12 years after battery cages were banned in Switzerland. Veterinary Record, 164: 203–207. doi:10.1136/vr.164.7.203
- Kim, J.N., Byun, S.H., Kim, M.J., Kim, J., Sung, H.W. & Mo, I.P. (2008) Outbreaks of hydropericardium syndrome and molecular characterization of Korean fowl adenoviral isolates. Avian Diseases, 52: 526–530. doi:10.1637/8178-112207-Case
- Kjos-Hanssen, B. (1950) Egg peritonitis in hens caused by pathogenic “cloacal bacteria”. Nordisk Veterinaermedicin, 2: 523–531.
- Lesser, E. (1961) In vitro cultivation of Histomonas meleagridis free of demonstrable bacteria. The Journal of Protozoology, 8: 228–230. doi:10.1111/jeu.1961.8.issue-2
- Liebhart, D., Ganas, P., Sulejmanovic, T. & Hess, M. (2016) Histomonosis in poultry: previous and current strategies for prevention and therapy. Avian Pathology, 1–43. in press. doi:10.1080/03079457.2016.1229458
- Lim, T.-H., Lee, H.-J., Lee, D.-H., Lee, Y.-N., Park, J.-K., Youn, H.-N., Kim, M.-S., Youn, H.-S., Lee, J.-B., Park, S.-Y., Choi, I.-S. & Song, C.-S. (2011) Identification and virulence characterization of fowl adenoviruses in Korea. Avian Diseases, 55: 554–560. doi:10.1637/9730-032011-Reg.1
- Lund, E.E. (1958) Growth and development of Heterakis gallinae in turkeys and chickens infected with Histomonas meleagridis. The Journal of Parasitology, 44: 297–301. doi:10.2307/3274594
- Lund, E.E. (1969) Histomoniasis. Advances in Veterinary Science and Comparative Medicine, 13: 355–390.
- Lund, E.E., Augustine, P.C. & Ellis, D.J. (1966) Immunizing action of in vitro-attenuated Histomonas meleagridis in chickens and turkeys. Experimental Parasitology, 18: 403–407. doi:10.1016/0014-4894(66)90041-5
- Maartens, L.H., Joubert, H.W., Aitchison, H. & Venter, E.H. (2015) Inclusion body hepatitis associated with an outbreak of fowl adenovirus type 2 and type 8b in broiler flocks in South Africa. Journal of the South African Veterinary Association, 85: e1–e5.
- Matos, M., Grafl, B., Liebhart, D. & Hess, M. (2016a) The outcome of experimentally induced inclusion body hepatitis (IBH) by fowl aviadenoviruses (FAdVs) is crucially influenced by the genetic background of the host. Veterinary Research, 47: 69. doi:10.1186/s13567-016-0350-0
- Matos, M., Grafl, B., Liebhart, D., Schwendenwein, I. & Hess, M. (2016b) Selected clinical chemistry analytes correlate with the pathogenesis of inclusion body hepatitis (IBH) experimentally induced by fowl aviadenoviruses (FAdVs). Avian Pathology, 45: 1–32.
- Matthes, S., Löliger, H.-C. & Schubert, H.J. (1969) Enzootisches Auftreten der Pasteurella haemolytica beim Huhn. Deutsche Tierärztliche Wochenschrift, 89: 98–102.
- Mazaheri, A., Prusas, C., Voß, M. & Hess, M. (1998) Some strains of serotype 4 fowl adenoviruses cause inclusion body hepatitis and hydropericardium syndrome in chickens. Avian Pathology, 27: 269–276. doi:10.1080/03079459808419335
- Mcdougald, L.R. (2005) Blackhead disease (histomoniasis) in poultry: a critical review. Avian Diseases, 49: 462–476. doi:10.1637/7420-081005R.1
- Mcferran, J.B. & Adair, B.M. (1977) Avian adenoviruses – a review. Avian Pathology, 6: 189–217. doi:10.1080/03079457708418228
- Mendelson, C., Nothelfer, H.B. & Monreal, G. (1995) Identification and characterization of an avian adenovirus isolated from a ‘spiking mortality syndrome’ field outbreak in broilers on the Delmarva Peninsula, USA. Avian Pathology, 24: 693–706. doi:10.1080/03079459508419108
- Mirle, C., Schöngarth, M., Meinhart, H. & Olm, U. (1991) Untersuchungen zu Auftreten und Bedeutung von Pasteurella haemolytica-Infektionen bei Hennen unter besonderer Berücksichtigung von Erkrankungen des Legeapparates. Monatshefte Veterinärmedizin, 46: 545–549.
- Mittal, D., Jindal, N., Tiwari, A.K. & Khokhar, R.S. (2014) Characterization of fowl adenoviruses associated with hydropericardium syndrome and inclusion body hepatitis in broiler chickens. Virusdisease, 25: 114–119. doi:10.1007/s13337-013-0183-7
- Müller, H. (1990) Enzootische Typhlohepatitis bei intensiv gehaltenen Junghennen. Monatshefte Veterinärmedizin, 45: 464–467.
- Mushin, R., Weisman, Y. & Singer, N. (1980) Pasteurella haemolytica found in the respiratory tract of fowl. Avian Diseases, 24: 162–168. doi:10.2307/1589775
- Naeem, K. & Akram, H.S. (1995) Hydropericardium syndrome outbreak in a pigeon flock. Veterinary Record, 136: 296–297. doi:10.1136/vr.136.12.296
- Nakamura, K., Mase, M., Yamaguchi, S., Shibahara, T. & Yuasa, N. (1999) Pathologic study of specific-pathogen-free chicks and hens inoculated with adenovirus isolated from hydropericardium syndrome. Avian Diseases, 43: 414–423. doi:10.2307/1592638
- Nakamura, K., Mase, M., Yamaguchi, S. & Yuasa, N. (2000) Induction of hydropericardium in one-day-old specific-pathogen-free chicks by adenoviruses from inclusion body hepatitis. Avian Diseases, 44: 192–196. doi:10.2307/1592524
- Nakamura, K., Mase, M., Yamamoto, Y., Takizawa, K., Kabeya, M., Wakuda, T., Matsuda, M., Chikuba, T., Yamamoto, Y., Ohyama, T., Takahashi, K., Sato, N., Akiyama, N., Honma, H. & Imai, K. (2011) Inclusion body hepatitis caused by fowl adenovirus in broiler chickens in Japan, 2009–2010. Avian Diseases, 55: 719–723. doi:10.1637/9813-052511-Case.1
- Nakamura, K., Ohyama, T., Yamada, M., Abe, T., Tanaka, H. & Mase, M. (2002) Experimental gizzard erosions in specific-pathogen-free chicks by serotype 1 group I avian adenoviruses from broilers. Avian Diseases, 46: 893–900. doi:10.1637/0005-2086(2002)046[0893:EGEISP]2.0.CO;2
- Nakamura, K., Shoyama, T., Mase, M., Imada, T. & Yamada, M. (2003) Reproduction of hydropericardium syndrome in three-week-old cyclophosphamide-treated specific-pathogen-free chickens by adenoviruses from inclusion body hepatitis. Avian Diseases, 47: 169–174. doi:10.1637/0005-2086(2003)047[0169:ROHSIT]2.0.CO;2
- Neubauer, C., De Souza-Pilz, M., Bojesen, A.M., Bisgaard, M. & Hess, M. (2009) Tissue distribution of haemolytic Gallibacterium anatis isolates in laying birds with reproductive disorders. Avian Pathology, 38: 1–7. doi:10.1080/03079450802577848
- Nguyen, D.T., Bilic, I., Jaskulska, B., Hess, M., Le, D.Q., Le Hua, L.N., Huynh, V.V., Nguyen, S.T. & Vu-Khac, H. (2015) Prevalence and genetic characterization of Histomonas meleagridis in chickens in Vietnam. Avian Diseases, 59: 309–314. doi:10.1637/10964-102414-Reg
- Niczyporuk, J.S. (2016) Phylogenetic and geographic analysis of fowl adenovirus field strains isolated from poultry in Poland. Archives of Virology, 161: 33–42. doi:10.1007/s00705-015-2635-4
- Ojkic, D., Krell, P.J., Tuboly, T. & Nagy, E. (2008a) Characterization of fowl adenoviruses isolated in Ontario and Quebec, Canada. Canadian Journal of Veterinary Research, 72: 236–241.
- Ojkic, D., Martin, E., Swinton, J., Vaillancourt, J.-P., Boulianne, M. & Gomis, S. (2008b) Genotyping of Canadian isolates of fowl adenoviruses. Avian Pathology, 37: 95–100. doi:10.1080/03079450701805324
- Okuda, Y., Ono, M., Shibata, I. & Sato, S. (2004) Pathogenicity of serotype 8 fowl adenovirus isolated from gizzard erosions of slaughtered broiler chickens. Journal of Veterinary Medical Science, 66: 1561–1566. doi:10.1292/jvms.66.1561
- Okuda, Y., Ono, M., Yazawa, S., Imai, Y., Shibata, I. & Sato, S. (2001a) Pathogenicity of serotype 1 fowl adenovirus in commercial broiler chickens. Avian Diseases, 45: 819–827. doi:10.2307/1592862
- Okuda, Y., Ono, M., Yazawa, S., Shibata, I. & Sato, S. (2001b) Experimental infection of specific-pathogen-free chickens with serotype-1 fowl adenovirus isolated from a broiler chicken with gizzard erosions. Avian Diseases, 45: 19–25. doi:10.2307/1593007
- Olsen, R.H., Stockholm, N.M., Permin, A., Christensen, J.P., Christensen, H. & Bisgaard, M. (2011) Multi-locus sequence typing and plasmid profile characterization of avian pathogenic Escherichia coli associated with increased mortality in free-range layer flocks. Avian Pathology, 40: 437–444. doi:10.1080/03079457.2011.592822
- Ono, M., Okuda, Y., Shibata, I., Sato, S. & Okada, K. (2004) Pathogenicity by parenteral injection of fowl adenovirus isolated from gizzard erosion and resistance to reinfection in adenoviral gizzard erosion in chickens. Veterinary Pathology, 41: 483–489. doi:10.1354/vp.41-5-483
- Ono, M., Okuda, Y., Shibata, I., Sato, S. & Okada, K. (2007) Reproduction of adenoviral gizzard erosion by the horizontal transmission of fowl adenovirus serotype 1. Journal of Veterinary Medical Science, 69: 1005–1008. doi:10.1292/jvms.69.1005
- Ono, M., Okuda, Y., Yazawa, S., Shibata, I., Sato, S. & Okada, K. (2003) Outbreaks of adenoviral gizzard erosion in slaughtered broiler chickens in Japan. Veterinary Record, 153: 775–779.
- Ono, M., Okuda, Y., Yazawa, S., Shibata, I., Tanimura, N., Kimura, K., Haritani, M., Mase, M. & Sato, S. (2001) Epizootic outbreaks of gizzard erosion associated with adenovirus infection in chickens. Avian Diseases, 45: 268–275. doi:10.2307/1593040
- Pallister, J., Wright, P.J. & Sheppard, M. (1996) A single gene encoding the fiber is responsible for variations in virulence in the fowl adenoviruses. Journal of Virology, 70: 5115–5122.
- Paudel, S., Alispahic, M., Liebhart, D., Hess, M. & Hess, C. (2013) Assessing pathogenicity of Gallibacterium anatis in a natural infection model: the respiratory and reproductive tracts of chickens are targets for bacterial colonization. Avian Pathology, 42: 527–535. doi:10.1080/03079457.2013.843160
- Paudel, S., Hess, C., Wernsdorf, P., Käser, T., Meitz, S., Jensen-Jarolim, E., Hess, M. & Liebhart, D. (2015) The systemic multiplication of Gallibacterium anatis in experimentally infected chickens is promoted by immunosuppressive drugs which have a less specific effect on the depletion of leukocytes. Veterinary Immunology and Immunopathology, 166: 22–32. doi:10.1016/j.vetimm.2015.05.001
- Paudel, S., Liebhart, D., Aurich, C., Hess, M. & Hess, C. (2014a) Pathogenesis of Gallibacterium anatis in a natural infection model fulfils Koch’s postulates: 2. Epididymitis and decreased semen quality are the predominant effects in specific pathogen free cockerels. Avian Pathology, 43: 529–534. doi:10.1080/03079457.2014.967176
- Paudel, S., Liebhart, D., Hess, M. & Hess, C. (2014b) Pathogenesis of Gallibacterium anatis in a natural infection model fulfils Koch’s postulates: 1. Folliculitis and drop in egg production are the predominant effects in specific pathogen free layers. Avian Pathology, 43: 443–449. doi:10.1080/03079457.2014.955782
- Pedersen, I.J., Pors, S.E., Bager Skjerning, R.J., Nielsen, S.S. & Bojesen, A.M. (2015) Immunogenic and protective efficacy of recombinant protein GtxA-N against Gallibacterium anatis challenge in chickens. Avian Pathology, 44: 386–391. doi:10.1080/03079457.2015.1069259
- Persson, G. & Bojesen, A.M. (2015) Bacterial determinants of importance in the virulence of Gallibacterium anatis in poultry. Veterinary Research, 46: 57. doi:10.1186/s13567-015-0206-z
- Pettit, J.R. & Carlson, H.C. (1972) Inclusion-body hepatitis in broiler chickens. Avian Diseases, 16: 858–863. doi:10.2307/1588767
- Popp, C., Hauck, R., Balczulat, S. & Hafez, H.M. (2011) Recurring histomonosis on an organic farm. Avian Diseases, 55: 328–330. doi:10.1637/9596-110810-Case.1
- Pors, S.E., Skjerning, R.B., Flachs, E.M. & Bojesen, A.M. (2016) Recombinant proteins from Gallibacterium anatis induces partial protection against heterologous challenge in egg-laying hens. Veterinary Research, 47: 36. doi:10.1186/s13567-016-0320-6
- Reid, W.M. (1967) Etiology and dissemination of the blackhead disease syndrome in turkeys and chickens. Experimental Parasitology, 21: 249–275. doi:10.1016/0014-4894(67)90087-2
- Saifuddin, M. & Wilks, C.R. (1991) Pathogenesis of an acute viral hepatitis: inclusion body hepatitis in the chicken. Archives of Virology, 116: 33–43. doi:10.1007/BF01319229
- Saifuddin, M., Wilks, C.R. & Murray, A. (1992) Characterisation of avian adenoviruses associated with inclusion body hepatitis. Newzealand Veterinary Journal, 40: 52–55. doi:10.1080/00480169.1992.35697
- Schachner, A., Marek, A., Grafl, B. & Hess, M. (2016) Detailed molecular analyses of the hexon loop-1 and fibers of fowl aviadenoviruses reveal new insights into the antigenic relationship and confirm that specific genotypes are involved in field outbreaks of inclusion body hepatitis. Veterinary Microbiology, 186: 13–20. doi:10.1016/j.vetmic.2016.02.008
- Schonewille, E., Singh, A., Göbel, T.W., Gerner, W., Saalmüller, A. & Hess, M. (2008) Fowl adenovirus (FAdV) serotype 4 causes depletion of B and T cells in lymphoid organs in specific pathogen-free chickens following experimental infection. Veterinary Immunology and Immunopathology, 121: 130–139. doi:10.1016/j.vetimm.2007.09.017
- Schulze, H.W. (1975) Über das Auftreten von Schwarzkopfkrankheit bei Legehennenaufzuchten in Norddeutschland. Der Praktische Tierarzt, 3: 164–168.
- Smith, T. (1895) An infectious disease among turkeys caused by protozoa (infectious entero-hepatitis). US Department of Agriculture, Bureau of Animal Industry Bulletin, 8: 1–38.
- Springer, W.T., Johnson, J. & Reid, W.M. (1970) Histomoniasis in gnotobiotic chickens and turkeys: biological aspects of the role of bacteria in the etiology. Experimental Parasitology, 28: 383–392. doi:10.1016/0014-4894(70)90106-2
- Steer, P.A., O’rourke, D., Ghorashi, S.A. & Noormohammadi, A.H. (2011) Application of high-resolution melting curve analysis for typing of fowl adenoviruses in field cases of inclusion body hepatitis. Australian Veterinary Journal, 89: 184–192. doi:10.1111/j.1751-0813.2011.00695.x
- Steer, P.A., Sandy, J.R., O’rourke, D., Scott, P.C., Browning, G.F. & Noormohammadi, A.H. (2015) Chronological analysis of gross and histological lesions induced by field strains of fowl adenovirus serotypes 1, 8b and 11 in one-day-old chickens. Avian Pathology, 44: 106–113. doi:10.1080/03079457.2015.1007919
- Stokholm, N.M., Permin, A., Bisgaard, M. & Christensen, J.P. (2010) Causes of mortality in commercial organic layers in Denmark. Avian Diseases, 54: 1241–1250. doi:10.1637/9375-041910-Reg.1
- Swayne, D., Glisson, J.R., Mcdougald, L.R., Nolan, L.K., Suarez, D.L. & Nair, V. (Eds). (2013) Diseases of poultry, 13th edn. (Ames, Wiley-Blackwell). ISBN:978-0-470-95899-5.
- Tanimura, N., Nakamura, K., Imai, K., Maeda, M., Gobo, T., Nitta, S., Ishihara, T. & Amano, H. (1993) Necrotizing pancreatitis and gizzard erosion associated with adenovirus infection in chickens. Avian Diseases, 37: 606–611. doi:10.2307/1591697
- Toro, H., Gonzalez, O., Escobar, C., Cerda, L., Morales, M.A. & Gonzalez, C. (2001) Vertical induction of the inclusion body hepatitis/hydropericardium syndrome with fowl adenovirus and chicken anemia virus. Avian Diseases, 45: 215–222. doi:10.2307/1593031
- Tyzzer, E.E. (1920) The flagellate character and reclassification of the parasite producing “blackhead” in turkeys-Histomonas (gen.nov.) meleagridis (Smith). The Journal of Parasitology, 6: 124–131. doi:10.2307/3271065
- Tyzzer, E.E. (1934) Studies on histomoniasis, or “Blackhead” infection, in the chicken and the turkey. Proceedings of the American Academy of Arts and Sciences, 69: 189–264. doi:10.2307/20023041
- Van Der Heijden, H.M. & Landman, W.J. (2011) High seroprevalence of Histomonas meleagridis in Dutch layer chickens. Avian Diseases, 55: 324–327. doi:10.1637/9609-120610-ResNote.1
- Von Bülow, V., Rudolph, R. & Fuchs, B. (1986) [Sequelae of concomitant infection of chicks with adenovirus or reovirus and the agent of avian infectious anemia (CAA)]. Zentralblatt Veterinärmedizin B, 33: 717–726.
- Wilson, F.D., Wills, R.W., Senties-Cue, C.G., Maslin, W.R., Stayer, P.A. & Magee, D.L. (2010) High incidence of glomerulonephritis associated with inclusion body hepatitis in broiler chickens: routine histopathology and histomorphometric studies. Avian Diseases, 54: 975–980. doi:10.1637/9050-090709-Reg.1
- Yates, V.J. & Fry, D.E. (1957) Observations on a chicken embryo lethal orphan (CELO) virus. American Journal of Veterinary Research, 18: 657–660.
- Zepeda, V.A., Calderón-Apodaca, N.L., Paasch, M.L., Martín, P.G., Paredes, D.A., Ramírez-Apolinar, S. & Soriano-Vargas, E. (2010) Histopathologic findings in chickens experimentally infected with Gallibacterium anatis by nasal instillation. Avian Diseases, 54: 1306–1309. doi:10.1637/9423-061410-ResNote.1
- Zhao, J., Zhong, Q., Zhao, Y., Hu, Y.-X., Zhang, G.-Z. & Devlin, J. (2015) Pathogenicity and complete genome characterization of fowl adenoviruses isolated from chickens associated with inclusion body hepatitis and hydropericardium syndrome in China. PLoS One, 10: e0133073. doi:10.1371/journal.pone.0133073
- Zsak, L. & Kisary, J. (1981) Studies on egg drop syndrome (EDS) and chick embryo lethal orphan (CELO) avian adenovirus DNAs by restriction endonucleases. Journal of General Virology, 56: 87–95. doi:10.1099/0022-1317-56-1-87

