 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
1. The two red grain sorghums were extensively characterised. Kafirin, polyphenolic compounds, free, conjugated and bound phenolic acids, phytate concentrations and starch pasting profiles were determined.
2. The experiment consisted of a 2 × 4 factorial array of dietary treatments comprising two red sorghum varieties (Tiger and Block I) ground through 4 hammer-mill screen sizes (2.0, 3.2, 4.8 6.0 mm) prior to incorporation into nutritionally equivalent diets. Eight steam-pelleted dietary treatments were each offered to 7 replicates (6 male Ross 308 birds per cage) from 7 to 28 d post-hatch.
3. Effects of dietary treatments on growth performance, relative gizzard and pancreas weights, nutrient utilisation, apparent starch and protein (N) digestibility coefficients and disappearance rates from 4 small intestinal segments were determined.
4. The 2.0-mm hammer-mill screen generated an average geometric mean particle size of 794 μm and the 6.0-mm screen a mean particle size of 1405 μm. However, hammer-mill screen size did not influence weight gain or FCR. The 6.0-mm screen size generated significantly higher starch and protein (N) digestibility coefficients in the distal jejunum and distal ileum than the 2.0-mm hammer-mill screen.
5. Tiger sorghum was superior to Block I sorghum, as significant advantages were observed for feed conversion ratios (3.25%), AME (0.37 MJ), ME:GE ratios (4.15%), AMEn (0.53 MJ), distal ileal starch digestibility coefficients (2.46%) and protein (N) digestibility coefficients in the distal jejunum (4.66%), proximal ileum (1.96%) and distal ileum (2.16%). The inferior Block I sorghum contained more kafirin (67.1 versus 51.3 g/kg), phytate (9.79 versus 8.40 g/kg), total phenolic compounds (4.68 versus 4.12 mg GAE/g), flavan-4-ols (7.98 versus 5.04 ABS/ml/g), total phenolic acids (554 versus 402 μg/g) and total ferulic acid (375 versus 281 μg/g) in comparison to Tiger sorghum.
Introduction
This study was part of a project designed to investigate the digestibility and utilisation of sorghum starch by broiler chickens. Reported digestibilities of sorghum starch in broiler chickens are inferior to those of maize (Truong et al., Citation2015a). The effects of grain particle size on broiler growth performance in steam-pelleted diets are less pronounced than in mash diets (Amerah et al., Citation2007). However, Liu et al. (Citation2013) suggested that particle size reductions of grain sorghum may enhance nutrient digestion, energy utilisation and growth performance in broiler chickens. This suggestion was based on a series of three separate, but very similar, feeding studies (Selle et al., Citation2012, Citation2013, Citation2014) in which identical diets were offered to broiler chickens that were based on the same white sorghum (Liberty) variety. This sorghum was ground through different hammer-mill screen sizes of 2.0, 3.2 and 6.0 mm in the three studies prior to being incorporated into complete steam-pelleted diets. Collectively, the outcomes indicated a quadratic relationship between hammer-mill screen size and feed conversion ratios (FCR) and for this particular grain sorghum a hammer-mill screen size in the order of 3.75 mm was optimal for FCR.
In practice, sorghums with red pericarps are far more common than white sorghums. Therefore, the primary objective of the present study was to confirm the effects of particle size on growth performance and nutrient utilisation and to determine optimal hammer-mill screen sizes for two red sorghum varieties, namely “Block I” and “Tiger”. The secondary objective was to compare the performance of broiler chickens offered diets based on these two sorghum varieties as they had been extensively characterised. The relevant data include concentrations of kafirin, polyphenols, phenolic acids, phytate and RVA starch pasting profiles of the two sorghum varieties.
Materials and methods
Block I sorghum was harvested in the Murrumbidgee Irrigation Area (NSW, Australia) in 2012 and Tiger was harvested in the same region the following year. Both sorghums were ground through 2.0-, 3.2-, 4.8- and 6.0-mm hammer-mill screen sizes prior to incorporation into nutritionally equivalent, steam-pelleted diets. It was anticipated that the optimal screen size would lie within this range.
The two sorghum varieties were very extensively characterised as is evident in and . This included quantification of kafirin, phytate, total phenolic compounds, various polyphenols, free, conjugated and bound phenolic acids, RVA starch pasting profiles and particle size index (PSI) grain textures (Symes, Citation1965). The analysis method to quantify kafirin was adapted from procedures developed by Wallace et al. (Citation1990) and Hamaker et al. (Citation1995) and has been described in Truong et al. (Citation2015b). The complex analytical methods to quantify a range of phenolic compounds have been described in detail in Khoddami et al. (Citation2013, Citation2015). The Clorox bleach test (Waniska et al., Citation1992) did not detect a pigmented testa in both grain sorghums which indicates that they did not contain condensed tannin. Phytate (IP6) and phytate-P concentrations were determined by a HPLC procedure and total phosphorus (P) and other minerals were determined by inductively coupled plasma mass spectrometry (ICP-MS).
Table 1. NIR AusScan results, concentrations of protein, amino acids, kafirin, minerals and phytate, grain texture and RVA starch pasting profiles of Block I and Tiger sorghum varieties.
Table 2. Concentrations of total phenolic compounds, polyphenols, free, conjugated, bound and total phenolic acids and ferulic acid in Block I and Tiger sorghum.
The starch pasting profiles were determined using a Rapid-Visco-Analyser as outlined by Hernandez et al. (Citation2008). A 28-g mixture of sorghum (or diet) and water (15:85 w/w) was prepared and held at 50°C temperature for 1 min and then heated from 50°C to 95°C. After holding the hot paste at 95°C for 2.5 min, the slurry was again cooled to 50°C, and then held at that temperature for 2 min with a total time interval of 13 min.
The geometric mean particle size of both sorghums following hammer-milling were determined as shown in . The mean particle size ranged from 794 μm with the 2.0-mm screen to 1405 μm with the 6.0-mm hammer-mill screen.
Table 3. Geometric mean particle sizes of two sorghum varieties, Tiger and Block I, following grinding through four hammer-mill screen sizes.
On the basis of the data, two nutritionally equivalent diets containing 620 g/kg of either Tiger or Block I sorghums were formulated on the basis of digestible amino acid concentrations with energy densities of 12.95 MJ/kg (). The diets contained 20.0 g/kg Celite® as an acid-insoluble-ash (AIA) inert dietary marker. Diets were steam pelleted at a conditioning temperature of 84°C and crumbled after both sorghum grains were ground through 4 hammer-mill screen sizes. Feather-sexed, male broiler chicks (Ross 308) were housed in an environmentally controlled facility and were initially offered a proprietary starter ration. Birds had unlimited access to feed and water under a “23-h on-1-h off” lighting regimen. The birds were individually identified (wing bands) and weighed at d 7 and distributed amongst cages so that mean body weights in each cage and their variations were nearly identical. The 8 dietary treatments were offered to 7 replicates (6 birds per replicate cage), or a total of 336 birds, from 7 to 28 d post-hatch. Body weights were determined on d 7 and 28 and feed intakes recorded over the entire period to calculate FCR with adjustments made from the weight of any dead or culled birds, which were monitored on a daily basis. Total excreta were collected from 23 to 26 d post-hatch from each cage to determine parameters of nutrient utilisation, which included apparent metabolisable energy (AME), metabolisable energy to gross energy ratios (ME:GE), nitrogen (N) retention and N-corrected AME (AMEn). On d 28, the birds were killed (intravenous injection of Na pentobarbitone) and digesta samples were collected in their entirety from 4 segments of the small intestine to determine nutrient (starch and crude protein) digestibility coefficients and nutrient disappearance rates (g/bird/d). The 4 small intestinal segments included the proximal jejunum, distal jejunum, proximal ileum and distal ileum.
Table 4. Dietary composition and nutrient specifications of sorghum-based diets based on Tiger and block I varieties.
The total excreta collection method over a 72-h period was used to determine AME on a dry matter basis, ME:GE ratios, N retention and AMEn. Total excreta were quantitatively collected from each cage and feed intakes recorded for the 72-h collection period. Excreta were dried in a forced-air oven at 80°C for 24 h and the GE of excreta and diets were determined using an adiabatic bomb calorimeter. The AME values of the diets on a dry matter basis were calculated from the following equation:
ME:GE Ratios were calculated by dividing AME by the GE of the appropriate diets. N contents of diets and excreta were determined using a nitrogen determinator (Leco Corporation, St Joseph, MI) and N retentions calculated from the following equation:
N-corrected AME (AMEn MJ/kg DM) values were calculated by correcting N retention to zero using the factor of 36.54 kJ/g N retained in the body (Hill and Anderson, Citation1958).
Acid insoluble ash (Celite) was included in diets at 2% as an inert marker to determine starch and N digestibility. The small intestines were removed from killed birds and samples of digesta were gently expressed from the proximal jejunum, distal jejunum, proximal ileum and distal ileum in their entirety and pooled for each cage. Proximal jejunal samples were taken from the end of the duodenal loop to the midpoint with Meckel’s diverticulum and distal jejunal samples from the midpoint to the diverticulum. Proximal ileal samples were taken from Meckel’s diverticulum to the midpoint with the ileocaecal junction and distal ileal samples were taken from below this midpoint. The digesta samples were freeze-dried to determine apparent digestibilities of starch and crude protein (N) using acid insoluble ash (AIA) as the inert dietary marker. Starch concentrations in diets and digesta were determined by a procedure based on dimethyl sulphoxide, α-amylase and amyloglucosidase as described by Mahasukhonthachat et al. (Citation2010). N concentrations were determined as already stated and AIA concentrations were determined by the method of Siriwan et al. (Citation1993). The apparent digestibility coefficients for starch and protein (N) at up to 4 small intestinal sites were calculated from the following equation:
Starch and protein (N) disappearance rates (g/bird/d) were deduced from feed intakes over the final phase of the feeding period from the following equation:
Ratios of starch to protein disappearance rates in the intestinal segments were calculated as this effectively cancels the potential confounding influence of feed intake.
Experimental data were analysed using the IBM® SPSS® Statistics 20 program (IBM Corporation. Somers, NY). The experimental units were cage means and statistical procedures included univariate analyses of variance using the general linear models procedure, Pearson correlations and single and multiple linear regressions and quadratic regressions. A probability level of less than 5% was considered to be statistically significant. The feeding studies complied with specific guidelines approved by the Animal Ethics Committee of Sydney University.
Results
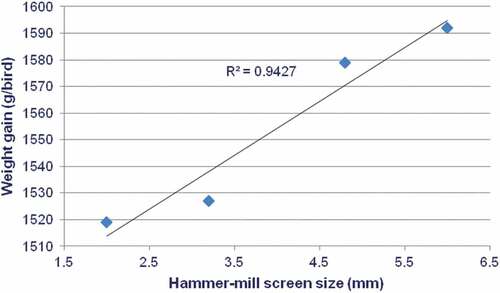
The effects of sorghum variety and hammer-mill screen size on 7 to 28 d post-hatch growth performance of broilers are shown in ; there was a mortality/cull rate of 6.25% but it was unrelated to treatment. Hammer-mill screen sizes did not significantly influence growth performance; however, the interaction between sorghum variety and hammer-mill screen size closely approached significance (P = 0.056) for weight gain. This was because weight gains for Block I sorghum remained relatively constant in diets with different particle sizes; whereas, weight gains with Tiger sorghum-based diets appeared to increase with larger hammer-mill screen sizes. When Tiger sorghum was ground through a 6.0-mm hammer-mill screen (particle size: 1402 μm) as opposed to a 2.0-mm screen (particle size: 783 μm), there was a 4.81% increase in weight gain (1592 versus 1519 g/bird; P < 0.02) which was significant on the basis of a pair-wise comparison. Indeed, when Tiger sorghum-based diets are considered in isolation, there were significant positive correlations between hammer-mill screen sizes (r = 0.971; P = 0.029), as shown in , and mean particles sizes (r = 0.951; P = 0.049) with weight gains.
Table 5. Effects of grain sorghum variety and hammer-mill screen sizes on growth performance of broilers from 7 to 28 days post-hatch.
Figure 1. Linear relationship (r = 0.971; P = 0.029) between hammer-mill screen-size and weight gain of broiler chickens offered Tiger sorghum-based diets.
The effects of dietary treatments on relative gizzard and pancreas weights and gizzard pH are shown in . There were no significant treatment effects on pancreas weights and gizzard pH. However, Block I sorghum supported 7.4% heavier gizzard weights (20.86 versus 19.43 g/kg; P < 0.001) than Tiger sorghum-based diets. Sorghum-based diets ground through a 2.0-mm hammer-mill screen generated significantly 4.94% lighter gizzards (19.43 versus 20.44 g/kg; P < 0.025) than the average of the three larger screens.
Table 6. Effects of grain sorghum variety and hammer-mill screen sizes on relative gizzard and pancreas weights and gizzard pH in broilers at 28 days post-hatch.
The nutrient utilisation data arising from this experiment are shown in . Tiger sorghum was superior to Block I sorghum-based diets by 0.37 MJ in AME (12.89 versus 12.52 MJ/kg; P < 0.01), by 0.53 MJ in N-corrected AME (12.02 versus 11.49 MJ/kg; P < 0.001) and by 4.15% in ME:GE ratios (0.753 versus 0.723; P < 0.001). Hammer-mill screen sizes did not influence any nutrient utilisation parameters and N retention was not influenced by treatments.
Table 7. Effects of grain sorghum variety and hammer-mill screen sizes on nutrient utilisation of broilers.
The effects of grain sorghum variety and hammer-mill screen sizes on starch digestibility at 4 small intestinal sites are shown in . Tiger sorghum-based diets supported numerically higher starch digestibilities in the three anterior small intestinal segments including a 3.97% increase in the proximal jejunum. However, the 2.46% advantage (0.918 versus 0.896; P < 0.005) of Tiger over Block I sorghum-based diets was significant in the distal ileum. Overall, larger hammer-mill screen sizes appear to be associated with enhanced starch digestibility. The effect of hammer-mill screen sizes was significant (P < 0.01) in the distal jejunum and distal ileum. In the distal jejunum there was a significant difference between 2.0 and 6.0 mm screen sizes of 8.85% (0.849 versus 0.780); similarly, in the distal ileum there was a significant difference of 4.04% (0.926 versus 0.890).
Table 8. Effects of grain sorghum variety and hammer-mill screen sizes on apparent starch digestibility coefficients at four small intestinal sites in broilers at 28 days post-hatch.
The effects of dietary treatments on starch disappearance rates (g/bird/d) at 4 small intestinal sites are shown in . Birds offered diets based on Tiger sorghum had 14.7% higher starch disappearance rates (33.51 versus 29.21 g/bird/d; P < 0.001) than their Block I counterparts at the proximal jejunum. Tiger sorghum-based diets held a similar advantage of 15.9% (37.44 versus 33.29 g/bird/d; P < 0.001) at the distal jejunum. However, hammer-mill screen sizes had a significant effect (P < 0.005) at this site where the 6.0-mm screen supported higher starch disappearance rates than the three smaller screens by up to 13.7% (38.07 versus an average of 33.47 g/bird/d). There were significant treatment interactions between sorghum variety and hammer-mill screen size in the proximal (P < 0.03) and distal ileum (P < 0.025). Overall, increasing hammer-mill screen sizes supported more rapid starch disappearance rates; however, significant increases were only observed with the 6.0-mm screen with Block I sorghum-based diets. In contrast, with Tiger sorghum-based diets, significant increases were observed with the 3.2-mm screen at the proximal ileum and with the 4.8-mm screen at the distal ileum.
Table 9. The effect of grain sorghum variety and hammer-mill screen size on apparent starch disappearance rates (g/bird/day) at four small intestinal sites in broilers at 28 days post-hatch.
The effects of sorghum variety and hammer-mill screen sizes on apparent protein (N) digestibility at 4 small intestinal sites are shown in where there were not any significant treatment interactions. Tiger sorghum-based diets supported higher N digestibilities in the distal jejunum by 4.66% (0.786 versus 0.751; P < 0.015), in the proximal ileum by 1.96% (0.781 versus 0.766; P < 0.04) and by 2.16% (0.804 versus 0.787; P < 0.01) in the distal ileum. The 6.0-mm screen supported a significantly higher N digestibility by 4.23% (0.788 versus 0.756) than the 2.0-mm screen in the proximal ileum and by 3.32% (0.809 versus 0.783) in the distal ileum.
Table 10. Effects of grain sorghum variety and hammer-mill screen sizes on apparent protein (N) digestibility coefficients at four small intestinal sites in broilers at 28 days post-hatch.
The effects of dietary treatments on apparent protein (N) disappearance rates are shown in , where, again, there were no significant interactions. Tiger sorghum-based diets supported more rapid protein disappearance rates (g/bird/d) in the distal jejunum by 4.53% (18.89 versus 18.07; P < 0.015), proximal ileum by 1.95% (18.79 versus 18.43; P < 0.04) and distal ileum by 2.17% (19.33 versus 18.92; P < 0.01). The 6.0-mm screen supported significantly faster protein disappearance rates by 4.18% (18.94 versus 18.18) than the 2.0-mm screen in the proximal ileum and by 3.24% (19.44 versus 18.83) in the distal ileum.
Table 11. Effects of grain sorghum variety and hammer-mill screen sizes on apparent protein (N) disappearance rates (g/bird/day) at four small intestinal sites in broilers at 28 days post-hatch.
Finally, the apparent starch:protein (N) disappearance rate ratios are shown in . In the proximal jejunum, the ratio of 2.66 for Tiger sorghum-based diets was significantly (P < 0.001) wider than 2.10 for diets based on Block I. There were significant treatment interactions in the three posterior small intestinal segments, especially in both segments of the ileum (P < 0.001). For Tiger sorghum-based diets, the narrowest ratio was observed with the 2.0-mm screen and the widest with the 4.8-mm screen. For Block I sorghum-based diets, the narrowest ratio was observed with the 4.8-mm screen and the widest with the 6.0-mm screen.
Table 12. The effect of grain sorghum variety and hammer-mill screen size on apparent starch:protein (N) disappearance rate ratios at four small intestinal sites in broilers at 28 days post-hatch.
Discussion
The effects of hammer-mill screen size, and resultant geometric mean sorghum particle size, were not expected as it was anticipated that there would be quadratic responses and a hammer-mill screen size of less than 6.0 mm would generate the best broiler performance. This assumption was based on three experiments previously completed at this institution, which involved a white sorghum variety as mentioned in the Introduction. This was not the case as hammer-mill screen size did not influence weight gain or FCR to significant extents. Indeed, with Tiger sorghum-based diets, increasing hammer-mill screen size was positively correlated with 7 to 28 d weight gain as illustrated in . Moreover, there was a linear correlation (r = 0.720; P = 0.044) between increasing hammer-mill screen sizes and distal ileal starch digestibility coefficients.
The overall distal ileal starch digestibility coefficient of 0.907 in sorghum-based diets recorded in this experiment is inferior to that of reported values for maize-based diets (Truong et al., Citation2015a). Moreover, starch digestibility coefficients significantly increased by 4.04% (0.926 versus 0.890) when hammer-mill screen sizes of 2.0 mm and 6.0 mm are compared in favour of the larger screen size. The 6.0-mm hammer-mill screen generated similar geometric mean particle sizes of 1402 and 1408 μm for Tiger and Block I sorghums, respectively, which were less than expected.
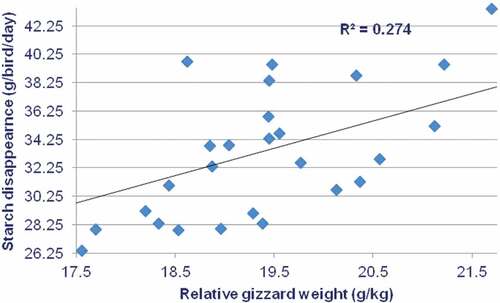
The influence of grain particle size on broiler performance has been competently reviewed by Amerah et al. (Citation2007). In this review, the authors suggested that grain particle size should range from 1100 to 1300 μm for 7 to 21 d-old broilers and from 1300 to 1500 μm for older birds. These suggestions are very consistent with the grain particle sizes generated by the larger hammer-mill screen sizes in the present study. Nir et al. (Citation1990) reported that the transition from “fine” to “coarse” sorghum particle sizes (unspecified) significantly increased weight gain by 4.95% and feed intake by 5.45%. Moreover, Nir et al. (Citation1994) found that the performance of 7 to 21 d-old broilers offered maize-, sorghum- and wheat-based diets with a mean grain particle size of 1130–1230 μm was superior to their counterparts offered diets with grain particle size of 570–670 μm. These findings are consistent with the outcomes of the present feeding study. The transition from sorghum ground through a 2.0-mm hammer-mill screen to three larger apertures resulted in a significant increase in relative gizzard weights from 19.25 g/kg to an average of 20.44 g/kg. However, the difference of 6.29% in relative gizzard weights (19.25 versus 20.46 g/kg) between the 2.0 and 6.0-mm hammer-mill screens appears subtle. Nevertheless, when Tiger sorghum-based diets are considered in isolation, there are positive correlations between relative gizzard weights and starch digestibility coefficients (r = 0.413; P = 0.029) and starch disappearance rates (r = 0.523; P = 0.004) in the proximal jejunum. The second linear relationship is illustrated in . One possible explanation is that as a result of gastroduodenal refluxes, digesta containing pancreatic amylase is being recycled back into the gizzard and starch digestibility was enhanced as a result of reverse peristalsis (Liu et al., Citation2015a). It is established that whole-grain feeding generates heavier relative gizzard weights as reviewed by Singh et al. (Citation2014) and Liu et al. (Citation2015a). Heavier relative gizzard weights are presumably indicative of more functional gizzards but they may not be a precise assessment. Interestingly, Nir et al. (Citation1994) found that the transition from fine-to-medium grain particle sizes in broiler diets significantly increased empty gizzard weights but gizzard contents were disproportionately increased to a large extent.
Figure 2. Linear relationship (r = 0.523; P = 0.004) between relative gizzard weights and starch disappearance rates at the proximal jejunum in broiler chickens offered Tiger sorghum-based diets.
Relative gizzard and pancreas weights were positively correlated (r = 0.278; P = 0.038) in the present study. However, with Tiger sorghum-based diets, gizzard weights were correlated with pancreas weights (0.407; P = 0.032), gizzard pH (r = −0.404; P = 0.033) and mean particle size (r = 0.472; P = 0.011). These relationships were not significant with Block I sorghum-based diets. Relative gizzard weights, or the “power of the gizzard”, is pivotal to the practice of whole-grain feeding but even in the context of standard diets in which the entire grain component has been ground, the influence of the gizzard and its impact on gut function and reverse peristalsis appears to hold importance.
Liu et al. (Citation2015b) suggested that the triad of kafirin, non-tannin phenolic compounds and phytate in grain sorghum negatively influence the utilisation of starch/energy in broilers offered sorghum-based diets. The rationale for this suggestion has been considered in detail elsewhere (Khoddami et al., Citation2015; Liu et al., Citation2015b; Truong et al., Citation2015b). Tiger was clearly superior to Block I sorghum in the present study as significant advantages for feed conversion ratios, energy utilisation, starch and protein (N) digestibility were observed. Therefore, it is noteworthy that Block I contained more kafirin, polyphenols, phenolic acids and phytate than Tiger sorghum. Kafirin is the dominant protein fraction in grain sorghum (Selle et al., Citation2010) and kafirin has often been implicated as having a deleterious effect on sorghum starch utilisation (Black et al., Citation2005) However, kafirin may be increasing as a proportion of sorghum protein in Australia as an inadvertent consequence of breeding programs (Selle, Citation2011). Sorghum contains far more phenolic compounds that other feed grains (Bravo, Citation1998) and non-tannin phenolic compounds may also have deleterious effects on sorghum starch utilisation (Khoddami et al., Citation2015). Sorghum contains at least as much phytate as other feed grains (Selle et al., Citation2003) and the anti-nutritive properties of this phosphorus-containing polyanionic molecule in poultry have been documented (Selle and Ravindran, Citation2007). Finally, it may be instructive that Tiger sorghum had a lower peak time and pasting temperature and higher RVA starch viscosities than Block I sorghum. This was particularly evident for peak RVA viscosity, which was higher for Tiger sorghum by a factor of 1.99 (4771 versus 2392 cP).
In conclusion, Tiger sorghum was superior to Block I sorghum as a feed grain for poultry as it supported an improvement of 3.25% in FCR (1.548 versus 1.600) in broiler chickens. The possibility exists that RVA starch pasting profiles may be predictive of the quality of sorghum as a feed grain in chicken-meat production. However, the effects of hammer-mill screen size, and geometric mean sorghum particle size of grain sorghum on broiler performance were not as expected. The numerically best weight gains and FCR were associated with a hammer-mill screen size of 6.0 mm and a geometric mean particle size in the order of 1400 μm; whereas, it was anticipated that smaller sizes would have been advantageous.
Acknowledgements
We would particularly like to thank Dr Karlie Nielsen for quantifying kafirin in sorghum while at the Australian Proteome Analysis Facility (Macquarie University) and Dr David Cadogan (Feedworks) for formulating the diets. We would like to acknowledge the Chicken-meat Committee of the Rural Industries Research and Development Corporation (RIRDC) for their ongoing encouragement and financial support and the Poultry CRC for their support of Ms Ha Truong’s PhD candidature.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Amerah, A.M., Ravindran, V., Lentle, R.G. & Thomas, D.G. (2007) Feed particle size: implications on the digestion and performance of poultry. Worlds Poultry Science Journal, 63: 439–455. doi:10.1017/S0043933907001560
- Black, J.L., Hughes, R.J., Nielsen, S.G., Tredrea, A.M., Macalpine, R. & Van Barneveld, R.J. (2005) The energy value of cereal grains, particularly wheat and sorghum, for poultry. Proceedings of Australian Poultry Science Symposium, 17: 21–29.
- Bravo, L. (1998) Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutrition Reviews, 56: 317–333. doi:10.1111/j.1753-4887.1998.tb01670.x
- Hamaker, B.R., Mohamed, A.A., Habben, J.E., Huang, C.P. & Larkins, B.A. (1995) Efficient procedure for extracting maize and sorghum kernel proteins reveals higher prolamin contents than the conventional method. Cereal Chemistry, 72: 583–588.
- Hernandez, J.R., Capareda, S.C., Hays, D.B., Portillo, O.R. & Rooney, W.L. (2008) Effect of grain sorghum protein digestibility on starch gelatinization and enzymatic conversion to glucose. Presented at the 2008 ASABE Annual International Meeting, Providence RI. American Society of Agricultural and Biological Engineers St Joseph, MI. USA.
- Hill, F.W. & Anderson, D.L. (1958) Comparison of metabolizable energy and productive energy determinations with growing chicks. Journal of Nutrition, 64: 587–603.
- Khoddami, A., Truong, H.H., Liu, S.Y., Roberts, T.H. & Selle, P.H. (2015) Concentrations of specific phenolic compounds in six red sorghums influence nutrient utilisation in broiler chickens. Animal Feed Science and Technology, 210: 190–199. doi:10.1016/j.anifeedsci.2015.09.029
- Khoddami, A., Wilkes, M.A. & Roberts, T.H. (2013) Techniques for analysis of plant phenolic compounds. Molecules, 18: 2328–2375. doi:10.3390/molecules18022328
- Liu, S.Y., Fox, G., Khoddami, A., Neilson, K.A., Truong, H.H., Moss, A.F. & Selle, P.H. (2015b) Grain sorghum: a conundrum for chicken-meat production. Agriculture, 5: 1224–1251. doi:10.3390/agriculture5041224
- Liu, S.Y., Selle, P.H. & Cowieson, A.J. (2013) Strategies to enhance the performance of pigs and poultry on sorghum-based diets. Animal Feed Science and Technology, 181: 1–14. doi:10.1016/j.anifeedsci.2013.01.008
- Liu, S.Y., Truong, H.H. & Selle, P.H. (2015a) Whole-grain feeding for chicken-meat production: possible mechanisms driving enhanced energy utilisation and feed conversion. Animal Production Science, 55: 559–572. doi:10.1071/AN13417
- Mahasukhonthachat, K., Sopade, P.A. & Gidley, M.J. (2010) Kinetics of starch digestion and functional properties of twin-screw extruded sorghum. Journal of Cereal Science, 51: 392–401. doi:10.1016/j.jcs.2010.02.008
- Nir, I., Hillel, R., Shefet, G. & Nitsan, Z. (1994) Effect of grain particle-size on performance .2. grain texture interactions. Poultry Science, 73: 781–791. doi:10.3382/ps.0730781
- Nir, I., Melcion, J.P. & Picard, M. (1990) Effect of particle-size of sorghum grains on feed-intake and performance of young broilers. Poultry Science, 69: 2177–2184. doi:10.3382/ps.0692177
- Selle, P.H. (2011) The protein quality of sorghum. Proceedings of Australian Poultry Science Symposium, 22: 147–160.
- Selle, P.H., Cadogan, D.J., Li, X. & Bryden, W.L. (2010) Implications of sorghum in broiler chicken nutrition. Animal Feed Science and Technology, 156: 57–74. doi:10.1016/j.anifeedsci.2010.01.004
- Selle, P.H., Liu, S.Y., Cai, J. & Cowieson, A.J. (2012) Steam-pelleting and feed form of broiler diets based on three coarsely ground sorghums influences growth performance, nutrient utilisation, starch and nitrogen digestibility. Animal Production Science, 52: 842–852. doi:10.1071/AN12026
- Selle, P.H., Liu, S.Y., Cai, J. & Cowieson, A.J. (2013) Steam-pelleting temperatures, grain variety, feed form and protease supplementation of mediumly-ground, sorghum-based broiler diets: influences on growth performance, relative gizzard weights, nutrient utilisation, starch and nitrogen digestibility. Animal Production Science, 53: 378–387. doi:10.1071/AN12363
- Selle, P.H., Liu, S.Y., Cai, J. & Cowieson, A.J. (2014) Steam-pelleting temperatures and grain variety of finely-ground, sorghum-based broiler diets. I. Influence on growth performance, relative gizzard weights, nutrient utilisation, starch and nitrogen digestibility. Animal Production Science, 54: 339–346. doi:10.1071/AN13080
- Selle, P.H. & Ravindran, V. (2007) Microbial phytase in poultry nutrition. Animal Feed Science and Technology, 135: 1–41. doi:10.1016/j.anifeedsci.2006.06.010
- Selle, P.H., Walker, A.R. & Bryden, W.L. (2003) Total and phytate-phosphorus contents and phytase activity of Australian-sourced feed ingredients for pigs and poultry. Australian Journal of Experimental Agriculture, 43: 475–479. doi:10.1071/EA02155
- Singh, Y., Amerah, A.M. & Ravindran, V. (2014) Whole grain feeding: methodologies and effects on performance, digestive tract development and nutrient utilisation of poultry. Animal Feed Science and Technology, 190: 1–18. doi:10.1016/j.anifeedsci.2014.01.010
- Siriwan, P., Bryden, W.L., Mollah, Y. & Annison, E.F. (1993) Measurement of endogenous amino-acid losses in poultry. British Poultry Science, 34: 939–949. doi:10.1080/00071669308417654
- Symes, K.J. (1965) The inheritance of grain hardness in wheat as measured by the particle size index. Australian Journal of Agricultural Research, 16: 113–123. doi:10.1071/AR9650113
- Truong, H.H., Liu, S.Y. & Selle, P.H. (2015a) Starch utilisation in chicken-meat production: the foremost influential factors. Animal Production Science, http://dx.doi.org/10.1071/AN15056
- Truong, H.H., Neilson, K.A., Mcinerney, B.V., Khoddami, A., Roberts, T.H., Liu, S.Y. & Selle, P.H. (2015b) Performance of broiler chickens offered nutritionally-equivalent diets based on two red grain sorghums with quantified kafirin concentrations as intact pellets or re-ground mash following steam-pelleting at 65 or 97°C conditioning temperatures. Animal Nutrition, 1: 220–228. doi:10.1016/j.aninu.2015.08.002
- Wallace, J.C., Lopes, M.A., Paiva, E. & Larkins, B.A. (1990) New methods for extraction and quantitation of zeins reveal a high content of gamma-zein in modified opaque-2 maize. Plant Physiology, 92: 191–196. doi:10.1104/pp.92.1.191
- Waniska, R.D., Hugo, L.F. & Rooney, L.W. (1992) Practical methods to determine the presence of tannins in sorghum. Journal of Applied Poultry Research, 1: 122–128. doi:10.1093/japr/1.1.122
