ABSTRACT
1. The avian embryo is an excellent model for studying embryology and the production of pharmaceutical proteins in transgenic chickens. Furthermore, chicken stem cells have the potential for proliferation and differentiation and emerged as an attractive tool for various cell-based technologies.
2. The objective of these studies is the derivation and culture of these stem cells is the production of transgenic birds for recombinant biomaterials and vaccine manufacture, drug and cytotoxicity testing, as well as to gain insight into basic science, including cell tracking.
3. Despite similarities among the established chicken stem cell lines, fundamental differences have been reported between their culture conditions and applications. Recent conventional protocols used for expansion and culture of chicken stem cells mostly depend on feeder cells, serum-containing media and static culture.
4. Utilising chicken stem cells for generation of cell-based transgenic birds and a variety of vaccines requires large-scale cell production. However, scaling up the conventional adherent chicken stem cells is challenging and labour intensive. Development of a suspension cell culture process for chicken embryonic stem cells (cESCs), chicken primordial germ cells (PGCs) and chicken induced pluripotent stem cells (ciPSCs) will be an important advance for increasing the growth kinetics of these cells.
6. This review describes various approaches and suggestions to achieve optimal cell growth for defined chicken stem cells cultures and use in future manufacturing applications.
Introduction
The avian embryo provides an excellent model for studying embryology, developmental biology and production of pharmaceutical proteins in transgenic chickens (Naito Citation2003; Farzaneh et al. Citation2016; Farzaneh et al. Citation2017b). The first chicken stem cells were derived from blastodermal cells (BCs) of fertilised eggs at embryonic stage X and were designated as chicken embryonic stem cells (cESCs) (Pain et al. Citation1996; van de Lavoir and Mather‐Love Citation2006; Intarapat and Stern Citation2013). Since the establishment of the first cESCs, rapid progress has been made and several groups have described the derivation and culture of chicken stem cells from various developmental stages (Petitte et al. Citation2004; ; Jung et al. Citation2007; Motono et al. Citation2008; Trefil et al. Citation2012; Bednarczyk Citation2014; Farzaneh et al. Citation2017a) . The second population of in vitro chicken stem cells, named embryonic germ cells (EGCs), were derived from primordial germ cells (PGCs) (Park and Han Citation2000; Guan et al. Citation2010). The third population are known as chicken-induced pluripotent stem cells (ciPSCs) and have been generated by in vitro reprogramming of chicken fibroblast cells (from 8–12dold embryos) on a feeder layer (Lu et al. Citation2011; Yu et al. Citation2013). Chicken stem cells with self-renewal capacity can be propagated in culture indefinitely and have the ability to differentiate into multiple cell types representing all primitive embryonic germ layers (Lavial and Pain Citation2010; Intarapat and Stern Citation2013). Chicken stem cells have provided an ideal opportunity for generating cell-based transgenic birds (Han et al. Citation2015; Farzaneh et al. Citation2017a) and a powerful source of cells for vaccine production for poultry and human viral diseases. Moreover, they provide insight into basic science including cell tracking, as well as drug and cytotoxicity testing (Aubrit et al. Citation2015; Gallo–Ramírez et al. Citation2015; Genzel Citation2015). For reliable use of chicken stem cells in basic and applied sciences, it is necessary to eliminate the variations in culture conditions required for their establishment. Standardised cell culture protocols are important not only for gene manipulation, but also for biological research carried out using these cells. Hence, this mini review describes different culture conditions required for chicken stem cell culture and development of serum/feeder-containing media to defined conditions for the purpose of large-scale and suspension culture of chicken stem cells for drug discovery and recombinant protein production.
Derivation of chicken stem cells
The chicken embryo spends approximately its first 20 h in the hen’s body and as the embryo traverses the oviduct cell division occurs in a meroblastic pattern to generate BCs (Jacobson Citation1989). By the time the egg is laid, there are approximately 40 000–50 000 BCs, which arise from the cleavage of a single fertilised ovum surrounded by islands of yolk cells (Lavial and Pain Citation2010). The entire embryo develops from the centre of the epiblast, and it has a remarkable regenerative capacity (Alev et al. Citation2013). cESCs were isolated from BCs at embryonic stage X; these have a relatively small amount of cytoplasm with a large translucent nucleus containing a single nucleolus (Wei et al. Citation2000; van de Lavoir and Mather‐Love Citation2006). In culture, cESCs grow on feeder cells and attach to each other with visible borders (Aubel and Pain Citation2013). Moreover, it was shown that cESCs are able to contribute to different cell lineages including somatic and germ cell lines (Etches et al. Citation1996; Pain et al. Citation1996). cESCs have been derived from chicken eggs of meat strains more efficiently compared with egg-laying strains (Aubel and Pain Citation2013). These cells are of interest for transgenic technology and vaccine manufacturing (Brown and Mehtali Citation2010; Olivier et al. Citation2010). However, some reports mentioned that cESCs lost their pluripotency after long-term culture (Yu et al. Citation2013). Therefore, it seems that their potential for germ line transmission into offspring was low (van de Lavoir et al. Citation2006; Intarapat and Stern Citation2013) and only a few laboratories have taken the risk of establishing cESCs for developmental studies (Jean et al. Citation2015).
Scientists have established long-term culture of chicken PGCs harvested from the circulatory system of 2.5–3 d old embryos (van de Lavoir et al. Citation2006) and embryonic gonads (5.5–6 d old) (Park and Han Citation2000). Approximately 30–130 PGCs are present in the extraembryonic region of the chicken embryo and unlike mammals, they use blood circulation as the migratory route and stop active migration as soon as they reach the genital ridges (Eyal-Giladi et al. Citation1981; Kuwana Citation1993; Petitte et al. Citation1997; Bernardo et al. Citation2012). PGCs are identified by their remarkably large size, large spherical nuclei and refractive cytoplasmic lipids with high glycogen content (Li et al. Citation2010; Pinkert Citation2014). These cells are a suitable vehicle for developing cell-based transgenic birds (Han et al. Citation2015; Taylor et al. Citation2017) and a cell-free culture system has been described (Whyte et al. Citation2015).
Research on iPSCs paved the way to identify the molecular mechanisms underlying pluripotency of all kinds of chicken stem cells (Lu et al. Citation2011) as well as providing a better tool for vaccine manufacturing (Shittu et al. Citation2016). Although feeder cell, serum and growth factor employment in culture conditions leads to the successful derivation and culture of chicken stem cells, it presents a risk of propagating contaminating virus particles making these supplements unsuitable for vaccine manufacturing and cell-based transgenic technology (Halme and Kessler Citation2006; Eaker et al. Citation2017; Gupta et al. Citation2017).
Culture medium for chicken stem cells
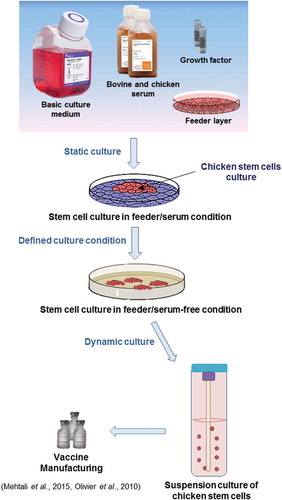
Knockout Dulbecco’s modified Eagle’s medium (Knockout DMEM or KO/DMEM) is a basic culture medium which can support the growth of chicken stem cells in vitro. The composition of KO/DMEM is suitable for all kinds of mammalian and non-mammalian stem cells and can increase their growth rate to produce an adequate population (van de Lavoir and Mather‐Love Citation2006; Amit and Itskovitz-Eldor Citation2016). Although Dulbecco’s modified Eagle’s medium (DMEM) can support the growth of chicken stem cells, its high osmolarity (320–350 mOsm) may have an adverse effect on proliferation of these cells in vitro (van de Lavoir and Mather‐Love Citation2006). Traditionally, KO/DMEM has been enriched with foetal bovine serum (FBS) to stimulate cell proliferation due to the presence of growth factors. The routine FBS used for mouse ESC (mESC) culture may not be suitable for chicken stem cell growth; however, FBS cannot be completely replaced by chicken serum since the foetal environment from which the FBS is derived is rich in growth factors (Horiuchi et al. Citation2006). Normally, it is preferred to keep both FBS and chicken serum at −70°C rather than 4°C (Pinkert Citation2014). One of the fundamental problems related to FBS or chicken serum is that both of them are highly complex containing unknown compounds including self-renewal and differentiation-inducing factors (Skottman and Hovatta Citation2006). All serums often have batch to batch and lot to lot variation that could affect in vitro chicken stem cell propagation (Stein Citation2007). Hence, the optimisation of a serum-free culture medium would be a suitable solution for these problems (Amit et al. Citation2004; Brunner et al. Citation2010). The commercial protein supplements such as knockout serum replacement (KSR) are often used in mammalian culture systems to replace FBS (Lin and Talbot Citation2011; Desai et al. Citation2015). KSR is also useful for proliferation and maintaining the undifferentiated state of long-term cultured chicken PGCs (Naito et al. Citation2015).
Feeder cells in chicken stem cell culture
The feeder layer is an important factor for derivation and maintenance of chicken stem cells. The establishment of cESCs was first described by using a Sandos inbred mouse (SIM)-derived 6-thioguanine- and ouabain-resistant (STO) cell line (mouse fibroblast cell line) feeder cells in a FBS-containing culture medium (Pain et al. Citation1996). Moreover, the first cEGCs were derived in the presence of chicken embryonic fibroblast (CEF) cells as the feeder layer (Park and Han Citation2000). Previous studies suggested that embryonic fibroblasts are capable of secreting paracrine factors including chicken leukaemia inhibitory factor (LIF) and basic fibroblast growth factor (bFGF); both factors inhibit differentiation of germ cells and increase their proliferation (De Felici et al. Citation2005; Tang et al. Citation2007). Mouse embryonic fibroblast cells (MEF) play a central role for derivation of ciPSCs (Lu et al. Citation2011). It seems that mitotically inactivated MEF cells are able to secret LIF as a factor that supports the self-renewal property of mouse stem cells (Lin and Talbot Citation2011), but its use was not beneficial for the culture of cESCs (van de Lavoir and Mather‐Love Citation2006). Utilisation of mitotically inactivated STO cells as feeder cells leads to the augmentation of alkaline phosphatase positive cESCs colonies and can also increase the proliferation rate of PGCs, but cEGCs are unable to proliferate on STO cells in culture (Park and Han Citation2000). Although, CEF has been successfully used for cEGC expansion and was one of the best substrates for proliferation of PGCs (Tang et al. Citation2007), it cannot support the growth of cESCs and ciPSCs (van de Lavoir and Mather‐Love Citation2006). cESCs can be grown on Buffalo rat liver (BRL) cells as well (van de Lavoir et al. Citation2006), but they attach more firmly to BRL layer compared to STO cells. However, it should be noticed that high concentrations of STO cells can impede the growth of cESCs and it is essential to seed these feeder cells at low concentrations (van de Lavoir et al. Citation2006).
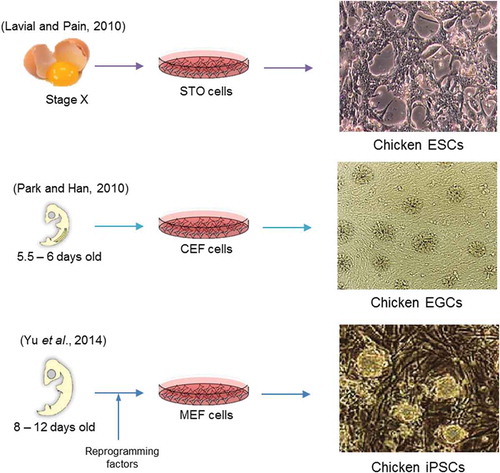
The presence of conditioned medium for chicken stem cell culture is also important. Conditioned medium derived from STO cells and BRL cells can maintain the self-renewal property of cESCs and cPGCs but not cEGCs or ciPSCs (Petitte et al. Citation2004). Taken together, the employment of feeder cells in chicken stem cells cultures can provide an appropriate attachment substrate and they also release the necessary growth factors to the culture media (Lavial and Pain Citation2010). However, some released factors may have unfavourable effects on chicken stem cell self-renewal or on the undifferentiated state. Moreover, derivation and maintenance of chicken stem cells in the presence of a feeder layer results in variation and ambiguity of culture composition which can affect the final characteristics of these cells (Intarapat and Stern Citation2013). It has been suggested that a feeder-free condition eliminates the influence of unknown factors and animal pathogens on optimisation procedures required for highly efficient and large-scale generation of functional stem cells (Kraus et al. Citation2011). Feeder-free culture of human stem cells is feasible on matrigel that contains laminin, collagen type IV and other substances that are each part of the extracellular matrix (ECM) including synthetic laminin or fibronectin (Desai et al. Citation2015). To date, long-term feeder-free culture of cESCs and ciPSCs has been conducted successfully (Reiter et al. Citation2014). For this purpose, chicken stem cells were cultured on matrigel or gelatin-coated dishes, the step-wise reduction of serum and growth factors was done and the cells were transferred to the suspension culture dish on a shaker in optimum culture conditions (Madeline et al. Citation2015; Shittu et al. Citation2016). However, the functional roles of natural or synthetic ECM in controlling stem cell behaviour are not clear and they cannot be a permanent substitute for feeder cells in the culture system (Hayashi and Furue Citation2016). Derivation and culture of chicken stem cells from various developmental stages are shown in (Park and Han Citation2000; Lavial and Pain Citation2010; Yu et al. Citation2014).
Figure 1. Derivation and culture of chicken stem cells from various developmental stages. Chicken ESCs derived from the blastodermal cells at embryonic stage X. In the culture, cESCs grow on the STO feeder layer and attach to each other with visible borders. PGCs have the ability to be reprogrammed into EGC colonies (from 5.5–6 d old embryo) on the CEF feeder layer. Chicken iPSCs have been generated by in vitro reprogramming of chicken fibroblast cells (from 8–12 d old embryo) on MEF feeder layer.
ESCs: embryonic stem cells; STO: mouse fibroblast cell line; CEF: chicken embryonic fibroblast cells; MEF: mouse embryonic fibroblast cells; EGCs: embryonic germ cells; iPSCs: induced pluripotent stem cells.
Culture medium supplements
The distinct role of growth factors in derivation and maintenance of chicken stem cells is undisputed. For instance, the presence of several growth factors such as chicken stem cell factor (SCF), bFGF, mouse IL-6, human IL-11, human insulin-like growth factor-1 (hIGF-1) and mouse LIF (mLIF) has been shown to be necessary for chicken stem cell proliferation (Pain et al. Citation1996). Several research groups have reported successful use of SCF, bFGF and mLIF in chicken PGC and EGC cultures (Park and Han Citation2000). LIF is a member of the cytokine family that by activation of the JAK/STAT pathway can result in long-term propagation of chicken stem cells (Petitte et al. Citation2004). Mammalian cytokines including human and mouse LIF (Petitte et al. Citation2004) and also avian cytokine such as chicken LIF (Nakano et al. Citation2011) are able to maintain chicken stem cells in an undifferentiated state. The role of other signalling pathways in maintenance of an undifferentiated state of these cells is not fully understood. bFGF is a key growth factor that turns the MEK/ERK signalling pathway on and is required to maintain proliferation-associated factors in chicken PGCs (Whyte et al. Citation2015). SCF also has an important role as a cofactor together with bFGF, since its presence in culture is critical for propagation of PGCs and its effect without bFGF is not significant (Miyahara et al. Citation2016). It seems that the effect of chicken SCF, specifically in its soluble form, which was extracted from the BRL cell line, can enhance proliferation rate more than the membrane-bound SCF (Miyahara et al. Citation2016). Insulin-like growth factor (IGF) is another growth factor which activates the insulin pathway and along with FGF increases self-renewal of chicken stem cells (Jean et al. Citation2013). Most probably, a central network of pluripotency is conserved between animals and the role of one or two pathways might be highlighted in one but reduced in the others (Jean et al. Citation2013).
Suspension culture of chicken stem cells for large-scale production
Chicken stem cells are master cells having the potential to develop cell-based transgenic birds and vaccines requiring large-scale cell technologies (Kraus et al. Citation2011; Genzel Citation2015; Farzaneh et al. Citation2017b). However, scaling up the conventional adherent chicken stem cell cultures is challenging and labour intensive (Sart et al. Citation2014). Therefore, development of a suspension cell culture process for these stem cells can be a very important step for increasing their dynamic culture (Kirouac and Zandstra Citation2008). Although current chicken stem cell culture dependency on feeder cells and serum has created major problems with suspension culture systems, this issue was recently addressed by the Vivalis Company (Bushell-Embling Citation2012). These authors demonstrated that suspension culture of chicken and duck ESCs (named as EBx and EB66, respectively) has a considerable potential for production of a variety of viral vaccines and therapeutic proteins in high yield (Olivier et al. Citation2010; Mehtali et al. Citation2015). EB66 cells can be maintained in a serum-free medium named EX-CELL EBx-GRO-I that is animal-component-free (Olivier et al. Citation2010). Hence, an efficient cell-based system has been provided and it can be applied as a safe, cheap and fast method for vaccine production (Merten et al. Citation2014). The optimum cell culture to maintain chicken stem cells involved the use of defined medium and dynamic culture in feeder and serum-free conditions is shown in (Olivier et al. Citation2010; Mehtali et al. Citation2015). Replacement of feeder and serum culture conditions with defined constituents that are free of animal-derived components has advanced the research on large-scale chicken stem cell culture for production of transgenic birds and vaccines (Mehtali et al. Citation2015).
Conclusion
The advent of techniques to culture chicken stem cells from different sources at various stages has enhanced the study of vaccine manufacture and production of transgenic chickens. There have been many attempts to develop suitable culture conditions for establishment of long-term culture of chicken stem cells and different methods have been used to create defined culture conditions. However, serum-free and feeder-free culture methods for these cells are preferable for reducing or eliminating undefined factors. The establishment of chicken stem cell culture systems offers an important advance for genetic, developmental and vaccine manufacture studies.
Acknowledgement
The authors would like to thank staff at the Royan Institute for Stem Cell Biology and Technology, Department of Stem Cells and Developmental Biology. The authors declare that there is no conflict of interest.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Alev, C., M. Nakano, Y. Wu, H. Horiuchi, and G. Sheng. 2013. “Manipulating the Avian Epiblast and Epiblast-Derived Stem Cells.” Epiblast Stem Cells: Methods and Protocols 1074: 151–173.
- Amit, M., C. Shariki, V. Margulets, and J. Itskovitz-Eldor. 2004. “Feeder Layer-And Serum-Free Culture of Human Embryonic Stem Cells.” Biology of Reproduction 70: 837–845. doi:10.1095/biolreprod.103.021147.
- Amit, M., and J. Itskovitz-Eldor. 2016. “Methods of Deriving Mammalian Pluripotent Stem Cell Lines.” US Patent 9,255,247.
- Aubel, P., and B. Pain. 2013. “Chicken Embryonic Stem Cells: Establishment and Characterization.” Epiblast Stem Cells: Methods and Protocols 1074: 137–150.
- Aubrit, F., F. Perugi, A. Léon, F. Guéhenneux, P. Champion-Arnaud, M. Lahmar, and K. Schwamborn. 2015. “Cell Substrates for the Production of Viral Vaccines.” Vaccine 33: 5905–5912. doi:10.1016/j.vaccine.2015.06.110.
- Bednarczyk, M. 2014. “Avian primordial germ cells and their application.” Proceedings of the Slovak Journal of Animal Science 47: 185–187.
- Bernardo, A. D. M., K. Sprenkels, G. Rodrigues, T. Noce, and S. M. C. D. S. Lopes. 2012. “Chicken Primordial Germ Cells Use the Anterior Vitelline Veins to Enter the Embryonic Circulation.” Biology Open BIO20122592: 1–6.
- Brown, S. W., and M. Mehtali. 2010. “The Avian EB66® Cell Line, Application to Vaccines, and Therapeutic Protein Production.” PDA Journal of Pharmaceutical Science and Technology 64: 419–425.
- Brunner, D., J. Frank, H. Appl, H. Schöffl, W. Pfaller, and G. Gstraunthaler. 2010. “Serum-Free Cell Culture: The Serum-Free Media Interactive Online Database.” Altex 27: 53. doi:10.14573/altex.2010.1.53.
- Bushell-Embling, D. 2012. “Year in Review: Big Deals.” Australian Life Scientist 9: 26.
- De Felici, M., M. L. Scaldaferri, and D. Farini. 2005. “Adhesion Molecules for Mouse Primordial Germ Cells.” Frontiers in Bioscience 10: 542–551. doi:10.2741/1550.
- Desai, N., P. Rambhia, and A. Gishto. 2015. “Human Embryonic Stem Cell Cultivation: Historical Perspective and Evolution of Xeno-Free Culture Systems.” Reproductive Biology and Endocrinology 13: 1. doi:10.1186/s12958-015-0005-4.
- Eaker, S., E. Abraham, J. Allickson, T. A. Brieva, D. Baksh, T. R. Heathman, B. Mistry, and N. Zhang. 2017. “Bioreactors for Cell Therapies: Current Status and Future Advances.” Cytotherapy 19: 9–18. doi:10.1016/j.jcyt.2016.09.011.
- Etches, R. J., M. E. Clark, A. Toner, G. Liu, and A. M. Verrinder Gibbins. 1996. “Contributions to Somatic and Germline Lineages of Chicken Blastodermal Cells Maintained in Culture.” Molecular Reproduction and Development 45: 291–298. doi:10.1002/(ISSN)1098-2795.
- Eyal-Giladi, H., M. Ginsburg, and A. Farbarov. 1981. “Avian Primordial Germ Cells are of Epiblastic Origin.” Development 65: 139–147.
- Farzaneh, M., S. Khoshnam, and P. Mozdziak. 2017a. “Concise Review: Avian Multipotent Stem Cells as a Novel Tool for Investigating Cell-Based Therapies.” Journal of Dairy Veterinary and Anim Research 5: 00125. doi:10.15406/jdvar.2017.05.00125.
- Farzaneh, M., S. E. Khoshnam, and M. Nokhbatolfoghahai. 2016. “First Scientific Record of Two Cases of Partial Twinning in the Chick Embryo, Gallus Gallus Domesticus.” Veterinary Record Case Reports 4: e000353. doi:10.1136/vetreccr-2016-000353.
- Farzaneh, M., S. N. Hassani, P. Mozdziak, and H. Baharvand. 2017b. “Avian Embryos and Related Cell Lines: A Convenient Platform for Recombinant Proteins and Vaccine Production.” Biotechnology Journal 12: 1–10. doi:10.1002/biot.201600598.
- Gallo–Ramírez, L. E., A. Nikolay, Y. Genzel, and U. Reichl. 2015. “Bioreactor Concepts for Cell Culture-Based Viral Vaccine Production.” Expert Review of Vaccines 14: 1181–1195.
- Genzel, Y. 2015. “Designing Cell Lines for Viral Vaccine Production: Where Do We Stand?” Biotechnology Journal 10: 728–740. doi:10.1002/biot.v10.5.
- Guan, W., Y. Wang, L. Hou, L. Chen, X. Li, W. Yue, and Y. Ma. 2010. “Derivation and Characteristics of Pluripotent Embryonic Germ Cells in Duck.” Poultry Science 89: 312–317. doi:10.3382/ps.2009-00413.
- Gupta, V., M. Sengupta, J. Prakash, and B. C. Tripathy. 2017. “Animal Cell Culture and Cryopreservation.” In Basic and Applied Aspects of Biotechnology, 59–75. Singapore: Springer.
- Halme, D. G., and D. A. Kessler. 2006. “FDA Regulation of Stem-Cell–Based Therapies.” The New England Journal of Medicine 16: 1730–1735. doi:10.1056/NEJMhpr063086.
- Han, J. Y., H. C. Lee, and T. S. Park. 2015. “Germline-Competent Stem Cell in Avian Species and Its Application.” Asian Journal of Andrology 17: 421.
- Hayashi, Y., and M. K. Furue. 2016. “Biological Effects of Culture Substrates on Human Pluripotent Stem Cells.” Stem Cells International 2016: 1–11. doi:10.1155/2016/5380560.
- Horiuchi, H., S. Furusawa, and H. Matsuda. 2006. “Maintenance of Chicken Embryonic Stem Cells in Vitro.” Embryonic Stem Cell Protocols: Volume 1: Isolation and Characterization 1: 17–34.
- Intarapat, S., and C. D. Stern. 2013. “Chick Stem Cells: Current Progress and Future Prospects.” Stem Cell Research 11: 1378–1392. doi:10.1016/j.scr.2013.09.005.
- Jacobson, A. G. 1989. “Chick Embryo: Early Development.” Developmental Biology 133: 44–57.
- Jean, C., N. M. Oliveira, S. Intarapat, A. Fuet, C. Mazoyer, I. De Almeida, K. Trevers, S. Boast, P. Aubel, and F. Bertocchini. 2015. “Transcriptome Analysis of Chicken ES, Blastodermal and Germ Cells Reveals that Chick ES Cells are Equivalent to Mouse ES Cells Rather than EpiSC.” Stem Cell Research 14: 54–67. doi:10.1016/j.scr.2014.11.005.
- Jean, C., P. Aubel, C. Soleihavoup, F. Bouhallier, S. Voisin, F. Lavial, and B. Pain. 2013. “Pluripotent Genes in Avian Stem Cells.” Development, Growth & Differentiation 55: 41–51. doi:10.1111/dgd.12021.
- Jung, J. G., Y. M. Lee, T. S. Park, S. H. Park, J. M. Lim, and J. Y. Han. 2007. “Identification, Culture, and Characterization of Germline Stem Cell-Like Cells in Chicken Testes.” Biology of Reproduction 76: 173–182. doi:10.1095/biolreprod.106.056275.
- Kirouac, D. C., and P. W. Zandstra. 2008. “The Systematic Production of Cells for Cell Therapies.” Cell Stem Cell 3: 369–381. doi:10.1016/j.stem.2008.09.001.
- Kraus, B., S. Von Fircks, S. Feigl, S. M. Koch, D. Fleischanderl, K. Terler, M. Dersch-Pourmojib, C. Konetschny, L. Grillberger, and M. Reiter. 2011. “Avian Cell line-Technology for Large Scale Vaccine Production.” BMC Proceedings 5: 1–3. doi:10.1186/1753-6561-5-S8-P52.
- Kuwana, T. 1993. “Migration of Avian Primordial Germ Cells toward the Gonadal Anlage.” Development, Growth & Differentiation 35: 237–243. doi:10.1111/j.1440-169X.1993.00237.x.
- Lavial, F., and B. Pain. 2010. “Chicken Embryonic Stem Cells as a Non‐Mammalian Embryonic Stem Cell Model.” Development, Growth & Differentiation 52: 101–114. doi:10.1111/j.1440-169X.2009.01152.x.
- Li, J., B. Zhang, H. Han, Z. Cao, Z. Lian, and N. Li. 2010. “Metabolic Properties of Chicken Embryonic Stem Cells.” Science China Life Sciences 53: 1073–1084. doi:10.1007/s11427-010-4055-8.
- Lin, S., and P. Talbot. 2011. “Methods for Culturing Mouse and Human Embryonic Stem Cells.” Embryonic Stem Cell Therapy for Osteo-Degenerative Diseases: Methods and Protocols 690: 31–56.
- Lu, Y., F. D. West, B. J. Jordan, J. L. Mumaw, E. T. Jordan, A. Gallegos-Cardenas, R. B. Beckstead, and S. L. Stice. 2011. “Avian-Induced Pluripotent Stem Cells Derived Using Human Reprogramming Factors.” Stem Cells and Development 21: 394–403. doi:10.1089/scd.2011.0499.
- Madeline, B., S. Ribaud, and A. Xenopoulus. 2015. “Culturing a Duck ES-derived Cell Line in Single-Use Bioreactors.” BioProcess International 13: 26–33.
- Mehtali, M., P. Champion-Arnaud, and A. Leon 2015. “Production of Viral Vaccines in Suspension on Avian Embryonic Derived Stem Cell Lines.” US Patent 8,148,132.
- Merten, O.-W., W. A. Bakker, J. Vorlop, M. Reiter, G. Visnovsky, V. Jäger, M. Merabishvili, and U. Reichl. 2014. “Virus Production under Suspension Conditions.” Industrial Scale Suspension Culture of Living Cells 14: 503–554.
- Miyahara, D., I. Oishi, R. Makino, N. Kurumisawa, R. Nakaya, T. Ono, H. Kagami, and T. Tagami. 2016. “Chicken Stem Cell Factor Enhances Primordial Germ Cell Proliferation Cooperatively with Fibroblast Growth Factor 2.” The Journal of Reproduction and Development 62: 143. doi:10.1262/jrd.2015-128.
- Motono, M., T. Ohashi, K.-I. Nishijima, and S. Iijima. 2008. “Analysis of Chicken Primordial Germ Cells.” Cytotechnology 57: 199–205. doi:10.1007/s10616-008-9156-x.
- Naito, M. 2003. “Development of Avian Embryo Manipulation Techniques and Their Application to Germ Cell Manipulation.” Animal Science Journal 74: 157–168. doi:10.1046/j.1344-3941.2003.00101.x.
- Naito, M., T. Harumi, and T. Kuwana. 2015. “Long-Term Culture of Chicken Primordial Germ Cells Isolated from Embryonic Blood and Production of Germline Chimaeric Chickens.” Animal Reproduction Science 153: 50–61. doi:10.1016/j.anireprosci.2014.12.003.
- Nakano, M., K. Arisawa, S. Yokoyama, M. Nishimoto, Y. Yamashita, M. Sakashita, R. Ezaki, H. Matsuda, S. Furusawa, and H. Horiuchi. 2011. “Characteristics of Novel Chicken Embryonic Stem Cells Established Using Chicken Leukemia Inhibitory Factor.” The Journal of Poultry Science 48: 64–72. doi:10.2141/jpsa.010102.
- Olivier, S., M. Jacoby, C. Brillon, S. Bouletreau, T. Mollet, O. Nerriere, and A. Angel et al. 2010. “EB66 Cell Line, a Duck Embryonic Stem Cell-derived Substrate for the Industrial Production of Therapeutic Monoclonal Antibodies with Enhanced ADCC Activity.” Monoclonal Antibodies 2:405–415.
- Pain, B., M. Clark, M. Shen, H. Nakazawa, M. Sakurai, J. Samarut, and R. Etches. 1996. “Long-Term in Vitro Culture and Characterisation of Avian Embryonic Stem Cells with Multiple Morphogenetic Potentialities.” Development 122: 2339–2348.
- Park, T. S., and J. Y. Han. 2000. “Derivation and Characterization of Pluripotent Embryonic Germ Cells in Chicken.” Molecular Reproduction and Development 56: 475–482. doi:10.1002/(ISSN)1098-2795.
- Petitte, J., G. Liu, and Z. Yang. 2004. “Avian Pluripotent Stem Cells.” Mechanisms of Development 121: 1159–1168. doi:10.1016/j.mod.2004.05.003.
- Petitte, J., L. Karagenc, and M. Ginsburg. 1997. “The Origin of the Avian Germ Line and Transgenesis in Birds.” Poultry Science 76: 1084–1092. doi:10.1093/ps/76.8.1084.
- Pinkert, C. A. 2014. Transgenic Animal Technology: A Laboratory Handbook. US: Gulf Professional Publishing.
- Reiter, M., D. Portsmouth, and P. N. Barrett. 2014. “Avian Suspension Culture Cell Lines for Production of Vaccines and Other Biologicals.” Industrial Scale Suspension Culture of Living Cells 11: 390–409.
- Sart, S., Y.-J. Schneider, Y. Li, and S. N. Agathos. 2014. “Stem Cell Bioprocess Engineering Towards cGMP Production and Clinical Applications.” Cytotechnology 66: 709–722. doi:10.1007/s10616-013-9687-7.
- Shittu, I., Z. Zhu, Y. Lu, J. M. Hutcheson, S. L. Stice, F. D. West, M. Donadeu, B. Dungu, A. M. Fadly, and G. Zavala. 2016. “Development, Characterization and Optimization of a New Suspension Chicken-Induced Pluripotent Cell Line for the Production of Newcastle Disease Vaccine.” Biologicals 44: 24–32. doi:10.1016/j.biologicals.2015.09.002.
- Skottman, H., and O. Hovatta. 2006. “Culture Conditions for Human Embryonic Stem Cells.” Reproduction 132: 691–698. doi:10.1530/rep.1.01079.
- Stein, A. 2007. “Decreasing Variability in Your Cell Culture.” Biotechniques 43: 228–229. doi:10.2144/000112561.
- Tang, X., C. Zhang, Y. Jin, C. Ge, and Y. Wu. 2007. “Pro-Proliferating Effect of Homologous Somatic Cells on Chicken Primordial Germ Cells.” Cell Biology International 31: 1016–1021. doi:10.1016/j.cellbi.2007.03.014.
- Taylor, L., D. F. Carlson, S. Nandi, A. Sherman, S. C. Fahrenkrug, and M. J. Mcgrew. 2017. “Efficient TALEN-mediated Gene Targeting of Chicken Primordial Germ Cells.” Development 144:928–934.
- Trefil, P., J. Mucksová, and J. Kalina. 2012. “Chicken Testicular Stem Cells.” The Journal of Poultry Science 49: 150–154. doi:10.2141/jpsa.011167.
- van de Lavoir, M. C., and C. Mather‐Love. 2006. “Avian Embryonic Stem Cells.” Methods in Enzymology 418: 38–64.
- van de Lavoir, M.-C., C. Mather-Love, P. Leighton, J. H. Diamond, B. S. Heyer, R. Roberts, L. Zhu, P. Winters-Digiacinto, A. Kerchner, and T. Gessaro. 2006. “High-Grade Transgenic Somatic Chimeras from Chicken Embryonic Stem Cells.” Mechanisms of Development 123: 31–41. doi:10.1016/j.mod.2005.10.002.
- Wei, Q., K. L. Woods, and R. J. Etches. 2000. “Long-Term Culture of Chicken Blastodermal Cells (Cbcs) and Selection of Transfected CBCs Using Antibiotic Resistance.” Developmental Biology Protocols: Volume II 136: 399–403.
- Whyte, J., J. D. Glover, M. Woodcock, J. Brzeszczynska, L. Taylor, A. Sherman, P. Kaiser, and M. J. Mcgrew. 2015. “FGF, Insulin, and SMAD Signaling Cooperate for Avian Primordial Germ Cell Self-Renewal.” Stem Cell Reports 5: 1171–1182. doi:10.1016/j.stemcr.2015.10.008.
- Yu, M., S. Lian, H. Han, K. Yu, G. Li, Z. Lian, and N. Li. 2013. “Four Recombinant Pluripotency Transcriptional Factors Containing a Protein Transduction Domain Maintained the in Vitro Pluripotency of Chicken Embryonic Stem Cells.” Science China Life Sciences 56: 40–50. doi:10.1007/s11427-012-4426-4.
- Yu, P., Y. Lu, B. J. Jordan, Y. Liu, J.-Y. Yang, J. M. Hutcheson, C. L. Ethridge, J. L. Mumaw, H. A. Kinder, and R. B. Beckstead. 2014. “Nonviral Minicircle Generation of Induced Pluripotent Stem Cells Compatible with Production of Chimeric Chickens.” Cellular Reprogramming (Formerly” Cloning and Stem Cells”) 16: 366–378.