Recombinant lactobacillin PlnK adjusts the gut microbiome distribution in broilers
L. Xu, J. Zhou, G. Qu, Z. Lin, Q. Fan, C. Wang and Q. Wang
British Poultry Science
Volume 61, Number 4, pages 390-399
DOI: https://doi.org/10.1080/00071668.2020.1752911
When this article was first published, errors were discovered in the following sections:
Materials and methods, Compliance with ethical standards section, page 391
The revised text is: The animal protocols used in this study were approved by the Research Ethics Committee of the College of Animal Science (College of Bee Science), Fujian Agriculture and Forestry University, Fujian, China.
Indicator bacteria section, page 391
The revised text is: S. aureus, E. coli and Salmonella pullorum were incubated in liquid nutrient agar culture medium and P. multocida was incubated in liquid nutrient agar culture medium with 10% bovine calf serum.
, page 391
Table 1 has been revised as follows:
Bacteriostatic effect of PlnK on indicator bacteria section, page 392
The revised text is: After resuscitation of the indicator bacteria (E. coli, S. aureus, Salmonella pullorum and Pasteurella multocida) the antibacterial effect of the recombinant PlnK, at concentrations of 0.50 × 10−2 mg/ml (the purified PlnK, 0.51 mg/ml, 9.8μl), 2.50 × 10−2 mg/ml (the purified PlnK, 0.51 mg/ml, 49 μl), 5.00 × 10−2 mg/ml (the purified PlnK, 0.51 mg/ml, 98 μl) in 1000 μl, was analysed using the McIntosh turbidimetric assay.
Table 2 footnote, page 392
The revised text is: NB. Nutrient levels in the table are all calculated. The feed formulation was designed for broilers aged 1 ~ 7 days. The premix included the following, per kilogram feed: Cu 8 mg, Fe 72 mg, Mn 78 mg, Zn 60 mg, I 0.36 mg, Se 0.24 mg, choline chloride 600 mg, retinoids 29.7 mg, cholecalciferol 0.055 mg, tocopherol 22 mg, menadione 2.2 mg, thiamine 2.2 mg, riboflavin 5.5 mg, pyridoxine 2.2 mg, cobalamin 0.0165 mg, niacin 22 mg, calcium pantothenate 11 mg, folic acid 1.1 mg, coenzyme R 0.088 mg, methionine 2364 mg.
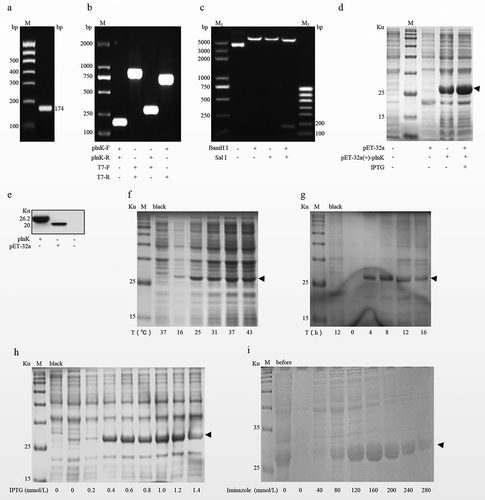
Results - Inducible expression of the pET-32a(+)-plnK protein in vitro, page 394
The revised text is: Specific bands of 26.2 ku and 20 ku were present in the pET-32a(+)-plnK recombinant plasmid group and the pET-32a(+) group, respectively, but there was no band in the mock control group ((e)).
Table 3 The bacteriostatic effect of PlnK on indicator bacteria in vitro
, Page 395
The revised table is:
Captions for , 3, 4, 5 and 6
These have been amended as follows:
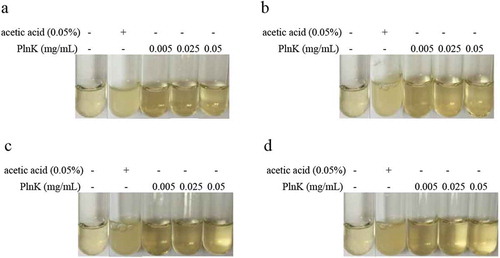
Figure 2. Inhibitory effect of recombinant PlnK on four indicator bacteria (a) Staphylococcus aureus, (b) Escherichia coli; (c) Salmonella pullorum were cultured with recombinant Plnk (0.005–0.05 mg/ml) in 1000 μL liquid nutrient agar culture medium, 0.05%, as the acetic acid control. (d) Pasteurella multocida was cultured with recombinant Plnk (0.005–0.05 mg/ml) in 1000 μL of liquid nutrient agar culture medium with 10% bovine serum, 0.05%, as the acetic acid control. The cuvettes were incubated at 37℃ for 24 h. The relative quantity of the four indicator bacteria were observed by perusal and examined using the McIntosh turbidimetric assay.
Figure 3. Relative abundance of intestinal microflora at the phylum level. Fifty four-day-old broilers were randomly divided into five treatment groups, in which 10 broilers were fed with a basal diet and administered three concentrations of PlnK (group III:5.00 × 10˗3 mg/ml, group Ⅳ: 2.5 × 10˗3 mg/ml, group V: 1.25 × 10˗3 mg/ml) respectively; 10 broilers were fed with a basal diet (group Ⅰ) and other 10 were administered acetic acid (group Ⅱ: final pH = 5.0) as the control, they were fed with water as the mock control group. After being fed with recombinant PlnK for one week, the total DNA of microflora 16 S in the caecal contents of each treatment group was extracted and sequenced by HiSeq. The relative abundance of the 10 most common intestinal microflora and the thermal map were presented as a and b. The significance between the treatments (c) was determined by One-Way ANOVA and the LSD test using SPSS software (Version 19.0).
Figure 4. Relative abundance of intestinal microflora at family level. Fifty four-day-old broilers were randomly divided into five treatment groups, in which 10 broilers were fed with a basal diet and administered three concentrations of PlnK (group III:5.00 × 10˗3 mg/ml, group Ⅳ: 2.5 × 10˗3 mg/ml, group V: 1.25 × 10˗3 mg/ml) respectively; 10 broilers were fed with a basal diet (group Ⅰ) and other 10 were administered acetic acid (group Ⅱ: final pH = 5.0) as the control, they were fed with water as the mock control group. After being fed with recombinant PlnK for one week, the total DNA of microflora 16 S in the caecal contents of each treatment group was extracted and sequenced by HiSeq. The relative abundance of the 10 most common intestinal microflora and the thermal map were presented as a and b. The significance between the treatments (c) was determined by One-Way ANOVA and the LSD test using SPSS software (Version 19.0).
Figure 5. Relative abundance of intestinal microflora at genus level. Fifty four-day-old broilers were randomly divided into five treatment groups, in which 10 broilers were fed with a basal diet and administered three concentrations of PlnK (group III:5.00 × 10˗3 mg/ml, group Ⅳ: 2.5 × 10˗3 mg/ml, group V: 1.25 × 10˗3 mg/ml) respectively; 10 broilers were fed with a basal diet (group Ⅰ) and other 10 were administered acetic acid (group Ⅱ: final pH = 5.0) as the control, they were fed with water as the mock control group. After being fed with recombinant PlnK for one week, the total DNA of microflora 16 S in the caecal contents of each treatment group was extracted and sequenced by HiSeq. The relative abundance of the 10 most common intestinal microflora and the thermal map were presented as a and b. The significance between the treatments (c) was determined by One-Way ANOVA and the LSD test using SPSS software (Version 19.0).
Figure 6. The content of sIgA in duodenum mucosa of broilers. In order to investigate whether the recombinant PlnK could increase mucosal immune function, 50 four-day-old broilers were randomly divided into five treatment groups, in which 10 broilers were fed with basal diet including drinking three concentrations of PlnK (group III:5.00 × 10˗3 mg/ml, group Ⅳ: 2.5 × 10˗3 mg/ml, group V: 1.25 × 10˗3 mg/ml), respectively;10 broilers were fed with a basal diet (group Ⅰ) and other 10 were administered acetic acid (group Ⅱ: final pH = 5.0) as the control, they were fed with water as the mock control group. After being fed with recombinant PlnK for one week, three broilers in each group were selected and the levels of sIgA in the duodenal mucus were examined by double antibody sandwich ELISA. All data are given as mean ± standard error (SE) of three independent experiments. Significance between the treatments was determined by One-Way ANOVA and the LSD test using SPSS software (Version 19.0).
Figures 1 and 2
The revised versions are shown here: