ABSTRACT
1. An in vitro test to study the effect of pH reduction on phytic acid degradation over time for four commercial phytases was conducted. Changing the pH level affected phytate degradation over time differently for the various phytases (P < 0.05).
2. The phytase with the largest response of pH reduction in the in vitro test and a feed pH level of 4.5 was chosen for the broiler experiment. The effect of intermittent feeding, addition of 500 FYT C. braakii-derived phytase and 1% formic acid were tested in a 2 x 2 x 2 factorial arrangement. Ten pens containing 10 birds each were fed each of the treatment combinations from 15 to 36 d of age. Ad libitum fed birds had two 4-h dark periods with 2-h light in-between, while intermittently fed birds in addition had restricted access to the feed through except for four 1-h and one 2-h feeding bouts.
3. In addition to assessing performance, excreta were collected on a pen basis. The tibia and contents from jejunum and ileum were collected from one bird per pen. In addition, crop contents were collected from the intermittently fed birds 80, 160 and 240 min after the start of feeding.
4. Phytase improved performance, ileal and jejunal P digestibility, P retention and tibia ash and P concentration (P < 0.001). Intermittent feeding increased jejunal P digestibility and P retention (P < 0.001), but ileal P digestibility increased only in the intermittently fed birds compared to the ad libitum fed birds without phytase addition (P = 0.025). Acidification increased jejunal P digestibility only in the ad libitum fed birds (P = 0.019). There was a considerable inositol hexakisphosphate degradation into lower isomers in the crop after 80 min for diets with phytase (InsP3 and 4:P < 0.001), and acidification further increased this degradation (InsP4:P = 0.007). After 160 min retention time, the effect of phytase and acidification was even higher with more significant (P < 0.05) interactions.
5. The current data showed that prolonged retention time in the crop combined with feed acidification increased phytase efficacy by improving the phytate degradation.
Introduction
A large part of phosphorus (P) in grains and legumes is found in the form of phytic acid, which cannot be degraded by enzymes secreted by chickens (Selle and Ravindran Citation2007). To increase P utilisation and avoid environmental pollution, exogenous phytase is commonly used as a feed additive in poultry nutrition. However, even with phytase addition, the degradation of phytate is incomplete, as indicated by Slominski (Citation2011) who found an increased liberation of P from 19% without phytase addition to 38% with phytase addition.
The optimal pH range for most new-generation phytases is between 4.0 and 5.0 (Menezes-Blackburn et al. Citation2015; Tamim et al. Citation2004; Vieira et al. Citation2018). In addition, phytate may be resistant to hydrolysis when the pH is raised above 4.0 due to the formation of mineral-phytate complexes (Angel et al., Citation2002; Selle and Ravindran Citation2007). Although the gizzard is considered an important site for phytase activity, the pH is usually too low, being between 2.0 and 4.0 (Svihus Citation2011) for phytases to exert optimal activity. Similarly, the pH in the small intestine is too high at between 6.5 and 7.5 (Svihus Citation2011). Due to this, the crop has been considered a major site for exogenous phytase activity (Sommerfeld et al. Citation2018; Zeller et al. Citation2015).
Exogenous enzymes that are added to the diet are activated and start to exert their effect in the crop, as demonstrated by Svihus et al. (Citation2010) and Zeller et al. (Citation2015). However, the pH in the crop is normally at the same level as in the feed by the time the feed enters the crop. Thus, since chicken feeds normally have a pH between 6.0 (Ao et al. Citation2008) and 6.5 (Sacranie et al. Citation2017), this will possibly be a limiting factor for phytase efficacy.
Phytase efficacy is dependent on the retention time in the favourable crop environment (Tamim et al. Citation2004). Svihus et al. (Citation2010) previously found increased degradation of myo-inositol hexakisphosphate (InsP6) in the crop over time. Depending on the feeding regime, the retention time in the crop may be considerable (Sacranie et al. Citation2017; Svihus et al. Citation2010). Birds adapted to intermittent feeding are able to store larger quantities of feed in their crop (up to 50 g DM), which gradually disappears from the crop over a period of up to 5 h (Sacranie et al. Citation2017). In contrast, Svihus et al. (Citation2010) found that 78% of ad libitum fed birds had under 5 g DM of feed in the crop. Thus, stimulating retention of feed in the crop could possibly increase phytate degradation when phytase is used. Svihus et al. (Citation2013) were not able to demonstrate an effect of intermittent feeding on phytase efficacy. However, Sacranie et al. (Citation2017) found a higher P and InsP6 digestibility in duodenum/jejunum with intermittent feeding.
Retention time in the crop is associated with considerable fermentation activity by Lactobacilli spp. and the subsequent production of lactic acid reduces the pH in crop over time (Cutler et al. Citation2005; Jozefiak et al. Citation2006). A reduction in the pH by acid addition, as demonstrated by Kim et al. (Citation2015), could be an effective way to improve phytase activity.
Previous research has shown that the simultaneous addition of organic acid and phytase improved phytase efficacy. Emami et al. (Citation2013) and Woyengo et al. (Citation2010) found increased ileal P digestibility by combining organic acids with phytase. In addition, Vieira et al. (Citation2017) performed a meta-analysis which showed that adding both phytase and citric acid improved bird weight gain and ash content in the tibia.
Therefore, the following experiment was performed to test the hypothesis that acidification of the diet could improve the efficacy of an exogenous phytase, which would be further improved by increased crop retention time caused by to intermittent feeding.
Materials and methods
In vitro test
A simple in vitro procedure simulating crop activity, based on the work of Zyla et al. (Citation1995), was used to test four commercial phytase products at selected pH levels and incubation times. The phytases came from the following source organisms: Escherichia coli, Buttiauxella sp., Aspergillus niger and Citrobacter braakii, arbitrarily denoted phytase A, B, C and D. A high-phytate diet () was mixed in a 6 l twin-shaft paddle mixer (Forberg, Sandefjord, Norway) from pre-ground raw materials. The feed had the same vitamin, mineral and enzyme content as a commercial chicken feed, with no phytase added.
Table 1. Composition and nutrient content of the basal diets used in the in vitro trial and broiler experiment (g/kg as fed unless otherwise stated)
Formic acid (98%) (Merck, Darmstadt, Germany) was used to adjust the pH. The level needed to achieve the desired pH levels was determined through a pilot trial, where formic acid was incrementally added to the experimental diet and pH was measured immediately and after 5 min when pH appeared to be stabilised. The pH was measured using a pH meter (pH 100, VWR International, Radnor, PA, USA). In both the pilot trial and the in vitro test, 1.0 g (±0.01 g) of the diet was added to a test tube containing 5.0 ml deionised water. The amount of formic acid added was 0, 4.0, 13.0 and 50.0 µl to reach pH 6.7, 5.5, 4.5 and 3.5, respectively. Phytase (0 or 500 FYT) and the specified amount of formic acid were added to the test tube and vortex mixed. The test tubes were incubated in a 40°C water bath and shaken by hand every 5 min during incubation. After allowing enough incubation time to be relevant to crop retention times of 10, 20, 30, 45 or 60 min, the enzymatic reaction was terminated by adding 5.0 ml 4% trichloroacetic acid (TCA) (Sigma-Aldrich, St. Louis, MO, USA). The test tubes were centrifuged at 3000 rpm for 15 min, followed by collection and freezing of the supernatant at −20°C until analysis for free phosphate. Each treatment combination was analysed four times, except for the negative control without phytase, which was only analysed in duplicate.
Broiler management
According to Polish law and EU directive (no 2010/63/EU), the experiments conducted within this study did not require the approval of the Local Ethical Committee for Experiments on Animals in Poznań, Poland. However, all activities complied with the guidelines of the Committee with respect to animal experimentation and care of the animals under the study.
Eight hundred, 1-d-old male Ross 308 chickens were randomly assigned to eight different treatments in 80 pens. The pens were arranged in the middle of an environmentally controlled broiler house (PIAST PASZE Sp. z o.o., Experimental Unit no. 0616, Olszowa, Poland) with 9,000 loose-housed birds hatched at the same time surrounding the pens, which were not included in the experiment. The birds were fed diets with or without phytase and with or without formic acid ad libitum or intermittently in a 2 × 2 × 2 factorial design with 10 replicate pens per treatment combination. The pens (100 cm x 100 cm) were made of wire, so birds had visual contact with surrounding birds. The pens with the ad libitum fed birds were located along one drinking line and the intermittently fed bird were located along a second drinking line to minimise behavioural influence between the treatments. Straw was used as bedding, and a temperature of 33°C was maintained during the first week and then reduced weekly by 2–3°C to 21°C on d 28. The birds were maintained on a commercial pelleted diet produced by the Piast Pasze Feed Mill (Lewkowiec, Poland) until d 15. From d 11, the intermittently fed birds had, based on several previously intermittent feeding schedules proposed by Sacranie et al. (Citation2012), access to feed between the hours of 08:00 to 09:00, 12:00 to 13:00, 16:30 to 17:30, 21:00 to 22:00 and from 02:00 to 04:00. The feeders were removed from the cages between these times, except from 22:00 to 02:00 and 04:00 to 08:00 when the light was turned off. All birds were given the experimental diets from d 15 to 36 and had ad libitum access to water throughout the experimental period.
Experimental diets
Based on the strong effect on pH from the in vitro, the Citrobacter braakii-derived phytase was chosen and the desired pH of the acidified feed was set to 4.5. A high-phytate diet () containing 5 g/kg titanium dioxide (TiO2) as a digestibility marker was produced at the Centre for Feed Technology (Ås, Norway). Half of the soy oil was added to the mash before pelleting, while the remaining amount was added after pelleting. For the two diets containing phytase, 500 FYT of Ronozyme® HiPhos (DSM, Denmark) was added per kg feed. The phytase was diluted in a small amount of water that did not influence the water content of the diet, to ensure even distribution and sprayed on the pellet before the soy oil and acid were added. The two diets with acid contained 1.0% of 85% formic acid (POCH, Avantor Performance Materials, Poland). The analysed activity was 520 FYT/kg for the feed with added phytase and 385 FYT/g for feed with both phytase and acid added, while phytase activity for the diets without phytase was below detection level. Diet pH was measured in samples taken from the feeders in the chicken house, using the same method as the in vitro test. The feed pH in the negative control (NC), phytase added (NC+phy), formic acid added (NC+acid) and formic acid and phytase added (NC+phy+acid) was 6.7, 6.6, 5.1 and 4.9, respectively. The level of the different inositol phosphate (InsP) isomers per g DM feed was 26.33 µmol InsP6, 1.1 µmol Ins(1,2,4,5,6)P5, 0.7 µmol Ins(1,2,3,4,5)P5, 0.4 µmol Ins(1,2,3,4,6)P5 and 0.15 µmol InsP4.
Performance and sample collection
Body weight (BW) and feed intake (FI) were recorded weekly on a per pen basis. Excreta was collected on d 28 by placing paper sheets on pen floors at the start of feeding at 12:00, followed by repeated manual collection of excreta without contamination during the next 4 h. The excreta from each pen were pooled and mixed before a representative sample was taken.
On d 36, one random bird from each pen of the ad libitum fed birds was killed by a blow to the head followed by cervical dislocation. Thereafter, a zip tie was tightened around the neck to avoid loss of crop content. Euthanasia was initiated 4 h after the light came on at 08:00 to give the birds sufficient feeding time. On d 37, one randomly selected intermittently-fed bird from each pen was killed at 80, 160 and 240 min after the start of feeding. On d 36, feeding was adjusted to 10 min between groups, to ensure that all intermittently fed birds were killed at the same time interval after feeding. The crop from each bird was emptied and pH was measured in the contents by inserting the electrode of a pH meter (pH 100, VWR International, Radnor, PA, USA) into the sampling container. For ad libitum and intermittently fed birds killed after 240 min, the gizzard pH was measured by placing the pH electrode directly into the gizzard. In addition, the left tibia and the contents of the jejunum and last two-thirds of the ileum were collected. Tibias were frozen at −20°C and stored until analysis. Other samples were immediately stored in dry ice and kept frozen at −20°C until lyophilising. Due to insufficient crop contents, pH could not be measured in two, 10 and 22 crops after 80, 160 and 240 min, respectively, with approximately equal numbers of missing values between treatments. A minimum of 0.5 g DM was required for the InsP analysis. Thus, the InsP values could not be determined in four, 15 and 27 crop samples after 80, 160 and 240 min, respectively.
Chemical analyses
Free phosphate was quantified by using a modification of the ammonium molybdate method (Heinonen and Lahti Citation1981). Briefly, a solution with ascorbic acid, sulphuric acid and water, and a second solution with ammonium molybdate and water was mixed with water in the ratio 5:1:10 to form a colour reagent. In total, 30 µl of supernatant was mixed with 240 µl colour reagent in microtitre plates, shaken on a microplate and incubated at 37°C for 60 min. After incubation, the absorbance at 820 nm was measured on a SpectraMax M2e (Molecular Devices, San Jose, CA, USA) and the amount of P released per kg of feed was calculated.
All digesta and excreta samples were lyophilised and homogenised. Lyophilised DM content was used for all calculations. Crude protein in the feed was determined by the Kjeldahl N method with a Kjel-Foss Automatic 16 210 (Foss Electric, Hillerød, Denmark), according to AOAC (Citation2005) no. 976.05. The excreta, digesta and feed were analysed for TiO2 content using the method described by Short et al. (Citation1996). The P in the diets and digest samples was analysed by adding a solution of HClO4, HNO3 and H2SO4 to the samples, which was mineralised until the solution became colourless. Thereafter, an ammonium molybdate solution was added and the samples were read on a Marcel Media spectrophotometer (Marcel S.A., Zielonka, Poland) at 720 nm. Ileal digesta were analysed enzymatically for starch content by the method of McCleary et al. (Citation1994). In short, the starch was depolymerised by thermostable α-amylase and amyloglucosidase to glucose. Thereafter, the concentration of glucose was read on a Halo SB-10 Spectrophotometer (Dynamica Scientific Ltd., Livingston, UK). One unit (FYT) of phytase was defined as the activity that released 1 μmol inorganic phosphate from 5.0 mM phytate per minute at pH 5.5 and 37°C, and the phytase activity in the diets was determined by the ISO Standard 30024 (Citation2009) method. Soft tissue from tibia was removed by hand, and dry matter and ash content were determined after drying for 16 h at 104°C and 16 h ashing at 550°C, respectively. The P was analysed from the tibia according to the FAO (Citation2011) method. Briefly, HCl was added to the samples and the solution was mineralised until it became colourless. Thereafter, ammonium molybdate solution was added and the samples were read on a MaxMat PL II Multi-analyser (MaxMat, France) at 340 nm. Inositol phosphates were extracted using the method described by Zeller et al. (Citation2015). Briefly, samples were extracted with a solution containing 0.2 M EDTA and 0.1 M NaF using a rotary shaker. The extracts were filtered through a 0.2-μm cellulose acetate filter (VWR, Darmstadt, Germany) into a Microcon® filter and centrifuged at 14,000 x g for 30 min. Filtrates were analysed using high-performance ion chromatography and UV detection at 290 nm after post-column derivatisation using an ICS-3000 system (Dionex, Idstein, Germany). InsPs with different degrees of phosphorylation (InsP3 to InsP6) and their positional isomers were separated without enantiomer differentiation on a CarboPac PA 200 column and corresponding guard column. An Fe(NO3)3 solution in HClO4 was used as the reagent for derivatisation in accordance with Phillippy and Bland (Citation1988). The elution order of InsPs was established using commercial standards, where available.
Calculations
The P retention and ileal and jejunal digestibility coefficient of P and starch were calculated using the following formula: Nutrient digestibility coefficient/retention = 1 – [([TiO2]diet/[TiO2] digesta) × ([nutrient]digesta/[nutrient]diet)]
Statistical analyses
The general linear model procedure in SAS software 9.4 (SAS Inst. Inc., Cary, NC, USA) was used with the Ryan–Einot–Gabriel–Welsh F-test to investigate differences (P < 0.05) between the different treatment groups. P-values between 0.05 and 0.1 were considered tendencies. The square root of means square error (√MSE) was used as a measure of random variation. Results for each phytase in the in vitro test were subjected to a two-way ANOVA, with pH and incubation time as main effects and each replicate as the experimental unit. Performance, digestibility and tibia data were subjected to a three-way ANOVA with phytase, acid and feeding regimen as the main effects. Pen was used as the experimental unit. Crop pH in intermittently fed birds was subjected to a two-way ANOVA, with time and acidification as the main effects. Crop dry matter content in intermittently fed birds and crop pH in ad libitum fed birds were subjected to a simple one-way ANOVA with time and acid as effects, respectively. Concentration of InsP isomers and InsP6 degradation was subjected to a two-way ANOVA with acid and phytase addition as the main effects.
Results
In vitro test
The amount of P in the form of free phosphate in the feed was 0.61 mg/g. The highest amount of P released without phytase addition (P < 0.05) occurred at pH 5.5, followed by pH 4.5, 6.7 and 3.5 () and the increase in P released with higher incubation time differed between the pH levels (P < 0.001).
Table 2. Released phosphorus (mg/g feed) from test feed in the negative control with no exogenous phytase added and with four different commercial phytases, at four different pH levels and five different incubation times (in vitro trial)
The numerical highest P released was found with phytase A at pH 4.5 and 60 min incubation time. For phytase B, D and C the highest amounts of P released occurred at pH 3.5 after 45, 45 and 60 min incubation time, respectively. The effect of pH reduction and increased incubation time was different for the four phytases, and there was a significant (Phytase A; P = 0.001, phytase B; P = 0.002, phytase C; P = 0.041, Phytase D; P < 0.001) interaction between pH and time. Phytase D had the largest increase in phosphorous released at a reduced pH and the largest response to increased incubation time. Phytase A had the smallest effect of reduced pH and increased incubation time.
Broiler performance
Intermittent feeding resulted in a lower FI and a lower body weight gain (BWG) (P < 0.001) than ad libitum feeding (). Acidification of the feed tended to reduce FI (P = 0.059). There was a tendency for an interaction (P = 0.057) between phytase and formic acid for BWG, whereby reduced BWG with acidification was only observed when no phytase was used. Phytase increased FI, BWG and feed conversion ratio (FCR; P < 0.001). Mortality (data not shown) was 4.25% and treatment had no impact on this parameter.
Table 3. Effects of intermittent feeding, formic acid and phytase addition on the performance of broilers from d 11 to 36
Dry matter content (data not shown) in the crop for intermittently fed birds was reduced (P < 0.001) from 16.4 g at 80 min after the start of feeding to 7.3 and 2.5 g after 160 and 240 min, respectively. In ad libitum fed birds, the average dry matter content in the crop was 8.8 g.
The pH in the crop for intermittently fed birds given feed containing acid was significantly lower (P < 0.001) than those given feed without acid (). The same significant pattern (P = 0.019) was seen for the ad libitum fed birds, with a crop pH of 4.3 in with acid and 4.9 without acid. For intermittently fed birds, crop pH was higher (P < 0.001) at 80 and 160 min after the start of feeding compared to 240 min after feeding. The pH in the gizzard (data not shown) was reduced (P = 0.004) from 2.3 to 2.0 with intermittent feeding and increased (P = 0.041) from 2.0 to 2.2 with phytase addition.
Table 4. The pH in crop of intermittently fed chickens with or without formic acid addition to the feed at 80, 160 and 240 min after the start of feeding.A
As shown in , phytase addition increased tibial ash and P content, when expressed per kg body weight (BW). The tendency of a three-way interaction for tibial P per kg BW (P = 0.083) was caused by acidification of the feed, which increased P mineralisation of the bone (from 0.13 to 0.16) but only when no phytase was used in the ad libitum fed birds. Intermittent feeding did not influence the phosphorus digestibility coefficient (PDC) in the ileum but increased P retention (P < 0.001). An interaction (P = 0.025) between phytase and feeding regimen was observed, whereby intermittent feeding increased ileal PDC, but only when phytase was not added to feed. An interaction (P = 0.019) between feeding regime and acidification was observed on jejunal PDC, whereby acidification of the feed increased jejunal PDC only in ad libitum fed birds. A tendency (P = 0.078) for an interaction between acidification and phytase was observed, whereby acid in feed increased jejunal PDC only when phytase was used. There was no difference (P > 0.05) in ileal starch digestibility coefficients between the different treatments.
Table 5. Effects of intermittent feeding, formic acid and phytase addition on bone mineralisation and P and starch digestibility coefficients in selected segments of the intestine
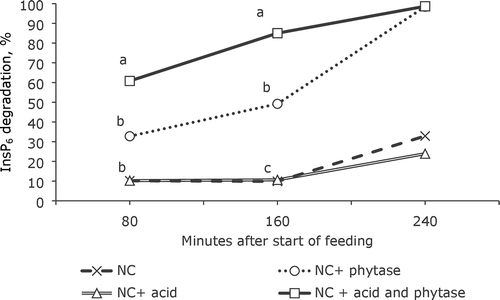
shows the InsP6 degradation in crop at 80, 160 and 240 min after the start of feeding. Phytase addition increased (P < 0.001) InsP6 degradation in the crop of intermittently fed birds at 80 and 160 min after the start of feeding (P-values not shown in the figure). After 80 min, acidification tended (P = 0.078) to increase InsP6 degradation, and this effect was significant (P = 0.028) after 160 min. A tendency (P = 0.079) for an interaction between acidification and phytase was observed after 80 min, whereby acid supplementation increased InsP6 degradation but only in diets containing phytase. This effect was significant (P = 0.034) after 160 min. After 240 min, only three samples from birds fed the phytase-containing diets contained enough material for analysis of InsP-isomers. The average degradation of InsP6 in these samples was 98.6%; however, the low number of samples precluded a proper evaluation of the significance of these data.
Figure 1. InsP6 degradation (%) in the crop digesta 80, 160 and 240 min after start of feeding. The InsP values were based on 10 NC, 7 NC+phy, 10 NC+acid and 9 NC+phy+acid crops at 80 min, 8 NC, 4 NC+phy, 8 NC+acid and 5 NC+phy+acid crops at 160 min, and 3 NC, 1 NC+phy, 7 NC+acid and 2 NC+phy+acid crops at 240 min. Treatment means within time with different letters are significantly different (P < 0.05), √MSE is 22.89 for 80 and 18.52 for 160 min. The InsP6 values for 240 min have not been included in the statistics
Phytase addition reduced the concentration (P < 0.001) of InsP6, Ins(1,2,4,5,6)P5 and Ins(1,2,3,4,6)P5 and increased the concentration (P < 0.001) of InsP4 and InsP3 in the crop 80 min after the start of feeding (). Acid addition increased the concentration of Ins(1,2,3,4,5)P5 (P = 0.007). The only interaction found between acid and phytase at 80 min after the start of the feeding was that acid addition increased the concentration of InsP4 (P = 0.007) when phytase was used, but not without phytase. After 160 min there was an interaction for all isomers except Ins(1,2,3,4,5)P5, whereby acid addition reduced the concentration only when phytase was added.
Table 6. Effect of acid and phytase addition on the concentration of different inositol phosphate isomers (InsPs) (µmol/g DM) in the crop digesta of intermittently fed birds at 80 and 160 min after the start of feeding
Discussion
As expected, with the high-phytate diet in this experiment, phytase increased performance, PDC and tibial ash. Phytase started to hydrolyse phytate already in the crop, as demonstrated in the previous research (Sommerfeld et al. Citation2018; Svihus et al. Citation2010). The current experiment showed contradictory results regarding the interaction between increased retention time and acidification on the efficacy of phytase, as there was an interaction between acid and phytase on phytate degradation in the crop, but no clear effect was found in P digestibility measurements.
The reduced feed intake and BWG seen with intermittent feeding were in agreement with the results obtained by Sacranie et al. (Citation2017), where intermittently fed birds only had access to feed 5 h per day. However, other experiments, where intermittently fed bird had 6 h access to feed per day, showed no difference in FI and BWG between intermittent and ad libitum feeding (Sacranie et al. Citation2012; Svihus et al. Citation2010).
The difference in contents in the crop between ad libitum and intermittently fed birds was smaller than expected, based on previous research. Sacranie et al. (Citation2017) found the crop contents of intermittently fed birds were significantly higher than for the ad libitum fed birds at 180 min after the start of feeding. However, in the current experiment, there was no difference in contents between ad libitum and intermittent fed groups after 160 min. The low amount of feed in the crop of the intermittently fed birds in the current experiment might have contributed to a small difference in the crop content between intermittently and ad libitum fed birds. The amount of feed in the crop for the intermittently fed birds after 240 min was higher in the experiment of Sacranie et al. (Citation2017) than in the current trial. The amount of crop contents in the ad libitum fed birds in the current experiment was higher than previously reported by Svihus et al. (Citation2010), who found that 78% of ad libitum fed birds (age 31 to 39 d) had less than 5 g of dry matter in their crop, whereas in the current experiment this was 49%. A different number of chickens in each pen compared to previous experiments, in addition to the fact that a commercial flock surrounded the pens, could have influenced the feeding behaviour and led to this difference.
The increased InsP6 degradation in the crop from 80 to 160 min after feeding in diets with phytase addition was in accordance with the findings of Svihus et al. (Citation2010). This suggested that the degradation of phytate in the anterior digestive tract was higher for the intermittently fed birds due to increased retention time. Similarly, increased P retention with intermittent feeding may indicate that even higher retention time alone without phytase addition may be favourable for total P retention.
It may be speculated that the higher ileal PDC without phytase addition for intermittently compared to ad libitum fed birds for diets without phytase was due to the prolonged time in the crop and, thus, intrinsic phytase could have had more time in the crop to degrade the phytate. In the in vitro test, the amount of released P increased with time (up to 60 min) even when no phytase was added. A 22% disappearance of InsP6 was previously reported in a wheat-based diet without phytase added in an in vitro trial by Sommerfeld et al. (Citation2017). A possible explanation for increased phytate degradation in vitro without the addition of exogenous phytase could be due to high intrinsic phytase activity in wheat (>1000 U/kg, Eeckhout and De Paepe Citation1994). The intrinsic phytase activity in corn is low (<100 U/kg; Eeckhout and De Paepe Citation1994) and hence the hypothesis was supported by the lack of InsP6 degradation in the corn-based diet without phytase addition (Sommerfeld et al. Citation2017). However, in the current experiment, the lack of increase in InsP6 degradation from 80 to 160 min after feeding in diets without exogenous phytase indicated that there was no phytase activity in the crop after 80 min. The difference in the effect of diets without phytase between the current in vivo and in vitro trials could partly be explained by the differences in incubation time, where the maximum time in the in vitro experiment was 60 min, and the measurements in the in vivo experiment started 80 min after feeding. In addition, the feed used in the in vitro trial was not heat-treated; hence, no inactivation of intrinsic phytase had taken place. Conversely, in the diet used in the in vivo experiment, no phytase activity was analysed, probably because of the presumed inactivation of intrinsic phytase with heat treatment (Esmaeilipour et al. Citation2012a).
Contrary to what was intended, a slight reduction in phytase was detected in the feed containing both formic acid and phytase. One possible explanation for this could be the addition of the acid together with the phytase on top of the pellet, which may have resulted in degradation and inactivation of the phytase in the final diet. This hypothesis was reinforced by phytase activity analyses, which was carried out on the feed before acid addition, where the phytase activity was 544 FYT/kg feed. The results of acidification and interaction between acid and phytase must be interpreted in consideration of the difference in phytase activity.
The tendency for reduced FI with acidification in the current study has been reported in other experiments, where high dosage of citric acid in feed decreased FI (Brenes et al. Citation2003; Esmaeilipour et al. Citation2012b). A possible explanation for this could be reduced palatability of the feed with a high amount of acid added; however, no literature on this has been found. In addition, the lowered gizzard pH may reduce gastric emptying rate, as this effect is well known in pigs (Van der Aar et al. Citation2017). The lowered emptying rate led to reduced feed intake, in addition to improved efficacy of digestion (Vieira et al. Citation2018). This relationship could be the reason why acidification did not reduce BWG when both acid and phytase were added in the feed, as the tendency was when only acid and no phytase was added. Conversely, there was no reduction in gizzard pH with acidification of the feed in the current experiment, which was in accordance with data described in a review by Kim et al. (Citation2015). However, lower gizzard pH for intermittently fed birds when feeding before 240 min cannot be ruled out, as acidification of the feed did not reduce the pH in the crop when measured after 240 min.
The increased P release with acidification and phytase addition in the current in vitro test and other in vitro experiments (Menezes-Blackburn et al. Citation2015; Tamim et al. Citation2004) was considered to be due to an increase in efficacy of phytase at lower pH. The increased degradation of InsP6 in the crop after 160 min by acid and phytase addition (compared to only phytase addition) indicated that this effect occurred in the current experiment and may have led to the tendency for increased jejunal P digestibility by acid, but only when used together with phytase. However, no effect on ileal P digestibility with acid addition and phytase was seen, which was contradictory to the result of Emami et al. (Citation2013) and Woyengo et al. (Citation2010), who found increased ileal P digestibility with organic acid and phytase addition in the feed.
A reduction in crop pH for intermittently fed birds over time without acid addition was reported by Sacranie et al. (Citation2017) and could have caused the interaction between feeding regime and acid, where increased jejunal PDC with acidification was seen only in ad libitum fed birds. It may be speculated that acidification had a positive effect on the ad libitum fed birds because of the presumed shorter crop retention time and, hence, less time at a lower pH without acid addition to the diet. The reduced pH with a longer retention time in the crop for the intermittently fed birds, even without acid addition, may compensate for the lack of instant pH reduction.
The decrease in the concentrations of InsP6 and two of the InsP5 isomers in the crop for diets with phytase addition which led to higher concentrations of InsP4 and InsP3 isomers have been demonstrated previously by Zeller et al. (Citation2016) and Sommerfeld et al. (Citation2018). Ins(1,2,3,4,5)P5 is the main InsP5 isomer in the degradation pathway for the C. braakii phytase (Pontoppidan et al. Citation2012), and the major degradation of insP6 in diets containing both acid and phytase might have led to the high concentration of Ins(1,2,3,4,5)P5. Hence, no phytase effect was seen after 80 min for this isomer. The increased significant interactions from 80 to 160 min for all isomers except Ins(1,2,3,4,5)P5, indicated that acidification and increased retention time in the crop improved phytase activity. A more rapid passage from the crop for some of the isomers cannot be ruled out, but selective retention of crop content has not been found in the literature.
The lack of any interaction between acidification, prolonged retention time in crop and phytase on performance, PDC and bone mineralisation implied that exogenous phytase activity is not dependent on these factors. The only tendency of a three-way interaction in the current experiment was for tibial P, which suggested that acidification increased P mineralisation of the bone only when no phytase was used in ad libitum fed birds. This tendency might simply have been a result of the slightly reduced BW for ad libitum fed birds without phytase compared to birds given diets containing phytase. However, the lack of three-way interactions could have been due to the low effect of feed manipulations in the current experiment, and that the amount of feed in the crop for the intermittently fed birds was relatively low, hence giving a shorter retention time than expected.
In conclusion, the current data showed that there was an effect of acidification in a diet containing phytase on the degradation of InsP6 and there were further reductions to decrease isomers after both 80 and 160 min in the crop. This indicated that acid addition combined with longer retention time in the crop can be beneficial for phytase activity in the anterior digestive system. However, the interaction effect between different retention times and acidification on phytate degradation needs further exploration.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Angel, R., N. M. Tamim, T. J. Applegate, A. S. Dhandu, and L. E. Ellestad. 2002. “Phytic Acid Chemistry: Influence on Phytin-Phosphorus Availability and Phytase Efficacy.” Journal of Applied Poultry Research 11: 471–480. doi:10.1093/japr/11.4.471.
- Ao, T., A. H. Cantor, A. J. Pescatore, and J. L. Pierce. 2008. “In Vitro Evaluation of Feed-Grade Enzyme Activity at pH Levels Simulating Various Parts of the Avian Digestive Tract.” Animal Feed Science and Technology 140: 462–468. doi:10.1016/j.anifeedsci.2007.04.004.
- AOAC. 2005. Official Method of Analysis. 18th ed. Washington DC: Assosiation of Officiating Analytical Chemists.
- Brenes, A., A. Viveros, I. Arija, C. Centeno, M. Pizarro, and C. Bravo. 2003. “The Effect of Citric Acid and Microbial Phytase on Mineral Utilization in Broiler Chicks.” Animal Feed Science and Technology 110: 201–219. doi:10.1016/S0377-8401(03)00207-4.
- Cutler, S. A., M. A. Rasmussen, M. J. Hensley, K. W. Wilhelms, R. W. Griffith, and C. G. Scanes. 2005. “Effects of Lactobacilli and Lactose on Salmonella typhimurium Colonisation and Microbial Fermentation in the Crop of the Young Turkey.” British Poultry Science 46: 708–716. doi:10.1080/00071660500393694.
- Eeckhout, W., and M. De Paepe. 1994. “Total Phosphorus, Phytate-Phosphorus and Phytase Activity in Plant Feedstuffs.” Animal Feed Science and Technology 47: 19–29. doi:10.1016/0377-8401(94)90156-2.
- Emami, N. K., S. Z. Naeini, and C. A. Ruiz-Feria. 2013. “Growth Performance, Digestibility, Immune Response and Intestinal Morphology of Male Broilers Fed Phosphorus Deficient Diets Supplemented with Microbial Phytase and Organic Acids.” Livestock Science 157: 506–513. doi:10.1016/j.livsci.2013.08.014.
- Esmaeilipour, O., H. Moravej, M. Shivazad, M. Rezaian, S. Aminzadeh, and M. M. Van Krimpen. 2012a. “Effects of Diet Acidification and Xylanase Supplementation on Performance, Nutrient Digestibility, Duodenal Histology and Gut Microflora of Broilers Fed Wheat Based Diet.” British Poultry Science 53: 235–244. doi:10.1080/00071668.2012.681771.
- Esmaeilipour, O., M. M. Van Krimpen, A. W. Jongbloed, L. H. De Jonge, and P. Bikker. 2012b. “Effects of Temperature, pH, Incubation Time and Pepsin Concentration on the in Vitro Stability of Intrinsic Phytase of Wheat, Barley and Rye.”.” Animal Feed Science and Technology 175: 168–174. doi:10.1016/j.anifeedsci.2012.05.007.
- FAO. 2011. Quality Assurance for Animal Feed Analysis Laboratories. Vol. 14. FAO Animal Production and Health maunal Rome: FAO Food and Agriculture Organization of the United Nations.
- Heinonen, J. K., and R. J. Lahti. 1981. “A New and Convenient Colorimetric Determination of Inorganic Orthophosphate and Its Application to the Assay of Inorganic Pyrophosphatase.” Analytical Biochemistry 113: 313–317. doi:10.1016/0003-2697(81)90082-8.
- ISO Standard 30024. 2009. Animal Feeding Stuffs – Determination of Phytase Activity. Geneva: International Organization for Standardization ISO.
- Jozefiak, D., A. Rutkowski, B. B. Jensen, and R. M. Engberg. 2006. “The Effect of Beta-Glucanase Supplementation of Barley- and Oat-Based Diets on Growth Performance and Fermentation in Broiler Chicken Gastrointestinal Tract.” British Poultry Science 47: 57–64. doi:10.1080/0071660500475145.
- Kim, J. W., J. H. Kim, and D. Y. Kil. 2015. “Dietary Organic Acids for Broiler Chickens: A Review.” Revista Colombiana De Ciencias Pecuarias 28: 109–123. doi:10.17533/udea.rccp.v28n2a01.
- McCleary, B. V., V. Solah, and T. S. Gibson. 1994. “Quantitative Measurement of Total Starch in Cereal Flours and Products.” Journal of Cereal Science 20: 51–58. doi:10.1006/jcrs.1994.1044.
- Menezes-Blackburn, D., S. Gabler, and R. Greiner. 2015. “Performance of Seven Commercial Phytases in an in Vitro Simulation of Poultry Digestive Tract.” Journal of Agricultural and Food Chemistry 63: 6142–6149. doi:10.1021/acs.jafc.5b01996.
- Phillippy, B. Q., and J. M. Bland. 1988. “Gradient Ion Chromatography of Inositol Phosphates.” Analytical Biochemistry 175: 162–166. doi:10.1016/0003-2697(88)90374-0.
- Pontoppidan, K., V. Glitsoe, P. Guggenbuhl, A. P. Quintana, C. S. Nunes, D. Pettersson, and A. S. Sandberg. 2012. “In Vitro and in Vivo Degradation of Myo-Inositol Hexakisphosphate by a Phytase from Citrobacter Braakii.” Archives of Animal Nutrition 66: 431–444. doi:10.1080/1745039X.2012.735082.
- Sacranie, A., X. Adiya, L. T. Mydland, and B. Svihus. 2017. “Effect of Intermittent Feeding and Oat Hulls to Improve Phytase Efficacy and Digestive Function in Broiler Chickens.” British Poultry Science 58: 442–451. doi:10.1080/00071668.2017.1328550.
- Sacranie, A., B. Svihus, V. Denstadli, B. Moen, P. A. Iji, and M. Choct. 2012. “The Effect of Insoluble Fiber and Intermittent Feeding on Gizzard Development, Gut Motility, and Performance of Broiler Chickens.” Poultry Science 91: 693–700. doi:10.3382/ps.2011-01790.
- Selle, P. H., and V. Ravindran. 2007. “Microbial Phytase in Poultry Nutrition.” Animal Feed Science and Technology 135: 1–41. doi:10.1016/j.anifeedsci.2006.06.010.
- Short, F. J., P. Gorton, J. Wiseman, and K. N. Boorman. 1996. “Determination of Titanium Dioxide Added as an Inert Marker in Chicken Digestibility Studies.” Animal Feed Science and Technology 59: 215–221. doi:10.1016/0377-8401(95)00916-7.
- Slominski, B. A. 2011. “Recent Advances in Research on Enzymes for Poultry Diets.” Poultry Science 90: 2013–2023. doi:10.3382/ps.2011-01372.
- Sommerfeld, V., M. Schollenberger, L. Hemberle, and M. Rodehutscord. 2017. “Modification and Application of an in Vitro Assay to Examine Inositol Phosphate Degradation in the Digestive Tract of Poultry.” Journal of the Science of Food and Agriculture 97: 4219–4226. doi:10.1002/jsfa.8297.
- Sommerfeld, V., M. Schollenberger, I. Kühn, and M. Rodehutscord. 2018. “Interactive Effects of Phosphorus, Calcium, and Phytase Supplements on Products of Phytate Degradation in the Digestive Tract of Broiler Chickens.” Poultry Science 97: 1177–1188. doi:10.3382/ps/pex404.
- Svihus, B. 2011. “The Gizzard: Function, Influence of Diet Structure and Effects on Nutrient Availability.” Worlds Poultry Science Journal 67: 207–223. doi:10.1017/s0043933911000249.
- Svihus, B., V. B. Lund, B. Borjgen, M. R. Bedford, and M. Bakken. 2013. “Effect of Intermittent Feeding, Structural Components and Phytase on Performance and Behaviour of Broiler Chickens.” British Poultry Science 54: 222–230. doi:10.1080/00071668.2013.772952.
- Svihus, B., A. Sacranie, V. Denstadli, and M. Choct. 2010. “Nutrient Utilization and Functionality of the Anterior Digestive Tract Caused by Intermittent Feeding and Inclusion of Whole Wheat in Diets for Broiler Chickens.” Poultry Science 89: 2617–2625. doi:10.3382/ps.2010-00743.
- Tamim, N. M., R. Angel, and M. Christman. 2004. “Influence of Dietary Calcium and Phytase on Phytate Phosphorus Hydrolysis in Broiler Chickens.” Poultry Science 83: 1358–1367. doi:10.1093/ps/83.8.1358.
- Van der Aar, P. J., F. Molist, and J. D. Van Der Klis. 2017. “The Central Role of Intestinal Health on the Effect of Feed Additives on Feed Intake in Swine and Poultry.” Animal Feed Science and Technology 233: 64–75. doi:10.1016/j.anifeedsci.2016.07.019.
- Vieira, B. S., J. G. Caramori Junior, C. F. S. Oliveira, and G. S. S. Correa. 2018. “Combination of Phytase and Organic Acid for Broilers: Role in Mineral Digestibility and Phytic Acid Degradation.” World’s Poultry Science Journal 74: 711–726. doi:10.1017/S0043933918000697.
- Vieira, B. S., F. G. Silva, C. F. S. Oliveira, A. B. Correa, J. G. Caramori Junior, and G. S. S. Correa. 2017. “Does Citric Acid Improve Performance and Bone Mineralization of Broilers When Combined with Phytase? A Systematic Review and Meta-Analysis.” Animal Feed Science and Technology 232: 21–30. doi:10.1016/j.anifeedsci.2017.07.016.
- Woyengo, T. A., B. A. Slominski, and R. O. Jones. 2010. “Growth Performance and Nutrient Utilization of Broiler Chickens Fed Diets Supplemented with Phytase Alone or in Combination with Citric Acid and Multicarbohydrase.” Poultry Science 89: 2221–2229. doi:10.3382/ps.2010-00832.
- Zeller, E., M. Schollenberger, I. Kühn, and M. Rodehutscord. 2015. “Hydrolysis of Phytate and Formation of Inositol Phosphate Isomers without or with Supplemented Phytases in Different Segments of the Digestive Tract of Broilers.” Journal of Nutritional Science 4: 4. doi:10.1017/jns.2014.62.
- Zeller, E., M. Schollenberger, I. Kühn, and M. Rodehutscord. 2016. “Dietary Effects on Inositol Phosphate Breakdown in the Crop of Broilers.” Archives of Animal Nutrition 70: 57–71. doi:10.1080/1745039X.2015.1112622.
- Zyla, K., D. R. Ledoux, A. Garcia, and T. Veum. 1995. “An in Vitro Procedure for Studying Enzymic Dephosphorylation of Phytate in Maize-Soyabean Feeds for Turkey Poults.” British Journal of Nutrition 74: 3–17. doi:10.1079/bjn19950102.
