?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
1. The effect of increasing the dose level of a novel consensus bacterial 6-phytase variant on apparent ileal digestibility (AID) of phosphorus (P), phytic acid (inositol hexa-phosphate, IP6) and ileal IP6 degradation profile was studied in diets containing varying phytate-P (PP) levels.
2. Ross 308, one-day-old males (n = 1,800) were allocated to cages (20 birds/cage, six cages/treatment) in a completely randomised design employing a 3 × 5 factorial arrangement (three PP levels: 2.45 (low) 2.95 (medium) and 3.45 g/kg (high); five dose levels of phytase (PhyG): 0, 500, 1,000, 2,000 and 4,000 FTU/kg). Phased diets were based on wheat, corn, soybean meal, rapeseed meal and rice bran (d 0 to 10; 2.60 g/kg digestible P, 7.6 g/kg calcium (Ca); d 11 to 21; 2.10 g/kg digestible P, 6.4 g/kg Ca). Ileal digesta was collected on d 21 for determination of P, IP6 and IP-esters content. Data were analysed by factorial ANOVA; means separation was achieved using Tukey’s HSD test.
3. Increasing PP reduced AID of IP6 and sum of IP3-6 (%) (P < 0.05) but absolute P-release (g/kg diet) above NC was increased (P < 0.05) at high vs. low PP. Increasing phytase dose exponentially increased (P < 0.001) AID IP6, sum of IP3-6 (%) and digestible IP3-6-P g/kg diet (P < 0.001). AID P was increased but there was an interaction with PP level (P < 0.001). Ileal accumulation of IP5-3-P was universally low with PhyG at ≥1,000 FTU/kg (<0.06 g/100 g DM). At 2,000 and 4,000 FTU/kg, AID IP6 was 97.2, 92.7, 92.6% and 100, 97.2, 97.1%, respectively, at low, medium and high PP. At 2,000 FTU/kg, phytate-P release estimated as the increase (above NC) in ileal digestible sum of IP3-6-P in the diet was 2.26, 2.59 and 3.10 g/kg in low, medium and high PP, respectively.
4. The data demonstrated that the novel phytase was effective in breaking down phytate to low IP-esters in diets with varied PP content but the optimal dose level for maximising P-release may differ in diets with varying PP content.
Introduction
Phytate (salt of phytic acid, myo-inositol hexakisphosphate, IP6) is a major potential source of phosphorus (P) in plant-based poultry diets. However, it has poor bioavailability because of insufficient endogenous phytase activity and antinutritional effects, due to interactions with calcium (Ca) that may be included at high concentrations in commercial diets to ensure nutritional requirements are met (Humer et al. Citation2015; Cowieson et al. Citation2016). In the digestive tract, phytate readily forms complexes with positively charged mineral ions, such as calcium and iron (Selle et al. Citation2009) and amino acids (Selle et al. Citation2012). This can have a negative impact on the digestibility and absorption of the complexed nutrient(s), as well as the accessibility of the phytate itself for digestion and release of inorganic P, which is a vital nutrient in many metabolic processes including bone mineralisation (Li et al. Citation2016a).
Exogenous phytase enzymes, with the capacity to dephosphorylate IP6 and release inorganic phosphate (Pi), are well established as a means to improve dietary P-availability and reduce its excretion in poultry (Selle and Ravindran Citation2007; Lei et al. Citation2013). In the digestive tract, phytase effects the sequential dephosphorylation of IP6 to lower inositol-phosphate esters (IP5, IP4, IP3, IP2, and eventually IP1), releasing an inorganic phosphate moiety at each step, which can subsequently be absorbed in the small intestine and used to support growth and maintenance (Greiner and Konietzny Citation2011). The 6-phytases (EC 3.13.26) preferentially cleave phosphate groups initially at the carbon 6 atom of the inositol ring, whereas 3-phytases cleave initially at the carbon 3 atom (EC 3.1.3.8). In vitro studies (Yu et al. Citation2012) have suggested that the capacity of phytate to aggregate with proteins and mineral cations and to resist the activity of digestive enzymes, decreases rapidly from higher IP-esters (IP6 and IP5) to lower IP-esters (IP1-4). Thus, for a phytase to be effective in reducing the antinutritional effects of phytate, it should ideally exhibit the capacity to rapidly and extensively degrade IP6 down to low IP-esters. This activity will have most benefit if it occurs in the upper gastrointestinal tract (proventriculus and gizzard) where phytate first encounters other nutrients in a low pH environment where phytate-amino-acids complexes may be formed. The pH in the proventriculus and gizzard may be as low as 2.5 to 3.0 (Shafey et al. Citation1991; Selle et al. Citation2009). Hence, high activity at low pH is a desirable characteristic of commercial phytases. Although the activity of individual phytases is measured at a pH of 5.5, activity varies quite markedly at pH levels below this (Menezes-Blackburn et al. Citation2015). For maximising P-release, it is desirable that the phytase has a wider functional pH range of 1.5 to 5.5, to completely breakdown phytase along the gastrointestinal tract (GIT). As well as the bio-characteristics of the phytase, e.g. pH activity profile, IP6 and lower inositol ester degradation, factors related to the diet (composition, actual and relative concentration and biological/physical properties of the nutrients therein) can impact phytase efficacy (Dersjant-Li et al. Citation2015). The phytate content of the diet is of particular relevance as the substrate for the enzyme and because of its potential for antinutritive effects. Commercial poultry diets have become more complex in recent years, reflecting use of a wider variety of cereal and cereal-derived ingredients beyond corn, wheat and soybean meal. Additional ingredients such as rapeseed meal, sunflower meal and rice bran may be included for cost saving or availability reasons, but tend to be high in phytate (Sanz-Penella and Haros, Citation2014; He et al. Citation2017). This may be of benefit for improving the potential for P-release by phytase, but may necessitate a higher dose level of phytase in the diet. The inherent accessibility of the phytate to phytase is an additional factor to consider. This can be affected by the tendency of phytate to complex with other nutrients in the digesta and inherent differences between phytate sources. For example, phytate in rice bran and canola meal are less accessible to phytase than in corn and soybean meal (Leske and Coon Citation1999). It is therefore relevant to consider the efficacy of a new exogenous phytase in the context of higher dietary phytate content and to pay regard to the accessibility of the phytate in the selected ingredients for inclusion.
Recent in vitro data from a simple substrate and buffer system demonstrated that a novel consensus bacterial 6-phytase variant, produced in Trichoderma reesei, was effective in rapidly breaking down IP6 to IP2 under low pH conditions (2.5 to 4.5; Christensen et al. Citation2020). The objective of the present study was to evaluate the effect of increasing the dose level of this phytase (0 to 4,000 FTU/kg) on the apparent ileal digestibility (AID) of P and IP6, and on the IP6 degradation profile in broilers when the phytase is added to complex diets containing three levels of phytate-P (PP); low, medium and high.
Materials and methods
Experimental design, birds and housing
The research was conducted at the University of Sydney, Australia. All experimental protocols were approved by the University of Sydney Animal Ethics Committee to ensure compliance with welfare and humane animal husbandry practices.
The study used a completely randomised design consisting of a 3 × 5 factorial arrangement of treatments comprising three levels of dietary PP (2.45 g/kg (low), 2.95 g/kg (medium) and 3.45 g/kg (high)) and five phytase dose levels (0, 500, 1,000, 2,000 and 4,000 FTU/kg). A total of 1,800 Ross 308, day-old, male broilers were obtained from a commercial hatchery and randomly allocated to 90 cohort cages with 20 birds/cage and six cages/treatment. Birds were housed in an environmentally controlled animal house in which the ambient temperature was maintained at 33 ± 1.0°C for the first three days and thereafter decreased by 1.0°C every two days to reach a final temperature of 23°C. The lighting regime and ventilation program were in accordance with breeder recommendations (Aviagen Inc. Citation2018).
Diets and phytase
Diets were based on wheat, soybean meal, corn, rapeseed meal and rice bran and were formulated in two phases to meet the nutritional requirements of the birds as set by the breeder (Aviagen Inc. Citation2019), except for reductions in Ca, digestible P, energy, AA and Na to account for the expected contribution of the phytase. Starter diets contained 2.60 g/kg digestible P and 7.6 g/kg total Ca. Grower diets contained 2.10 g/kg digestible P and 6.4 g/kg Ca. Celite (20 g/kg), a source of acid insoluble ash, was added to all diets as an indigestible marker. The three levels of dietary PP (2.45, 2.95 and 3.45 g/kg) were selected based on what might be representative of a low, medium, and high level of PP used in commercial broiler diets. These were achieved by varying the content of broken rice, canola meal and rice bran in the diet whilst maintaining an equivalent caloric value and digestible amino acids (AA) content. Full details of the ingredient and calculated nutrient composition of the diets are given in .
Table 1. Ingredient and calculated nutrient content (g/kg, as fed basis) of the basal negative control diets, by phase1
The phytase was a commercial bacterial consensus 6-phytase variant expressed in Trichoderma reesei (PhyG, Axtra® PHY GOLD, Danisco Animal Nutrition, IFF). It was added to each of the three PP diets at a level of 0, 500, 1,000, 2,000 and 4,000 FTU/kg, to give 15 treatments. Diets were steam-pelleted at 80°C. Grower diets were fed as a pellet, whereas starter diets were crumbled. Birds had ad libitum access to feed and water for the duration of the study (21 d).
Sampling
At 21 d of age, six birds per cage were euthanised by intravenous injection of sodium pentobarbitone. The small intestine was removed and the digesta gently expressed and pooled per replicate cage. Digesta samples were homogenised, freeze-dried and stored at −20°C for later analysis. Acid-insoluble-ash (AIA) was measured both in diets and digesta samples as an indigestible marker. Samples of the final diets (low, medium and high dietary PP) were analysed for crude protein, total P, phytate, total Ca and phytase activity.
Chemical analyses of feed and ileal digesta
Acid insoluble ash in feed and digesta was determined according to the method described by Siriwan et al. (Citation1993). Calcium in final feed and P in final feed and digesta were analysed by inductively coupled plasma-optical emission spectrometry (ICP-OES). Nitrogen in feed was determined by combustion analysis of a 0.25 g sample in a combustion analyser (Leco model FP-2000 N Analyzer, Leco Corp., St. Joseph, MI) using EDTA as a calibration standard. Crude protein was calculated by multiplying the percentage of N by a correction factor (6.25). These analyses were conducted at the University of Sydney, Australia. Phytase in feed was determined by Danisco Animal Nutrition Research Centre, Brabrand, Denmark, using an internally validated method adapted from ISO method 30024:2009 (Gizzi et al. Citation2008). One phytase unit (FTU) was defined as the amount of enzyme that liberated 1 µmol of inorganic phosphate from phytate per minute at pH5.5 and 37°C.
Determination of IP6 and inositol phosphate esters (IP3-6) in feed and ileal digesta
The extraction of IP6 and lower phosphorylated inositol esters (IP5, IP4 and IP3) from digesta samples was carried out at a sample concentration of 0.05 g/ml using 1.0 M HCL as a solvent, in 10 ml total volume. Samples were rotated on a mixer for 1 h at 5°C and subsequently centrifuged (3,700 × g for 60 min) to separate the precipitate. For complete extraction, the initial extraction step was repeated by addition of 5 ml of 1.0 M HCL to the precipitate (after removal of the supernatant) and subsequently combining soluble fractions from both steps. Combined supernatants were filtered (0.45 μm) using centrifugation (3,700 x g for 60 min) and subjected to high performance ion chromatography (HPIC) analysis for the determination of myo-inositol phosphate isomers (IP3-6) according to the procedure described in Christensen et al. (Citation2020). It was not possible to analyse IP2 and IP1 with this method. This same method was used for the determination of phytate in final feed samples. For the feed and digesta samples, the concentration of IP6 and IP3-5 isomers was calculated as a percentage (grams of IPx per 100 g of feed or freeze-dried digesta).
Calculations
Apparent digestibility percentages were calculated according to the following equation:
where ‘nutrient’ referred to either P, IP6, or the sum of IP3-6, AIA acid insoluble ash, (nutrient/AIA)diet the ratio of the nutrient and AIA in the experimental diet and (nutrient/AIA)digesta the ratio of the nutrient and AIA in the ileal digesta.
The calculated digestibility percentages and the determined values of dietary total P and IP6 content were used to calculate the content of ileal digestible P and P release from the sum of IP3-6–P as g/kg feed. The phytate content in the ingredients of the diets is mainly in the form of IP6, with the content of lower esters being very low (Bello et al. Citation2019). Therefore, the determined IP6–P content of the diet was used to calculate the digestible IP3-6–P as g/kg feed.
Statistical analyses
Data were analysed as a 3 × 5 factorial Analysis of Variance (ANOVA), with dietary PP content (high, medium or low) and phytase level included as fixed effects. Where the ANOVA identified significant differences (at a probability level of P < 0.05), Tukey’s test was used for means separation. Cage was the experimental unit and all calculations were generated based on cage averages. Dose response relationships between dietary PP level or analysed phytase dose level and AID percentages or IPx content in g/100 g freeze-dried DM were tested using linear or exponential curve fitting. All statistical analyses were conducted in JMP14.0 (JMP, 2019; SAS Institute Inc., Cary, NC). Differences or effects were considered significant at P < 0.05. Where 0.05 < P < 0.1, this was considered a tendency.
Results
Diet analysis
Analysed concentrations of Ca and P in the diets were close to (within 10% of) formulated values (). Values of analysed PP were slightly higher than formulated in all diets and phases (range +11.1% to +19.8%). Nevertheless, there were clear differences between the analysed PP values of the low, medium and high PP diets. Analysed phytase activity in the basal diets ranged from 330 to 416 FTU/kg (). After subtracting this endogenous activity, analysed phytase activities in the treatment diets were consistently close to (within 30% of) target dose levels, with good separation between adjacent dose levels.
Table 2. Analysed nutrient content and phytase activities of the treatment diets, by phase
Apparent ileal digestibility of IP6, sum of IP3-6 and P
According to the data presented in , both dietary PP level (P < 0.01) and phytase dose level (P < 0.001) exerted significant main effects on AID IP6, AID sum of IP3-6 and AID P (%). The AID IP6 and AID sum of IP3-6 were lower in the high compared with the low PP diet, on average by −9.0 and −11.4 percentage points, respectively. Increasing the phytase dose level from 0 (NC) to 4,000 FTU/kg led to exponential (P < 0.001) increases in AID IP6 and AID sum of IP3-6 up to 100% (an increase of 69 percentage points vs. 0 FTU/kg) and 96.8% (an increase of 78 percentage points. vs. 0 FTU/kg), respectively, in the low PP diets with PhyG at 4,000 FTU/kg. For AID P (but not AID IP6 or AID sum of IP3-6), there was an interaction (P < 0.01) between dietary PP and phytase dose level, such that the positive effect of increasing the dose level was magnified with increasing dietary PP content. At 2,000 FTU/kg, phytase increased AID P by 26, 29 and 42 percentage points vs 0 FTU/kg, for low, medium and high PP diets, respectively.
Table 3. Apparent ileal digestibility (AID) of IP6, sum IP3-6 and P at 21 d of age1
Ileal content of IP6 and lower IP-esters
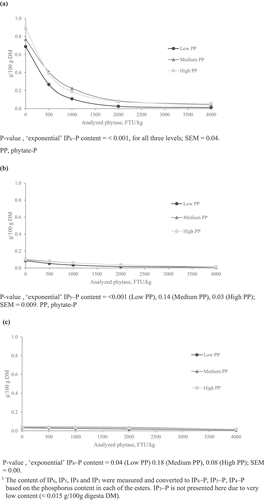
The amount of IP6–P remaining in the digesta and the extent of its degradation to IP5–P, IP4–P and IP3–P, is presented in . No interaction was observed between phytase dose level and dietary PP level for any of these IP-esters. Across PP levels, increasing phytase dose from 0 to 4,000 FTU/kg exponentially decreased (P < 0.001) ileal IP6–P content from 0.79 to 0.02 g/100 g DM and IP5–P from 0.09 to 0.01 g/100 g DM.
Table 4. Ileal content (g/100 g DM) of individual IP3-6 esters at 21 days of age1
Although factorial ANOVA did not detect any interactions, the reduction in ileal IP6–P concentration with increasing phytase dose was numerically greatest in the high PP level treatment. The reductions in ileal content of IP6–P within the dose range of 0 to 4,000 FTU/kg were from 0.91 (NC) to 0.03 g/100 g DM (4,000 FTU/kg) in the high PP diet, from 0.77 to 0.03 g/100 g DM in the medium PP diet, and from 0.69 g/100 g DM to non-detectable in the low PP diet. These reductions equate to IP6 disappearances of 96.7%, 96.1% and 100%, respectively. Across phytase doses, increasing dietary PP level increased ileal IP6-P content from 0.22 to 0.34 g/100 g DM and linearly increased (P < 0.05) IP5–P from 0.05 to 0.06 g/100 g DM. The ileal content of IP4–P and IP3–P was universally very low.
The fitted exponential curves illustrating the relationship between ileal IP-ester content and analysed phytase dose at low, medium and high PP are shown in . At all three PP levels, the concentration of these IP esters decreased exponentially with increasing analysed phytase dose (P < 0.001). Ileal content of IP5–P was considerably lower than IP6–P () vs. (a)) and ranged from 0.08 to 0.1 g/100 g DM at 0 FTU/kg across dietary PP levels (). It decreased exponentially with increasing analysed phytase dose level in high (P < 0.05) and low (P < 0.001) PP diets, to levels very close to zero ()). The ileal content of IP4-P was yet lower than that of IP5-P at all phytase dose levels () vs. (b)), but similarly exhibited an exponential decrease with increasing phytase dose level in low PP diets (P < 0.05) and a tendency in high PP diets (P < 0.1). Levels of IP3–P were extremely low (≤0.01 g/100 g DM) across all treatments and were unaffected by phytase dose or dietary PP level ().
Calculated dietary content of ileal digestible P and IP3-6–P
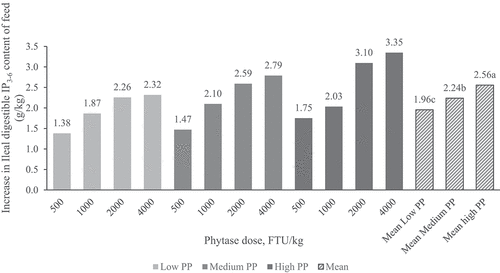
The calculated dietary content of ileal digestible P and IP3-6–P (grams per kilogram of diet) in relation to dietary PP content and phytase dose level are presented in . Ileal digestible IP3-6–P content of the diet increased exponentially with increasing phytase dose level (P < 0.001) but was unaffected by dietary PP level. Dietary PP level and phytase dose had significant main effects (P < 0.001 in both cases) on the calculated improvement in ileal digestible IP3-6–P content of the feed (above NC), but the level of improvement by phytase tended to be higher with increasing PP level (interaction between PP level and phytase dose, P = 0.055): In the high PP diet, ileal digestible IP3-6–P was increased above NC by +3.10 g/kg or +3.35 g/kg with PhyG at 2,000 or 4000 FTU/kg, respectively, whereas the corresponding increases in the medium PP diets were +2.59 g/kg and +2.79 g/kg, respectively, and for the low PP diets were +2.26 g/kg and 2.32 g/kg, respectively.
Table 5. Calculated dietary content (g/kg diet) of ileal digestible P and sum of IP3-6 – P at 21 d of age1
This effect can be seen more clearly in which shows that the phytate P release, based on calculated ileal digestible IP3-6-P content above NC, was greater at high PP compared to medium or low PP (P < 0.05) across phytase doses.
Figure 2. Calculated increase (above NC) in ileal digestible IP3-6–P content (g/kg feed) of diets with low, medium or high dietary phytate-P content and phytase added at different dose levels, determined in broilers at 21 d of age.
The calculated content of ileal digestible P in the diet was increased by phytase (P < 0.001) as well as by increasing dietary PP level (P = 0.055; ). There was a significant interaction (P < 0.01) for these parameters, such that the magnitude of improvement in ileal digestible P with increasing phytase dose level became larger at higher dietary PP level. In the low PP diet, ileal digestible P was increased from 2.26 g/kg (NC without phytase) to 3.50 (+ 1.24 g/kg) or 3.81 (+ 1.55 g/kg) with PhyG at 2,000 or 4,000 FTU/kg, respectively. However, in the high PP diet, ileal digestible P was increased from 1.8 g/kg (NC without phytase) to 4.11 g/kg (+ 2.31 g/kg) or 4.46 (+ 2.66 g/kg) with PhyG at 2,000 or 4,000 FTU/kg, respectively ().
Discussion
Few previous studies have reported the in vivo IP6 degradation profile of microbial phytases in broilers (Zeller et al. Citation2015; Li et al. Citation2016b, Citation2017; Beeson et al. Citation2017; Bello et al. Citation2019). Most have simply reported IP6 disappearance or digestibility, which provide information about the capacity of the phytase to hydrolyse the parent molecule (phytic acid, IP6), but not about the further breakdown of IP5 to other esters. Rapid and complete breakdown of phytate by phytase to low IP-esters, is desirable for minimising anti-nutritional effects and maximising P-release. The impact of dietary PP content on phytase efficacy to degrade IP6 has not been intensively studied, but may be relevant for optimising the enzyme-to-substrate ratio in the diet by adjusting the dose level of the phytase to maximise phytate hydrolysis and P-release. With broiler diets often containing ingredients having high phytate content, understanding the capacity of a phytase to release P from diets of varied PP content and containing different cereal ingredient sources of phytate is important. This study focused on the total P release along the GIT and measured IP6 degradation profile at ileal level.
The analysed PP content of the basal low, medium and high PP diets was higher than expected. As this was a consistent feature of all three diets and good separation between the diets in their analysed PP content was retained, it was considered unlikely to have compromised the integrity of the study. However, it did mean that the PP content of the ‘low’ PP diet was above what might be considered ‘low’ in a commercial context. This variation from expected values most likely resulted from the use of feed composition tables, rather than actual measured values to calculate the PP content of the feed ingredients, as is common practice in feed formulation. Thus, it likely reflected what might occur in practice.
Across phytase dose levels, the general effect of increasing the PP content of the diet within the range 2.98 to 3.98 g/kg (analysed values) was to reduce AID IP6 and of partially dephosphorylated IP6 (as estimated by AID sum of IP3-6) as a proportion of intake. However, it should be noted that the absolute amount of P released by phytase from the diet, as estimated by calculation of the ileal digestible IP3-6–P content of the diet above NC, was higher in high PP vs. low PP diets (). Studies by Li et al. (Citation2016b, Citation2017) similarly observed a reduction in ileal IP6 disappearance (as a percentage of IP6 in the diet) with increasing PP concentration across phytase dose levels (0, 500 or 1,000 FTU/kg of a Buttiauxella spp. phytase). Li et al. (Citation2017) considered this finding was due to the combined influences of the higher PP dietary content and a reduced availability of the PP due to the inclusion of rice bran. In vivo studies by Leske and Coon (Citation1999) previously demonstrated that different cereal sources of phytate were not equally accessible to a phytase from Aspergillus niger. The authors observed that a lower percentage of the phytate in rice bran and canola was hydrolysed by the phytase than that hydrolysed in phytate from corn and soybean meal (Leske and Coon Citation1999). Rice bran and canola meal were the main ingredients for increasing the PP content of diets in the present study, and their inclusion could have reduced the overall accessibility of the phytate in the medium and high PP diets. However, if this effect was manifested, it did not appear to have prevented the phytase from achieving greater P-release in absolute terms in the high compared with the low PP diet. On the other hand, it was logical that, at a given phytase dose level, the proportion of IP6 degraded could be lower with increasing PP in the diet. The estimated ileal digestible IP3-6-P release value could be more meaningful when evaluating the phytase effects at different PP levels.
Across dietary PP levels, increasing the phytase dose level (between 0 and 4,000 FTU/kg) resulted in an exponential increase in AID IP6 and AID sum of IP3-6, leading to an exponential increase in AID P, such that the magnitude of the increase in these response measures declined with increasing phytase dose level. Numerous previous studies have observed a positive dose-response relationship of microbial phytase supplementation on ileal digestibility or disappearance of phytate and on AID P (Dersjant-Li and Dusel Citation2019; Ajuwon et al. Citation2020; Dersjant-Li et al. Citation2020), and the present findings support these earlier observations. Direct comparisons between individual studies are problematic because of the differences between studies in factors known to affect phytase efficacy (different generation of phytases, diet composition, bird age and genetics). However, it was noted that with the phytase dosed at 1,000 FTU/kg, the obtained AID IP6 values of ≥80% in the low- (2.98 g/kg) and medium- (3.45 g/kg) PP diets and of ≥70% in the high- (3.98 g/kg) PP diet were at the upper limit of those reported in a review by Dersjant-Li et al. (Citation2015) for broiler studies involving other phytases at equivalent dose level. Values of AID IP3-6 were only 5 to 10 percentage points below values of AID IP6, indicating a high capacity of the phytase at 1,000 FTU/kg to extensively degrade IP6 to IP3 or lower IP esters in diets with varied PP content. At 2,000 FTU/kg, the AID IP6 and AID of sum of IP3-6 were high, at 94.2% and 89.8% respectively, on average across dietary phytate-P levels. At 4,000 FTU/kg these values were increased further.
There was no interaction between phytase dose level and dietary PP level on AID IP6 or AID IP3-6, which suggested that the degree of improvement in these response measures with increasing phytase dose level was similar across dietary PP levels, although there were different starting points in the respective NCs. However, for AID P there was an interaction which might have been explained by the different direction of (AID P) response of the NC treatments compared to the phytase supplemented treatments to variation in the dietary PP level. In the NC treatments, AID P was lowest in the high PP diet and highest in the low PP diet, reflecting a lower proportion of P in the high PP diet being digested due to the higher phytate content with no phytase present. Meanwhile, the improvement in AID P with increasing phytase dose was greater with the high PP diet than with the medium and low PP diets, reflecting a greater capacity of the phytase to improve the proportion of P digested in the high PP diet because of the greater availability of substrate.
In the NC, there was very limited IP6 degradation, therefore the IP5–P, IP4–P and IP3–P content was low. Ileal digesta concentrations of IP6–P, IP5–P and IP4–P decreased markedly and substantially with increasing phytase dose level between 0 and 4,000 FTU/kg, regardless of dietary PP level. This indicated the in vivo activity of the phytase in hydrolysing IP6 to low IP-esters. Compared to the NC, ileal IP6 concentrations were reduced by 73% (across PP levels, by 83, 75 and 66% at low, medium and high PP, respectively) with phytase at 1,000 FTU/kg, and the ileal accumulation of IP5–P, IP4–P and IP3–P was very low at all phytase dose levels and all dietary PP levels (<0.1, <0.05 and ≤0.01 g/100 g digesta DM, respectively). This suggested that, by the terminal ileum, IP6 had been rapidly degraded to IP3 or lower. These reductions were higher than those reported in previous studies with other phytases applied to diets with lower or similar PP content. For example, Beeson et al. (Citation2017) reported an ileal IP6 reduction of 71.4% vs. NC at d 21 in birds fed diets containing (formulated values) 1.8 g/kg PP and 1,500 FTU/kg of an E. coli phytase. Bello et al. (Citation2019) reported an IP6 reduction of 73.5% vs. NC in diets containing 2.7 to 3.0 g/kg PP and 1,000 FTU/kg of a Buttiauxella spp. phytase. The IP6 degradation profile of the phytase observed in the present in vivo study was consistent with that previously described by Christensen et al. (Citation2020) from in vitro studies, where an IP6 substrate was rapidly and entirely hydrolysed to IP2 at low pH (2.5 to 4.5). It was unclear from the present results whether and to what extent the phytase degraded IP6 to IP-esters compared to IP3. The lack of apparent accumulation of IP4–P and IP3–P raised this as a possibility, but the analytical HP-IC method did not detect the peak for lower IP esters (IP2, IP1). There is some evidence from in vitro studies of accumulation of IP1 during phytate hydrolysis by a phytase which suggested that exogenous phytase can hydrolyse IP2 to IP1 (Wyss et al. Citation1999; Hirvonen et al. Citation2019). However, in vivo studies measuring IP2 and IP1 are lacking, and there is no evidence to suggest that exogenous phytase can break down IP1 to release free inositol. Nevertheless, there is evidence from studies in pigs and poultry that endogenous phosphatases present in the intestinal mucosa can have some IP-ester degrading capacity (Hu et al. Citation1996; Zeller et al. Citation2015) which might be involved in hydrolysis of low IP-esters (IP3-1) in vivo. Alternatively, or in addition, low IP-esters could potentially be absorbed directly in the ileum. These are areas warranting further research.
Calculation of the content of ileal digestible IP3-6–P in the diets from the sum of IP3-6 ileal digestibility data was used to estimate the P-releasing capacity of the novel phytase compared to the NC. This measure was considered preferable to using the calculated dietary content of ileal digestible P, because the latter may have underestimated the P-release capacity of the phytase. Firstly, due to the potential for adaptation to the low P content of the NC diet, this would have led to a higher-than-expected AID P in the NC, and therefore a lower estimated percentage improvement above NC by the phytase. It has previously been shown that broilers can adapt to a P deficient diet by increasing intestinal absorption of P and other nutrients such as crude protein and amino acids (Yan et al. Citation2005; Li et al. Citation2014, Citation2015). Secondly, using the digestibility of IP6 alone (as distinct from digestibility of sum of IP3-6) did not account for the capacity of the phytase to continue to hydrolyse IP-esters. For these reasons, the digestible P release by the phytase was estimated from the improvement in ileal digestible IP3-6 content of the diet above NC. These estimates were 1.87, 2.10 and 2.03 g/kg of diet for low, medium and high PP diets with the phytase at 1,000 FTU/kg, and 2.26, 2.59 and 3.10 g/kg of diet for low, medium and high PP diets with the phytase at 2,000 FTU/kg.
The observed interaction between phytase dose and dietary PP level on calculated ileal digestible P and the tendency for an interaction between these variables on ileal digestible sum of IP3-6-P in the diet, suggested that the optimum dose level of the phytase to maximise P-release may have been related to dietary PP content. In the high PP diet, ileal digestible P or IP3-6 was low in the NC. However, increasing the phytase dose increased ileal digestible P and IP3-6 P to a greater extent in the high PP diet, presumably due to the higher substrate levels. On the other hand, the optimal dose of phytase based on AID IP3-6 data showed that, in the low PP diet, 2,000 FTU/kg broke down 95% of the sum of IP3-6, whereas 4,000 FTU/kg was needed in the medium and high PP diets to breakdown 94% of the sum of IP3-6. As discussed above, due to inherent differences in the accessibility of phytate to phytase among feedstuffs, the proportion of IP6 degradation could be different when other ingredients are used. However, the data indicated that, to maximise phytate degradation, a higher dose of phytase may be needed with increasing PP level in the diets. In addition, it needs to be considered that, at a high phytase dose level, the P made available by the phytase may not be 100% absorbed. This would occur if the P requirement has already been met or the balance of Ca:P is no longer favourable to enable further P absorption. This hypothesis was supported by the observation that increasing the phytase dose from 2,000 to 4,000 FTU/kg did not further increase ileal digestible IP3-6-P as a percentage of total ileal digestible P. In the NC, the amount of P released from PP as a percentage of total ileal digestible P varied from 25% in the low PP diet to 15% in the high PP diet. With phytase (within the dose range 500 to 4,000 FTU/kg), the percentage range was 66% (500 FTU/kg, all PP levels) to 88% (4,000 FTU, medium PP level). This implied that, when formulating diets containing phytase, in order to maximise PP utilisation and minimise P excretion, the optimal phytase-to-substrate ratio should be considered.
In conclusion, the novel consensus bacterial 6-phytase variant, PhyG, was effective in extensively breaking down IP6 to IP3 or lower IP-esters in the ileum of broilers at 21 d of age, with little accumulation of IP5–P, IP4–P or IP3–P when the phytase was dosed at 1,000 FTU/kg or higher. Independent of phytase dose level, increasing the dietary PP level within the range 2.98 g/kg to 3.98 g/kg reduced the proportion of absorption of IP6, sum of IP3-6 and P in the ileum, whereas the estimated dietary content of ileal digestible P and breakdown of phytate-P was higher in high PP diets. Across all diets, increasing the phytase dose level between 0 and 4,000 FTU/kg exponentially increased AID IP6, AID sum of IP3-6 and AID P, and improved ileal digestible P content of the diet and estimated P-release across all diets, with maximum dose-response improvements above NC seen in the high PP diet. The phytase was effective in breaking down phytate in diets with varied PP content, but the optimal dose level to maximise degradation may differ for diets of different PP content.
Acknowledgments
The authors would like to thank Dr Joelle Buck (Newbury, UK) for her assistance with the writing of this manuscript, which was sponsored by Danisco Animal Nutrition (IFF), The Netherlands, in accordance with Good Publication Practice guidelines.
Disclosure statement
Y. Dersjant-Li, T. Christensen, S. Knudsen, A. Bello and L. Marchal are employees of Danisco Animal Nutrition (IFF).
References
- Ajuwon, K. M., V. Sommerfeld, V. Paul, M. Däuber, M. Schollenberger, I. Kühn, O. Adeola, and M. Rodehutscord. 2020. “Phytase Dosing Affects Phytate Degradation and Muc2transporter Gene Expression in Broiler Starters.” Poultry Science 99 (2): 981–991. doi:https://doi.org/10.1016/j.psj.2019.10.016.
- Aviagen Inc. 2018. “Ross 308 Broiler Management Handbook.” Accessed 9 April 2021. https://en.aviagen.com/assets/Tech_Center/Ross_Broiler/Ross-BroilerHandbook2018-EN.pdf.
- Aviagen Inc. 2019. “Ross 308 Broiler: Nutrition Specifications.” Accessed 9 April 2021. https://en.aviagen.com/assets/Tech_Center/Ross_Broiler/RossBroilerNutritionSpecs2019-EN.pdf.
- Beeson, L. A., C. L. Walk, M. R. Bedford, and O. A. Olukosi. 2017. “Hydrolysis of Phytate to Its Lower Esters Can Influence the Growth Performance and Nutrient Utilisation of Broilers with Regular or Super Doses of Phytase.” Poultry Science 96 (7): 2243–2253. doi:https://doi.org/10.3382/ps/pex012.
- Bello, A., Y. Dersjant-Li, and D. R. Korver. 2019. “The Efficacy of 2 Phytases on Inositol Phosphate Degradation in Different Segments of the Gastrointestinal Tract, Calcium and Phosphorus Digestibility, and Bone Quality of Broilers.” Poultry Science 98 (11): 5789–5800. doi:https://doi.org/10.3382/ps/pez375.
- Christensen, T., Y. Dersjant-Li, V. Sewalt, R. Mejldal, S. Haaning, S. Pricelius, I. Nikolaev, R. A. Sort, and A. De Kreij. 2020. “In Vitro Characterisation of a Novel Consensus Bacterial 6-phytase and One of Its Variants.” Current Biochemical Engineering 6 (3): 156–171. doi:https://doi.org/10.2174/2212711906999201020201710.
- Cowieson, A. J., J. P. Ruckebusch, I. Knap, P. Guggenbuhl, and F. Fru-Nji. 2016. “Phytate-free Nutrition: A New Paradigm in Monogastric Animal Production.” Animal Feed Science and Technology 222: 180–189. doi:https://doi.org/10.1016/j.anifeedsci.2016.10.016.
- Dersjant-Li, Y., A. Awati, H. Schulze, and G. Partridge. 2015. “Phytase in Non-ruminant Animal Nutrition: A Critical Review on Phytase Activities in the Gastrointestinal Tract and Influencing Factors.” Journal of Science of Food and Agriculture 95 (5): 878–896. doi:https://doi.org/10.1002/jsfa.6998.
- Dersjant-Li, Y., G. Archer, A. M. Stiewert, A. A. Brown, E. G. Sobotik, A. Jasek, L. Marchal, et al. 2020. “Functionality of a Next Generation Biosynthetic Bacterial 6-phytase in Enhancing Phosphorus Availability to Broilers Fed a Corn-soybean Meal-based Diet.” Animal Feed Science and Technology 264: 114481. doi:https://doi.org/10.1016/J.anifeedsci.2020.114481.
- Dersjant-Li, Y., and G. Dusel. 2019. “Increasing the Dosing of a Buttiauxella Phytase Improves Phytate Degradation, Mineral, Energy, and Amino Acid Digestibility in Weaned Pigs Fed a Complex Diet Based on Wheat, Corn, Soybean Meal, Barley, and Rapeseed Meal.” Journal of Animal Science 97 (6): 2524–2533. doi:https://doi.org/10.1093/jas/skz151.
- Gizzi, G., P. Thyregod, C. von Holst, G. Bertin, K. Vogel, M. Faurschou-Isaksen, R. Betz, R. Murphy, and B. Brandt Andersen. 2008. “Determination of Phytase Activity in Feed: Interlaboratory Study.” Agricultural Materials 91: 259–267 doi:https://doi.org/10.1093/joac/91.2.259.
- Greiner, R., and U. Konietzny. 2011. “Phytase: Biochemistry, Enzymology and Characteristics Relevant to Animal Feed Use.” In Enzymes in Farm Animal Nutrition, edited by M. R. Bedford and G. G. Partridge, 96–128. 2nd ed. London, UK: CAB International.
- He, O., C. E. Rodrigues Reis, F. Wang, and B. Hu. 2017. “Phytate Extraction from Coproducts of the Dry-grind Corn Ethanol Process.” RSC Advances 7: 5466–5472. doi:https://doi.org/10.1039/C6RA27409A.
- Hirvonen, J., J. Liljavirta, M. T. Saarinen, M. J. Lehtinen, I. Ahonen, and P. Nurminen. 2019. “Effect of Phytase on in Vitro Hydrolysis of Phytate and the Formation of myo-Inositol Phosphate Esters in Various Feed Materials.” Journal of Agricultural and Food Chemistry 67 (41): 11396–11402. doi:https://doi.org/10.1021/acs.jafc.9b03919.
- Hu, H. L., A. Wise, and C. Henderson. 1996. “Hydrolysis of Phytate and Inositol Tri-, Tetra-, and Penta- Phosphates by the Intestinal Mucosa of the Pig.” Nutrition Research 16 (5): 781–787. doi:https://doi.org/10.1016/0271-5317(96)00070-X.
- Humer, E., C. Schwarz, and K. Schedle. 2015. “Phytate in Pig and Poultry Nutrition.” Journal of Animal Physiology and Animal Nutrition 99 (4): 605–625. doi:https://doi.org/10.1111/jpn.12258.
- Lei, X. G., J. D. Weaver, E. Mullaney, A. H. Ullah, and M. J. Azain. 2013. “Phytase, a New Life for an “Old” Enzyme.” Annual Review of Animal Biosciences 1 (1): 283–309. doi:https://doi.org/10.1146/annurev-animal-031412-103717.
- Leske, K. L., and C. N. Coon. 1999. “A Bioassay to Determine the Effect of Phytase on Phytate Phosphorus Hydrolysis and Total Phosphorus Retention of Feed Ingredients as Determined with Broilers and Laying Hens.” Poultry Science 78 (8): 1151–1157. doi:https://doi.org/10.1093/ps/78.8.1151.
- Li, W., R. Angel, S.-W. Kim, E. Jiménez-Moreno, M. Proszkowiec-Weglarz, and P. Plumstead. 2015. “Age and Adaptation to Ca and P Deficiencies: 2. Impacts on Amino Acid Digestibility and Phytase Efficacy in Broilers.” Poultry Science 94 (12): 2917–2931. doi:https://doi.org/10.3382/ps/pev273.
- Li, W., R. Angel, S.-W. Kim, E. Jiménez-Moreno, M. Proszkowiec-Weglarz, and P. W. Plumstead. 2014. “Age and Adaptation Effects to Ca and P Deficiencies. Effect on P Digestibility.” Poultry Science 93: 84(Abstr.).
- Li, W., R. Angel, S.-W. Kim, K. Brady, S. Yu, and P. W. Plumstead. 2016b. “Impacts of Dietary Calcium, Phytate, and Nonphytate Phosphorus Concentrations in the Presence or Absence of Phytase on Inositol Hexakisphosphate (IP6) Degradation in Different Segments of Broilers Digestive Tract.” Poultry Science 95 (3): 581–589. doi:https://doi.org/10.3382/ps/pev354.
- Li, W., R. Angel, S.-W. Kim, K. Brady, S. Yu, and P. W. Plumstead. 2017. “Impacts of Dietary Calcium, Phytate, and Phytase on Inositol Hexakisphosphate Degradation and Inositol Phosphate Release in Different Segments of Digestive Tract of Broilers.” Poultry Science 96 (10): 3626–3627. doi:https://doi.org/10.3382/ps/pex170.
- Li, X., D. Zhang, T. Y. Yang, and W. L. Bryden. 2016a. “Phosphorus Bioavailability: A Key Aspect for Conserving This Critical Animal Feed Resource with Reference to Broiler Nutrition.” Agriculture 6 (2): 25. doi:https://doi.org/10.3390/agriculture6020025.
- Menezes-Blackburn, D., S. Gabler, and R. Greiner. 2015. “Performance of Seven Commercial Phytases in an in Vitro Simulation of Poultry Digestive Tract.” Journal of Agricultural Food Chemistry 63 (27): 6142–6149. doi:https://doi.org/10.1021/acs.jafc.5b01996.
- Sanz-Penella, J. M., and M. Haros. 2014. “Chapter 2: Whole Grain and Phytate-degrading Human Bifidobacteria.” In Wheat and Rice in Disease Prevention and Health, edited by R. R. Watson, V. R. Preedy, and S. Zibadi, 17–31. Washington, DC: Academic Press.
- Selle, P. H., A. J. Cowieson, N. P. Cowieson, and V. Ravindran. 2012. “Protein–phytate Interactions in Pig and Poultry Nutrition: A Reappraisal.” Nutrition Research Reviews 25 (1): 1–17. doi:https://doi.org/10.1017/S0954422411000151.
- Selle, P. H., A. J. Cowieson, and V. Ravindran. 2009. “Consequences of Calcium Interactions with Phytate and Phytase for Poultry and Pigs.” Livestock Science 124 (1–3): 126–141. doi:https://doi.org/10.1016/j.livsci.2009.01.006.
- Selle, P. H., and V. Ravindran. 2007. “Microbial Phytase in Poultry Nutrition.” Animal Feed Science and Technology 135: 1–41.
- Shafey, T. M., M. W. McDonald, and J. G. Dingle. 1991. “Effects of Dietary Calcium and Available Phosphorus Concentration on Digesta pH and on the Availability of Calcium, Iron, Magnesium and Zinc from the Intestinal Contents of Meat Chickens.” British Poultry Science 32 (1): 185–194. doi:https://doi.org/10.1080/00071669108417339.
- Siriwan, P., W. L. Bryden, Y. Mollah, and E. F. Annison. 1993. “Measurement of Endogenous Amino Acid Losses in Poultry.” British Poultry Science 34 (5): 939–949. doi:https://doi.org/10.1080/00071669308417654.
- Wyss, M., R. Brugger, A. Kronenberger, R. Rémy, R. Fimbel, G. Oesterhelt, M. Lehmann, and A. P. G. M. Van Loon. 1999. “Biochemical Characterization Of Fungal Phytases (myo-Inositol Hexakisphosphate Phosphohydrolases): Catalytic Properties.” Applied and Environmental Microbiology 65 (2): 367–373. doi:https://doi.org/10.1128/AEM.65.2.367-373.1999.
- Yan, F., R. Angel, C. M. Ashwell, A. Mitchell, and M. Christman. 2005. “Evaluation of the broiler’s Ability to Adapt to an Early Moderate Deficiency of Phosphorus and Calcium.” Poultry Science 84 (8): 1232–1241. doi:https://doi.org/10.1093/ps/84.8.1232.
- Yu, S., A. Cowieson, C. Gilbert, P. Plumstead, and S. Dalgaard. 2012. “Interactions of Phytate and Myo-inositol Phosphate Esters (IP1-5) Including IP5 Isomers with Dietary Protein and Iron and Inhibition of Pepsin.” Journal of Animal Science 90 (6): 1824–1832. doi:https://doi.org/10.2527/jas.2011-3866.
- Zeller, E., M. Schollenberger, I. Kühn, and M. Rodehutscord. 2015. “Hydrolysis of Phytate and Formation of Inositol Phosphate Isomers without or with Supplemented Phytases in Different Segments of the Digestive Tract of Broilers.” Journal of Nutrition Science 4: e1. doi:https://doi.org/10.1017/jns.2014.62.