 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
1. It was hypothesised that dietary N-acetyl-L-cysteine (NAC) in feed, as a source of cysteine, could improve the performance of heat-stressed finisher broilers by fostering glutathione (GSH) synthesis. GSH is the most abundant intracellular antioxidant for which the sulphur amino acid cysteine is rate limiting for its synthesis.
2. In the first experiment, four levels of NAC: 0, 500, 1000 and 2000 mg/kg were added to a diet with a suboptimal level of sulphur amino acids in the finisher phase. In the second experiment, NAC was compared to other sulphur amino acid sources at equal molar amounts of digestible sulphur amino acids. Birds were allocated to four groups: control, 2000 mg/kg NAC, 1479 mg/kg L-cystine, and 2168 mg/kg Ca-salt of 2-hydroxy-4-(methylthio)butanoic acid. A chronic cyclic heat stress model (temperature was increased to 34°C for 7 h daily) was initiated at 28 d of age.
3. In the first experiment, growth performance and feed efficiency in the finisher phase were significantly improved by graded NAC. ADG was 88.9, 92.2, 93.7 and 97.7 g/d, and the feed-to-gain ratio was 2.18, 1.91, 1.85 and 1.81 for the 0, 500, 1000 and 2000 mg/kg NAC treatments, respectively. However, liver and heart GSH levels were not affected by NAC. On d 29, liver gene transcript of cystathionine-beta-synthase like was reduced by NAC, which suggested reduced trans-sulphuration activity. The second experiment showed that L-cystine and Ca-salt of 2-hydroxy-4-(methylthio) butanoic acid were more effective in improving performance than NAC.
4. In conclusion, N-acetyl-L-cysteine improved dose-dependently growth and feed efficiency in heat-stressed finishing broilers. However, this was not associated with changes in tissue GSH levels, but more likely worked by sparing methionine and/or NAC’s and cysteine’s direct antioxidant properties.
Introduction
Heat stress in poultry is a challenge for producers in tropical and subtropical regions as well as in temperate areas, by the occurrence of heat waves. This phenomenon has triggered extensive research due to the rapid development of poultry production globally, and in particular in tropical and subtropical areas, to fulfil the high demand for chicken meat as a predominant protein source (Mottet and Tempio Citation2017). Heat stress results in important economic losses, as it impairs broiler productivity, particularly during the grower and finisher phase (Rath et al. Citation2015; Rosa et al. Citation2007). Heat exposure reduces feed consumption, a primary physiological response to maintain homoeostasis by decreasing metabolic heat (Lara and Rostagno Citation2013; Pertiwi et al. Citation2022). Chronic heat stress seriously decreases feed efficiency, carcass and meat quality, health status, and survival rate (Aksit et al. Citation2006; Lu et al. Citation2018).
The role of heat stress in generating oxidative stress has gained significant attention in recent years. It has been observed that there is an increase in electron leakage within the mitochondrial electron transport chain during heat stress (Akbarian et al. Citation2016). This can lead to mitochondrial dysfunction which may contribute to metabolic diseases in broiler chickens.
Reactive oxygen species (ROS) are produced when molecular oxygen is not fully reduced to water, which can degrade important cellular components such as lipids, carbohydrates, proteins, and DNA. If the production of ROS exceeds the capacity of antioxidants to neutralise them, it can lead to apoptosis and tissue damage (Bottje et al. Citation2006). Glutathione (L-γ-glutamyl-L-cysteinylglycine, GSH) is the main endogenous intracellular antioxidant compound. It is moderately stable in intracellular milieus, interacting with ROS and reactive nitrogen species and with electrophiles as a cofactor for various enzymes, including glutathione peroxidase (GPx; Cooper et al. Citation2011; Lushchack Citation2012). High GSH levels in tissues can promote productivity by decreasing oxidative stress associated with higher metabolic activities from the rapid growth in broilers (Enkvetchakul and Bottje Citation1995).
In the endogenous synthesis of GSH, L-cysteine is the limiting amino acid that restricts the rate of production. L-cysteine can be derived from the diet or through the transmethylation and trans-sulphuration pathways, originating from L-methionine. N-acetyl-L-cysteine (NAC) is a form of L-cysteine that is resistant to changes in oxidation-reduction reactions. It is easily converted into bioavailable L-cysteine by mucosal deacetylation and subsequent absorption, although NAC can be absorbed intact (Sommer et al. Citation2020). In turn, the intestinal epithelium, next to the liver, is known to be a main site of GSH synthesis. NAC is commonly found in allium plants, particularly in Allium cepa (onion), which has 45 mg NAC per kg. It is not naturally found in animal tissues. Administration of NAC to human and rats increased mitochondrial and cytosolic GSH concentration and protected against cell toxicity and various pathological condition related to GSH cellular homoeostasis (Attia et al. Citation2019). In pigs, NAC enhanced cysteine utilisation for GSH synthesis and immune system metabolites and preserved morphological integrity of intestinal mucosa in the postweaning phase (Rakhshandeh et al. Citation2019; Zhu et al. Citation2013). It helps resolve ulcerative colitis through regulating oxidative stress, gene expression (EGF, TLR4), tight junction signalling and cell apoptosis (Hou Citation2015; Wang et al. Citation2013). However, adding NAC to the drinking water of weaned piglets failed to exert any beneficial response on animal performances and the GSH redox system (Degroote et al. Citation2019).
It was hypothesised that dietary of NAC supplemented in feed could improve the performance of heat-stressed finisher broilers by enhancing GSH levels in tissues. It is known that older poultry generally need to release higher hepatic GSH amounts than younger birds to support their growth (Enkvetchakul et al. Citation1995). Up to now, studies on the application NAC in the heat-stressed broiler have been limited (He et al. Citation2019; Yi, Hou, Tan, et al. Citation2016). The current work included two experiments with heat-stressed broilers. First, graded levels of NAC were added to a basal diet with a level of digestible sulphur amino acids equal or below the recommendations (depending on literature source) for ideal protein for as-hatched finisher broiler chickens. Responses in terms of animal performance and GSH redox status in various tissues were assessed. As the first experiment did not show a response on tissue GSH levels, in a second experiment the efficacy of NAC was compared with other sulphur amino acid sources, namely L-cystine (L-CYS), and Ca-salt of 2-hydroxy-4-(methylthio) butanoic acid (CaHMTBa). The former is another stable source of L-cysteine, while the latter is precursor of methionine (Baker Citation2009). Only performances were recorded from the second experiment.
Material and methods
Birds, heat stress, and performances
The study was conducted in compliance with the ethical principles and guidelines for the housing and treatment of laboratory animals as stated in the European Directive (2010/63/EU) for the protection of animals used for scientific purposes, and the Belgian royal decree (KB29.05.13) on the use of animals in experimental research.
In the first experiment (Exp I), 680 one-d-old male broilers (Ross 308; Hatchery Vervaeke-Belavi, Tielt, Belgium) were assigned to 34 pens with 20 birds per pen at a density of 14.7 birds per m2. In the second (Exp II), 720 male one-d-old broilers (Ross 308) were divided to 36 pens with 20 birds per pen. The flooring was coated with fresh wood saw dust (1.5 kg/m2) and no refilling or cleaning of the floor was conducted during the experiment. Water distribution was through nipple drinkers and feeding by feed towers.
On the first day of life, broilers were vaccinated towards Newcastle Disease (NDC2) and Infectious Bronchitis (IBMAS) at the hatchery’s facilities. At 17 d of age, a second dose of NDC2 vaccination did using Nobilis ND Clone 30 (MSD Animal Health, Boxmeer, the Netherlands). In addition, on the same day, the animals were sprayed with Nobilis Gumboro D78 (MSD Animal Health, Boxmeer, the Netherlands) to protect against Gumboro. These vaccination programs were conducted according to conventional production standards in Belgium.
The light scheme was 23 h light/1 h dark and 18 h light/6 h dark from d 0–7 and 7–41 d, respectively. The 18 h light is referred to light with a minimum of 20 lux between 4 am and 10 pm. The initial chamber temperature was 34°C, which decreased linearly to 22°C on d 28. During the first five days, one infrared lamp was used per pen to provide additional heating. From d 28 until the end of the investigation, which was d 41 in Experiment I and d 40 in Experiment II, a particular temperature and humidity regime was used to initiate chronic cyclic heat stress (Akbarian et al. Citation2014; Majdeddin et al. Citation2020; Zhang et al. Citation2023). The baseline temperature was 22°C. Daily, between 8:00 and 9:00 a.m., excluding sampling days (between 6:00 and 7:00 a.m.), the temperature was progressively increased to 34°C and maintained for 7 h (until 4:00 p.m).
The temperature was then reduced to its baseline level. During periods of heat stress, the relative humidity of the air was maintained between 50 and 60% through nebulisation of water. Body weight (BW) and food intake were recorded per pen. For each period of rearing, the average daily gain (ADG), average daily feed intake (ADFI), feed-to-gain ratio (F:G, adjusted for mortality and calculated as total feed intake divided by total gain including the weight of birds lost per pen), and mortality were determined. The European Production Efficiency Factor (EPEF) was determined using the following equation:
where MR was the mortality rate for the total period (%), BWend the BW at d 41 (Exp I) or 40 (Exp II) (kg) and F:G the feed-to-gain ratio (g/g) for the total period.
Dietary treatments
Broiler chickens were fed starter (d 0–10), grower (d 10–25) and finisher (d 25–41 in Exp I and day 25–40 in Exp II) which were formulated using wheat-soybean diets as mash. The ingredient and calculated nutrient composition of the basal diets are shown in .
Table 1. Ingredient and calculated nutrient composition of wheat-soybean-based basal diets for the starter (day 0–10), grower (day 10–25), and finisher (day 25–41 in Exp I and day 25–40 in Exp II) phase in Exp I and II (as-is basis).
All pens received the same basal starter and grower diet. In the finisher period, pens were assigned to one of four dietary group treatments according to a randomised block design. Dietary treatments were replicated in eight or nine pens. In Exp I, the treatments included a control (CTRL, basal diet), and three diets with increasing levels of NAC i.e., 500, 1000 and 2000 mg/kg (CAS 616-91-1, 99% purity; 74.3% L-cysteine; Zambon, Jette, Belgium). The NAC was added ‘on top’ of the basal diet. In Exp II, the treatments included a control (CTRL, basal diet), and basal diet supplemented ‘on top’ with either 2000 mg/kg NAC, 1479 mg/kg L-CYS (L-CYS; CAS 56-89-3; 98.5% purity; 100.8% L-cysteine; Sigma-Aldrich, Overijse, Belgium), or 2168 mg/kg Ca-salt of 2-hydroxy-4-(methylthio)butanoic acid (CaHMTBa; CAS 4857-44-7; 84% methionine activity; Novus, Brussels, Belgium). The basal diet in the finisher period was formulated to have a content of apparent faecal digestible lysine (Lys) of 0.95% and a ratio digestible methionine + cysteine (M+C) to digestible Lys of 0.73. This ratio was equal or below the recommendations for ideal protein for as-hatched finisher broiler chickens depending on literature source (e.g., 0.73, Tabellenboek Veevoeding 2018, Centraal Veevoederbureau, the Netherlands, www.cvbdiervoeding.nl; 0.77, Amino Acids in Animal Nutrition 2014, FEFANA, fefana.org; 0.78, Broiler Ross Nutrition Specifications 2019, Aviagen, aviagen.com). Theoretically, if NAC was completely converted to L-cysteine and absorbed, this would raise the digestible M+C to digestible Lys ratio to 0.77, 0.81 and 0.89 following the graded levels of NAC, and concomitantly the ratio digestible M to digestible M+C was reduced stepwise from 0.63 to 0.52.
The analysed nutrient composition of basal diets is presented in supplementary Table S1. The dry matter (DM) content of diets was measured by dehydration at 103°C until constant weight (SO 6496:1999). Crude ash was analysed by incineration at 550°C for 4 h in a combustion oven (ISO 5984:2002). The total nitrogen (N) content was calculated using the Kjeldahl method (ISO 5983–1:2005), and crude protein content was determined by multiplying total N by 6.25. After extraction with diethyl ether, ether extract (EE), as a measure of crude fat, was analysed gravimetrically using the Soxhlet system (ISO 6492:1999). Using HPLC on hydrolysed and oxidised samples, the amino acid composition of protein-bound amino acids was determined (ISO 13903:2005). Since this amino acid is destroyed by acid hydrolysis (ISO 13904:2016), tryptophan determination required separate analysis. Although the levels of lysine and methionine levels by analysis were higher and lower than formulated () for experiment I and II, respectively, the ratio M+C to lysine of total amino acids remained close to 0.73.
Sample collection
In Exp I, two birds per pen (within a similar weight range) were selected for sampling, both on d 19, to evaluate the acute heat stress response, as well as d 42, to evaluate the chronic heat stress response. Birds were euthanised by cervical dislocation followed by exsanguination. Blood was collected in two tubes with EDTA as anticoagulant to assess haematocrit and malondialdehyde (MDA). The digestive tract was excised and a 5 cm piece of mid-jejunum was fixated in formalin for histo-morphological examination on d 41. Subsequently, heart and liver were taken for GSH, and GSSH analysis, GPx in liver samples on d 29 and 41. Samples from liver for MRNA extraction were taken on d 29, snap frozen and stored at −80°C.
On d 42, after overnight fasting and no further implementation of heat stress protocol, two birds per pen were euthanised to measure carcass characteristic and MDA. In Exp II, on d 29 (acute heat stress) and d 40 (chronic heat stress), two birds per pen (close to average weight) were selected for determination of rectal temperature. No birds were sacrificed for sampling.
Histo-morphological indices of mid-jejunum
Formalin-fixed mid-jejunum samples were dehydrated, embedded, sliced into 5-μm transects and stained with haematoxylin and eosin. Subsequently, villus height and adjacent crypt depth of at least ten, well-oriented villi were measured and the ratio of the villus height to the crypt depth was calculated (Van Nevel et al. Citation2003). Observations were conducted using a microscope (Olympus BX61, Olympus, Aartselaar, Belgium) and analysed by image capture software (AnalySIS Pro, Olympus, Aartselaar, Belgium).
Oxidative and glutathione redox status in plasma, liver, and heart
Spectrophotometry at 532 nm was used to determine total MDA concentration in plasma using the TBARS assay developed by Grotto et al. (Citation2007) with minor modifications. Buffered aqueous extracts of liver samples were prepared after mixture with 1% Triton X-100 phosphate buffer (pH 7, 50 mM), homogenisation, centrifugation and filtration. The GPx activity in buffered aqueous extracts of liver was quantified based on the dynamical change in the oxidation of NADPH and reaction time using Multi-Mode Microplate Readers at 340 nm (Hernandez et al. Citation2004).
The Yoshida (Citation1996) method was applied to measure GSH and GSSG in liver and heart samples, with modifications (Degroote et al. Citation2019). Briefly, iodoacetic acid was used as a thiol quenching agent, and the acid solution’s pH was adjusted to 8–9 by adding a potassium hydroxide-potassium bicarbonate buffer. Incubation overnight with 1-fluoro-2,4-dinitrobenzene produced stable thiol derivatives that were separated on an aminopropyl column using reverse-phase HPLC. Chromatographic separations were conducted with water/methanol (1:4 v/v) and acetic acid (0.5 M)/methanol (1:1.78 v/v) as the mobile phases and UV absorption at 365 nm was measured. The concentrations of GSH and GSSG were determined relative to -glutamyl-glutamate as the internal standard and GSH and GSSG external standard solutions, respectively. The results were expressed based on the weight of wet tissue.
Gene expression in liver samples
The PureLinkTM RNA mini kit (ThermoFisher, Merelbeke, Belgium) combined with TRIzol (ThermoFisher) was used as per the manufacturer’s instructions to extract total RNA from liver samples. When isolating the RNA, genomic DNA was removed using the kit’s gDNA eliminator spin. The concentration (ranging from 200 to 1200 ng/ml) and purity (optical density 260A/280A ranging from 1.95 to 2.25) of the sample were analysed using the NanoDrop ND-2000 (ThermoFisher). To synthesise cDNA, the QuantiTect Reverse Transcription Kit (Qiagen, Hilden, Germany) instructions were followed. Specifically, 1 µg of RNA was mixed with 2 µl of gDNA Wipeout buffer and the volume was adjusted to 14 µl using RNase-free water.
The reverse transcription quantitative PCR (RT-qPCR) analysis was performed on a Step One Plus Real-Time System (ThermoFisher) using Maxima SYBR Green/ROX qPCR Master Mix (2X) in a 10 µl reaction volume that contained 5 µl of 2X SYBR green master mix, 1 µl of forward and reverse primers (10 nM each), 2 µl of water and 1 µl of template cDNA. The thermal cycling conditions included an initial denaturation at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 s and annealing/elongation at 60°C for 1 min. Amplification of non-targeted fragments was checked via dissociation curve analysis. Qiagen software version 2.0.2 of Rotor-Gene Q was utilised for data collection. The qPCR analysis was conducted to identify mRNA levels of genes cystathionine-beta-synthase like (CBSL), glutamate-cysteine ligase catalytic subunit (GCLC), and glutathione synthetase (GSS). The data were normalised by introducing two internal reference genes: actin beta (ACTB) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Specific primers for each gene were designed utilising the cross-exon strategy and version 5.0 of the software Primer Premier (Premier Biosoft, San Francisco, U.S.A.) (Supplementary Table S2).
Statistical analysis
The experimental unit for all performance variables was the pen. For variables from the digestive tract and other tissues, carcass and breast meat characteristics, two birds per pen were collected, and average values for both chickens for each variable were taken for statistical analysis. Data obtained were subjected to analysis of variance, using SPSS software program package (SPSS 27.0), with dietary supplementation in finisher diet as main factor and block as random factor. The non-parametric Kruskal–Wallis test was utilised to analyse the mortality data. The BW at d 25, the start of feeding the finisher diets, was introduced as a covariate for analysis. As the time of sampling after starting the heat stress protocol may have affected rectal temperature, haematocrit and oxidative and glutathione redox status parameters, this was incorporated as a covariate where significant effects were seen. Means were separated by Tukey test. In Exp I, orthogonal contrasts were used to examine the linear and quadratic impacts of NAC supplementation. Data were assumed to be statistically significant when P < 0.05, and to prove a trend when 0.05 ≤ P < 0.10.
Results
Broiler performance and rectal temperature
In Exp I, final BW and ADG in the finisher period when chronic cyclic heat stress was prevailing were linearly increased by graded levels of NAC in the feed (both P < 0.05; ). The ADG was nearly 10% higher in birds fed NAC at 2000 mg/kg when compared to CTRL (P < 0.05). Remarkably, 500, 1000 and 2000 mg/kg of NAC decreased feed intake substantially (quadratic effect, P < 0.05). It should be noted that in some pens containing birds on the CTRL treatment, chickens seemed to waste the mash feed, likely leading to overestimation of feed consumption. Hence, feeding NAC resulted in large improvements of F:G as compared with CTRL (P < 0.05; linear effect). Mortality was high during the chronic cyclic heat stress period and variable among treatments, yet without statistical significance. For the total period (d 0–41), similarly ADG and F:G were largely improved (both P < 0.05, linear effect) and ADFI tended to decrease (P = 0.067, quadratic effect) with supplemental NAC, resulting in higher EPEF values (P < 0.05, linear effect).
Table 2. Effect of N-acetyl-L-cysteine (NAC) supplementation on body weight (BW), average daily gain (ADG), average daily feed intake (ADFI), feed gain ratio (F:G), mortality, and European production efficiency factor (EPEF) of male broilers subjected to chronic cyclic heat stress in the finisher phase in Exp I.a
Analogously to Exp I, in Exp II, NAC at 2000 mg/kg stimulated growth and final BW in the finisher period as compared to CTRL (both P < 0.05; ). This improvement equalled 6.6% and 3.3%, respectively, thus lower than in Exp I. Growth responses were higher in groups L-CYS (+8.0%, P < 0.05) and CaHMTBa (+11.1%, P < 0.05). Feeding the sulphur amino acid sources did not change ADFI. Feed efficiency was better for birds fed L-CYS and CaHMTBa in comparison to the CTRL (P < 0.05), while NAC was intermediate but not significantly different from either treatment. Again, mortality was high during the episode of chronic cyclic heat stress but not affected by dietary supplement. For d 0–40, ADG, F:G and EPEF were all positively affected by L-CYS and CaHMTBa compared to CTRL (P < 0.05). On the other hand, the NAC treatment did not exhibit similar effects.
Table 3. Effect of supplementary sulphur amino acid sources on body weight (BW), average daily gain (ADG), average daily feed intake (ADFI), feed gain ratio (F:G), mortality, and European production efficiency factor (EPEF) of male broilers subjected to chronic cyclic heat stress in the finisher phase in Exp II.a
Rectal temperatures of birds selected at d 29 did not reveal significant treatment effects in either experiment (Supplementary Figure S1). There was a tendency for a linear reduction in rectal temperature in line with graded NAC on d 41 in Exp I (P = 0.070). It should be emphasised that there was large variation among replicate measurements within treatment occurred.
Carcass and breast meat characteristics
Characteristics, such as skinless eviscerated carcass and carcass percentage, breast meat percentage, pH at 0 and 24 h, L*, a*, b* and TBARS of breast meat were not influenced by incremental NAC (Exp I, ).
Table 4. Effect of N-acetyl-L-cysteine (NAC) supplementation on carcass and breast meat characteristics of male broilers subjected to chronic cyclic heat stress in the finisher phase after overnight fasting and sampled at day 42 in Exp I.a
Haematocrit
In Exp I, haematocrit on d 29 (30.0% ± 0.9) and d 41 (28.8% ± 0.6) was not affected by treatment (). On d 41, haematocrit was negatively affected by time of sampling after beginning the heat stress protocol that day, irrespective of treatment (P < 0.05; ).
Figure 1. Effect of N-acetyl-L-cysteine supplementation (Exp I) on haematocrit in male broilers subjected to chronic cyclic heat stress in the finisher phase.
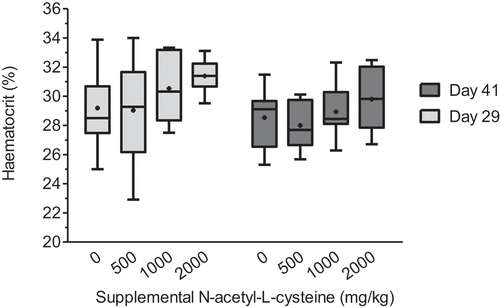
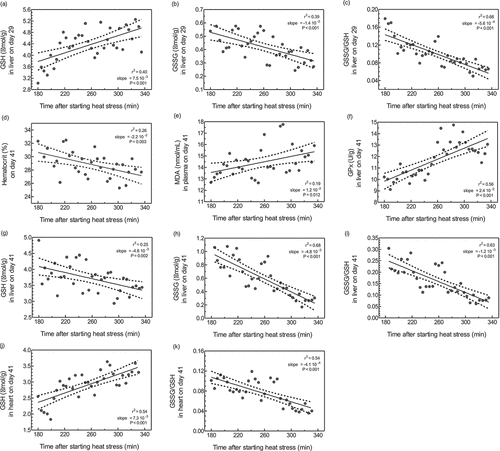
Figure 2. GSH (a), GSSG (b), and GSSG/GSH (c) in liver on day 29 and haematocrit (d) and MDA (e) in plasma, GPx activity (f), GSH (g), GSSG (h), and GSSG/GSH (i) in liver, and GSH (j) and GSSG/GSH (k) in heart on day 41 of male broilers subjected to chronic cyclic heat stress sampled on day 25 as affected by time of sampling after starting the heat stress protocol that day (Exp I). GSH, glutathione; GSSG, glutathione disulphide; MDA, malondialdehyde; GPx, glutathione peroxidase.
Histo-morphological indices of mid-jejunum
No significant differences were found due to treatment for villus length, crypt depth, and the ratio of villus height to the crypt depth (data not shown, Exp I).
Oxidative and glutathione redox status in plasma, liver, and heart
Plasma MDA (P = 0.076) and GPx activity (P < 0.05) were linearly reduced by incremental NAC in the diet on d 29 (Exp I, ). The MDA level in the 2000 mg/kg NAC treatment group, 12.7 nmol/ml, was smaller than for the CTRL (13.3 nmol/ml; P < 0.05). However, GSH neither in liver nor heart was affected by treatment. In contrast, GSSG was linearly increased in liver (trend, P = 0.062) and heart (P < 0.05). Obviously, tissue GSH levels were higher in liver than heart.
Table 5. Effect of N-acetyl-L-cysteine (NAC) supplementation on variables of oxidative status in male broilers subjected to chronic cyclic heat stress in the finisher phase and sampled at day 29 in Exp I.a
In contrast to d 29, on d 41 a linear trend suggested an increase in MDA in plasma when feeding graded NAC (P = 0.083, ). Similarly, as on d 29, GPx activity in plasma showed a trend for linear reduction in birds fed NAC on d 41 (P = 0.058). Again, GSH in liver and heart were not altered by feeding NAC, and clearly the difference between GSH concentrations in liver and heart on d 41 was smaller than on d 29. However, GSH in liver decreased while GSH in heart increased with age and chronic cyclic heat stress. Furthermore, covariance analysis revealed that oxidative and GSH redox status in liver and heart were significantly affected by the time after starting heat stress on the respective days irrespective of treatment (). An upregulation of GSH levels in liver can be seen on d 29 (), while GSSG decreases (). Obviously, on d 41 both MDA and GPx activity in plasma increased upon time under heat stress, emphasising the association between heat and oxidative stress (). Conversely to d 29, on d 41 GSH in liver declines substantially when heat stress progresses. Interestingly, heart GSSG levels increased sharply.
Table 6. Effect of N-acetyl-L-cysteine (NAC) supplementation on variables of oxidative status in male broilers subjected to chronic cyclic heat stress in the finisher phase and sampled at day 41 in Exp I.a
Gene expression in liver samples
The CBSL mRNA level in liver samples of chickens on d 29 in Exp I was linearly and quadratically reduced, with the 500 and 2000 mg/kg NAC groups showing lower gene expression as compared to CTRL (P < 0.05; ). The GCLC mRNA level was not altered by treatment, while a trend indicated a linear reduction in GSS mRNA levels (P = 0.072). It should be noted that large variation among replicate measurements within treatment occurred.
Table 7. Effect of N-acetyl-L-cysteine (NAC) supplementation on mRNA level of genes involved in transsulphuration and glutathione synthesis in liver in male broilers subjected to chronic cyclic heat stress in the finisher phase and sampled at day 29 in Exp I.ab
Discussion
Although numerous trials have underscored the beneficial role of sulphur amino acids (SAA) on growth performance and health of heat-stressed broilers (Del Vesco et al. Citation2015; Willemsen et al. Citation2011), limited reports are available concerning the consequences of NAC supplementation. In the present study, increased levels of NAC added to a basal diet with suboptimal level of sulphur amino acids improved linear growth due to largely better feed conversion. Yi, Hou, Tan, et al. (Citation2016) reported similar results, showing that supplementation with 1000 mg/kg of NAC could ameliorate the negative impact of heat stress on feed efficiency and intestinal function in chronic cyclic heat-stressed Cobb male chickens. NAC supplementation at similar dosage in Arbor Acres broilers reversed the adverse effects of chronic high temperatures on weight gain and feed intake (He et al. Citation2019). Furthermore, Omid et al. (Citation2018) proved that NAC at 5000 mg/kg mitigated heat stress in breeder Japanese quail under constant high temperatures. Feeding NAC increased feed intake and egg production, optimised feed efficiency (P < 0.05), hatchability and Haugh units, and was associated with changes immune and antioxidant responses. In the above studies, NAC was added to a nutrient adequate diet. In an aflatoxin B1 challenge, Valdivia et al. (Citation2001) found that NAC at daily dosage of 800 mg/kg BW alleviated the detrimental impact on performance, biochemical parameters and liver activities in birds. Similarly, Attia et al. (Citation2019) who administrated 100 mg/kg NAC in feed proved that NAC protected the digestive system against toxins from feed, decreased the feed-to-gain ratio and reduced mortality. These studies demonstrated that NAC may protect poultry when the metabolism is stressed by either environment or diet. However, in the present study NAC administration lowered feed intake in broilers dramatically, even at 500 mg/kg (Exp I), though, whilst improving growth. Yet in Exp II no reduction in feed consumption was seen with 2000 mg/kg NAC. Nonetheless, in young animals, L-cysteine treatment has been related with decreased feed consumption which has been linked to its bitter taste (Lee et al. Citation2013). In birds, a large dietary excess of methionine or cysteine typically depresses growth (Baker Citation2009). For instance, inclusion of L-cysteine, L-Cys, and N-acetyl-L-cysteine at a concentration of 2.5–3.0% in the diet caused slight suppression of growth in young chicks, in contrast an iso-sulphureous concentration of DL-methionine led to more significant performance suppression (Dilger and Baker Citation2007a). The toxic effect of L-Cys was ascribed to a marked increase in anion gap and metabolic acidosis, yet the effects of N-acetyl-L-cysteine and L-Cys were not tested. It should be noted that these dosages extended far beyond the levels applied in the current study, and hence could not explain the reduction in feed consumption. Conversely, Dilger and Baker (Citation2007b) demonstrated that a slight surplus of cyst(e)ine (e.g., 1000 mg/kg) in a methionine deficient diet was growth suppressing. Thus, it is plausible that the low performance of the CTRL in Exp I was due to the suboptimal level of methionine/sulphur amino acids.
Interestingly, supplementation of NAC in this study decreased rectal temperature of heat-stressed birds on d 41. Feeding NAC is known to inhibit NF-κB pathways (Lou and Kaplowitz Citation2007; Oka et al. Citation2000) which can induce the regulation of various pro-inflammatory genes, such as the encoding cytokines (Wrotek et al. Citation2020). Therefore, NAC could act as an antipyretic in heat-stressed broilers. This activity has been associated with the activation and increased synthesis of the endogenous antipyretic cytokines such us IL-10 (Wrotek et al. Citation2020). Other mechanisms for NAC in enhancing body functions may relate to the production ATP and catalase and trypsin activity. Furthermore, it has been reported that NAC upregulated critical genes associated with AMPK, TLR4/NF-κB, EGFR, and type I IFN cell signalling pathways, and enhanced the levels of both total and phosphorylated proteins for mTOR and P70S6K in intestinal epithelial cells (Li et al. Citation2022; Yi, Hou, and Wang Citation2016). In addition, mTOR has been widely recognised as a promoter of protein synthesis (Suryawan et al. Citation2009).
Exp II was executed to further clarify the mechanism of NAC. Efficacy was compared to other sulphur amino acid sources, namely L-Cys and CaHMTBa. The former is another stable source of L-cysteine, while the latter is precursor of methionine (Baker Citation2009). The largest growth-promoting effects were found for CaHMTBa. This observation pointed to the fact that supplemental NAC had a methionine sparing, rather than a cysteine effect.
In the second study, a linear response to NAC, L-CYS and CaHMTBa suppressed any detrimental effect of HS, especially on the final BW and the ADG and FCR from d 25–40 and d 0–40. Similar to NAC, L-cysteine acts as an antioxidant, mitigating oxidative stress triggered by heat, whereas CaHMTBa produces cysteine and taurine that are mostly used for supporting cellular integrity and epithelial barrier function in the intestine during heat stress (Bauchart-Thevret et al. Citation2011; Martín-Venegas et al. Citation2006; Wang et al. Citation2019). These amino acids have been seen to improve nutrient utilisation, enhance growth performance, reduce mortality and boost immune function in heat-stressed broilers (Wang et al. Citation2019; Willemsen et al. Citation2011).
Plasma MDA, liver GPx and heart GSSG level on d 29 changed significantly with incremental NAC, whereas on d 41, only liver GPx was influenced. These observations indicated a higher need of GSH in oxidatively challenged tissues such as heart, and a failure of the liver to keep up with GSH synthesis after chronic cyclic heat stress. However, supplemental NAC did not support higher GSH levels in tissues such as liver and heart, contrary to the current hypothesis. To some extent, these results are similar with Degroote et al. (Citation2019) who reported that 200 mg/l NAC in drinking water for weaned piglets did not influence GSH level in any tissue. This indicated that NAC may not necessarily assist GSH levels in the body or the amount of NAC utilised in the study may not have been sufficient to observe a favourable effect. As an oxidative stress indicator, MDA in blood can show the amount of ROS overproduction and lipid peroxidation in the body. Reduced plasma MDA level seen was in line with He et al. (Citation2019), who administered 1000 mg/kg NAC in feed to chronic constant heat-stressed broilers. They demonstrated that NAC could partially reverse elevated MDA levels induced by heat on d 28, 35 and 42, however the ability to inhibit hydroxyl radicals was only improved d day 28 and 35. The study by Yi, Hou, Tan, et al. (Citation2016) showed that 1000 mg/kg NAC in heat-stressed broilers elevated the GSH and cysteine concentrations in the duodenum and decreased MDA concentration in the small intestine. They argued that the main role of NAC was to provide Cys for GSH generation, thus sparing other antioxidants such as catalase (CAT) and superoxide dismutase (SOD). This consequently improved intestinal antioxidative capacity and attenuated intestinal oxidative stress (Yi et al. Citation2014). When 65 d-old Japanese quail were heat-stressed and fed 5000 mg/kg NAC, they exhibited higher concentrations of the antioxidant enzymes and lower MDA compared to a control group (Omid, Amirali, and Ahmad Citation2018). This showed that lipid peroxides produced under heat stress circumstances can be partially neutralised, along with restoration of antioxidant enzyme activities, potentially fostering GSH synthesis by including NAC in the diet. Elmasry et al (Citation2020) reported that supplementation with an NAC chit nanocomposite (60 and 120 μg/kg feed) in thermoneutral broilers improved antioxidant status (MDA and glutathione-S-transferase) in kidney and liver tissue. Nonetheless, the impact of NAC on oxidative status remains controversial, as some investigations have failed to observe any effect. Wang et al. (Citation2017) found that administering NAC at a dose of 50 mg/kg body weight did not lead to significant alterations in malondialdehyde (MDA) levels in porcine blood plasma.
Although dietary supplementation with NAC in the current study was hypothesised to increase the performance of finishing broilers under heat-stressed condition by enhancing GSH, levels in heart and liver were not affected. Yet some evidence shows improved antioxidant status. It could be that GSH was elevated in other tissues such as the intestines (Yi, Hou, Tan, et al. Citation2016), which were not measured in the current trial. It has been indicated that NAC might have evoked a Met sparing effect. Besides this mode of action, NAC and L-Cys both might act as an antioxidant per se. As a thiol molecule, NAC can act as an antioxidant or pro-oxidant associated with three distinct mechanisms: 1) an antioxidative impact on highly oxidising free radicals (Samuni et al. Citation2013), 2) an indirect antioxidant effect due to NAC’s capacity to function as a precursor of Cys that in turn can act as antioxidant or furnish GSH synthesis, and 3) a disulphide-breaking effect and the potential to replenish thiol pools, which, in turn, modulates the redox status (Aldini et al. Citation2018). The compound NAC is thought to primarily act as a precursor of cysteine (Nakagawa et al. Citation2014). Its deacetylation to Cys is catalysed by acylase I, a cytosolic enzyme that is highly expressed (Uttamsingh et al. Citation2000). This process is not the primary factor in GSH production and NAC augments GSH through indirect routes. A potential mechanism by which NAC can increase Cys levels and subsequently boost glutathione (GSH) production is through thiol exchange interactions with cysteine that is bound to plasma or tissue proteins (Nakagawa et al. Citation2014). In accordance with this notion, earlier research demonstrated a quick release of protein-bound endogenous cysteine following NAC treatment (Radtke et al. Citation2012; Ventura et al. Citation1999). In an in vitro plasma study, Radtke et al. (Citation2012) found that binding Cys in plasma was significantly reduced when treated with different concentrations of NAC, ranging from 10 to 1000 g/ml. They observed that almost all the unbound Cys in plasma was released within 5 min of NAC treatment. Cys is a strong antioxidant with the capacity to neutralise free radical species (Oshimura and Sakamoto Citation2017). As other types of sulphur-containing compounds, taurine and hydrogen sulphide, are generated from L-Cys supplementation as well, they have crucial function to mitigate oxidative stress and defence against a variety of environmental pollutants (Das et al. Citation2012).
The current study assessed mRNA expression of CBSL, GCLC, and GSS in broiler liver on d 29 and found that NAC administration decreased the expression level of CBSL and GSS. The former results suggested that broilers under heat stress condition with NAC supplementation have lower trans-sulphuration activities as cystathionine-beta-synthase catalyses the first reaction in this pathway, namely the condensation of homocysteine (HC) and serine to generate cystathionine. Less HC may be redirected to form Cys and be available for remethylation, underscoring the potential Met sparing effect of NAC. Downregulation of GSS may hamper the second step in GSH synthesis. Glutathione synthetase condenses γ-glutamylcysteine and glycine to form GSH. This may explain the minimum effect on GSH levels in liver. Additionally, lower trans-sulphuration can induce hyperhomocysteinaemia. Werstuck et al (Citation2001) reported that hyperhomocysteinaemia can be the clinical indication of cystathionine-beta-synthase insufficiency. Previous research (Bravo et al. Citation2011; Dahlhoff et al. Citation2013; Louiselle et al. Citation2021) indicated that downregulation hepatic CBSL could be stimulated in liver failure conditions caused by oxidative stress that were associated with protein level changes and inhibition of trans-sulphuration.
Conclusions
In conclusion, these findings suggested that the improvement of performance of chronic cyclic heat-stressed finisher broilers supplemented with NAC was Met mediated by changes in tissue GSH levels, but likely operated by sparing methionine and/or NAC and Cys direct antioxidant properties.
Author contributions
J.M. conceived the idea, and J.M., M.M., and H.P. designed the study. H.P., M.M., J.D.G., and H.Z. conducted the experiment, collected the samples, and analysed samples. H.P, J.M., and M.M. analysed the data and drafted the first version of the manuscript. All authors read, revised, and approved the definitive version of the manuscript.
Supplemental Tables 1-3 and Figure 1
Download MS Word (41.1 KB)Acknowledgments
The authors would like to thank Sabine Coolsaet, Erik Claeys, and Tessa Van Der Eecken for their technical support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/00071668.2023.2264234.
Additional information
Funding
References
- Akbarian, A., J. Michiels, J. Degroote, M. Majdeddin, A. Golian, and S. de Smet. 2016. “Association Between Heat Stress and Oxidative Stress in Poultry; Mitochondrial Dysfunction and Dietary Interventions with Phytochemicals.” Journal of Animal Science and Biotechnology 7 (1): 37. (Review). https://doi.org/10.1186/s40104-016-0097-5.
- Akbarian, A., J. Michiels, A. Golian, J. Buyse, Y. Wang, and S. de Smet. 2014. “Gene Expression of Heat Shock Protein 70 and Antioxidant Enzymes, Oxidative Status, and Meat Oxidative Stability of Cyclically Heat-Challenged Finishing Broilers Fed Origanum Compactum and Curcuma Xanthorrhiza Essential Oils.” Poultry Science 93 (8): 1930–1941. https://doi.org/10.3382/ps.2014-03896.
- Aksit, M., S. Yalcin, S. Ozkan, K. Metin, and D. Ozdemir. 2006. “Effects of Temperature During Rearing and Crating on Stress Parameters and Meat Quality of Broilers.” Poultry Science 85 (11): 1867–1874. https://doi.org/10.1093/ps/85.11.1867.
- Aldini, G., A. Altomare, G. Baron, G. Vistoli, M. Carini, L. Borsani, and F. Sergio. 2018. “N-Acetylcysteine as an Antioxidant and Disulphide Breaking Agent: The Reasons Why.” Free Radical Research 52 (7): 751–762. https://doi.org/10.1080/10715762.2018.1468564.
- Attia, K. M., M. H. Asser, F. A. Tawfeek, and H. A. Basuney. 2019. “Efficacy of N-Acetylcysteine and Hydrated Sodium Calcium Aluminosilicate to Reduce the Effect of Aflatoxin B1 Intoxication in Broiler Chicken.” Alexandria Journal of Veterinary Science 61 (2): 18–31. https://doi.org/10.5455/ajvs.34945.
- Baker, D. H. 2009. “Advances in Protein–Amino Acid Nutrition of Poultry.” Amino Acids 37 (1): 29–41. https://doi.org/10.1007/s00726-008-0198-3.
- Bauchart-thevret, C., J. Cottrell, B. Stoll, and D. G. Burrin. 2011. “First-Pass Splanchnic Metabolism of Dietary Cysteine in Weanling Pigs.” Journal of Animal Scence 89 (12): 4093–4099. https://doi.org/10.2527/jas.2011-3944.
- Bottje, W., N. R. Pumford, C. Ojano-dirain, M. Iqbal, and K. Lassiter. 2006. “Feed Efficiency and Mitochondrial Function.” Poultry Science 85 (1): 8–14. (Review). https://doi.org/10.1093/ps/85.1.8.
- Bravo, E., S. Palleschi, P. Aspichueta, X. Buqué, B. Rossi, A. Cano, and M. Napolitano. 2011. “High Fat Diet‐Induced Non-Alcoholic Fatty Liver Disease in Rats is Associated with Hyperhomocysteinemia Caused by Down Regulation of the Transsulphuration pathway.” Lipids in Health and Disease 10 (1): 60. https://doi.org/10.1186/1476-511X-10-60.
- Cooper, A. J. L., J. T. Pinto, and P. S. Callery. 2011. “Reversible and Irreversible Protein Glutathionylation: Biological and Clinical Aspects.” Expert Opinion on Drug Metabolism and Toxicology 7 (7): 891–910. https://doi.org/10.1517/17425255.2011.577738.
- Dahlhoff, C., C. Desmarchelier, M. Sailer, R. W. Fürst, A. Haag, S. E. Ulbrich, B. Hummel, et al. 2013. “Hepatic Methionine Homeostasis is Conserved in C57BL/6N Mice on High-Fat Diet Despite Major Changes in Hepatic One-Carbon Metabolism.” PLoS ONE 8 (3): e57387. https://doi.org/10.1371/journal.pone.0057387.
- Das, J., A. Roy, and P. C. Sil. 2012. “Mechanism of the Protective Action of Taurine in Toxin and Drug Induced Organ Pathophysiology and Diabetic Complications: A Review.” Food & Function 3 (12): 1251–1264. https://doi.org/10.1039/c2fo30117b.
- Degroote, J., N. van Noten, W. Wang, S. de Smet, and J. Michiels. 2019. “Effects of N-Acetyl-Cysteine Supplementation Through Drinking Water on the Glutathione Redox Status During the Weaning Transition of Piglets.” Antioxidants 8 (1): 24. https://doi.org/10.3390/antiox8010024.
- Del Vesco, A. P., E. Gasparino, D. O. Grieser, V. Zancanela, D. M. Voltolini, A. S. Khatlab, S. E. Guimarães, M. A. Soares, A. R. Oliveira Neto, and T. D. Dinkova. 2015. “Effects of Methionine Supplementation on the Expression of Protein Deposition-Related Genes in Acute Heat Stress-Exposed Broilers.” PLoS One 10 (2): e0115821. https://doi.org/10.1371/journal.pone.0115821.
- Dilger, R. N., and D. H. Baker. 2007a. ““DL-Methionine is as Efficacious as L-Methionine, but Modest L-Cystine Excesses are Anorexigenic in Sulfur Amino Acid-Deficient Purified and Practical-Type Diets Fed to Chicks.” Poultry Science 86 (11): 2367–2374. https://doi.org/10.3382/ps.2007-00203.
- Dilger, R. N., and D. H. Baker. 2007b. “Oral N-Acetyl-L-Cysteine is a Safe and Effective Precursor of Cysteine.” Journal of Animal Science 85 (7): 1712–1718. https://doi.org/10.2527/jas.2006-835.
- Elmasry, D. M. A., S. M. Hegazi, M. EL-Sabagh, and S. A. Nasef. 2020. “Impact of N-Acetyl Cysteine (NAC) Chit Nanocomposite as Antioxidant on Broiler Chicks.” African Journal of Biological Science 16 (1): 33–42. https://doi.org/10.21608/ajbs.2020.75930.
- Enkvetchakul, B., N. B. Anthony, and W. G. Bottje. 1995. “Liver and Blood Glutathione in Male Broiler Chickens, Turkeys, and Quail.” Poultry Science 74 (5): 885–889. https://doi.org/10.3382/ps.0740885.
- Enkvetchakul, B., and W. G. Bottje. 1995. “Influence of Diethyl Maleate and Cysteine on Tissue Glutathione and Growth in Broiler Chickens.” Poultry Science 74 (5): 864–873. https://doi.org/10.3382/ps.0740864.
- Grotto, D., L. D. Santa Maria, S. Boeira, J. Valentini, M. F. Charão, A. M. Moro, and P. C. Nascimento. 2007. “Rapid Quantification of Malondialdehyde in Plasma by High Performance Liquid Chromatography–Visible Detection.” Journal of Pharmaceutical and Biomedical Analysis 43 (2): 619–624. https://doi.org/10.1016/j.jpba.2006.07.030.
- He, S., J. Ding, Y. Xiong, D. Liu, S. Dai, and H. Hu. 2019. “Effects of Dietary N-Acetylcysteine on Rectal Temperature, Respiratory Rate, Growth Performance and Blood Redox Parameters in 22- to 42-Day Old Broilers Exposed to Chronic Heat Stress.” European Poultry Science (EPS) 83. https://doi.org/10.1399/eps.2019.288.
- Hernandez, P., L. Zomeño, B. Ariño, and A. Blasco. 2004. “Antioxidant, Lipolytic and Proteolytic Enzyme Activities in Pork Meat from Different Genotypes.” Meat Science 66 (3): 525–529. https://doi.org/10.1016/S0309-1740(03)00155-4.
- Hou, Y. 2015. “N-Acetylcysteine and Intestinal Health a Focus on Its Mechanism of Action.” Frontiers in Bioscience 20 (5): 872–891. https://doi.org/10.2741/4342.
- Lara, L. J., and M. H. Rostagno. 2013. “Impact of Heat Stress on Poultry Production.” Animals 3 (2): 356–369. https://doi.org/10.3390/ani3020356.
- Lee, S., K. H. Han, Y. Nakamura, S. Kawakami, K.-I. Shimada, T. Hayakawa, and H. Onoue. 2013. “Dietary L -Cysteine Improves the Antioxidative Potential and Lipid Metabolism in Rats Fed a Normal Diet.” Bioscience, Biotechnology, and Biochemistry 77 (7): 1430–1434. https://doi.org/10.1271/bbb.130083.
- Li, Z., Y. Zhao, Y. Zhuang, Z. Xu, C. Wu, P. Liu, G. Hu, et al. 2022. “Effects of N-Acetyl-L-Cysteine on Serum Indices and Hypothalamic AMPK-Related Gene Expression Under Chronic Heat Stress.” Frontiers of Veterinary Science 9:936250. https://doi.org/10.3389/fvets.2022.936250.
- Lou, H., and N. Kaplowitz. 2007. “Glutathione Depletion Down-Regulates Tumor Necrosis Factor α-Induced NF-Κb Activity via Iκb Kinase-Dependent and -Independent Mechanisms.” Journal of Biological Chemistry 282 (40): 29470–29481. https://doi.org/10.1074/jbc.M706145200.
- Louiselle, A. E., S. M. Niemiec, C. Zgheib, and K. W. Liechty. 2021. “Macrophage Polarization and Diabetic Wound Healing.” Translational Research 236:109–116. https://doi.org/10.1016/j.trsl.2021.05.006.
- Lu, Z., X. He, B. Ma, L. Zhang, J. Li, Y. Jiang, G. Zhou, and F. Gao. 2018. “Dietary Taurine Supplementation Improves Breast Meat Quality in Chronic Heat‐Stressed Broilers via Activating the Nrf2 Pathway and Protecting Mitochondria from Oxidative Attack.” Journal of the Science of Food and Agriculture 99 (3): 1066–1072. https://doi.org/10.1002/jsfa.9273.
- Lushchack, V. I. 2012. “Glutathione Homeostasis and Functions: Potential Targets for Medical Interventions.” Journal of Amino Acids. 2012:1–26. ID 736837. https://doi.org/10.1155/2012/736837.
- Majdeddin, M., U. Braun, A. Lemme, A. Golian, H. Kermanshahi, S. de Smet, and J. Michiels. 2020. “Guanidinoacetic Acid Supplementation Improves Feed Conversion in Broilers Subjected to Heat Stress Associated with Muscle Creatine Loading and Arginine Sparing.” Poultry Science 99 (9): 4442–4453. https://doi.org/10.1016/j.psj.2020.05.023.
- Martín-venegas, R., J. F. Soriano-garcía, M. P. Vinardell, P. A. Geraert, and R. Ferrer. 2006. “Oligomers are Not the Limiting Factor in the Absorption of DL-2-Hydroxy-4-(Methylthio) Butanoic Acid in the Chicken Small Intestine.” Poultry Science 85 (1): 56–63. https://doi.org/10.1093/ps/85.1.56.
- Mottet, A., and G. Tempio. 2017. “Global Poultry Production: Current State and Future Outlook and Challenges.” World’s Poultry Science Journal 73 (2): 1–12. https://doi.org/10.1017/S0043933917000071.
- Nakagawa, Y., T. Suzuki, K. Nakajima, A. Inomata, A. Ogata, and D. Nakae. 2014. “Effects of N-Acetyl-L-Cysteine on Target Sites of Hydroxylated Fullerene-Induced Cytotoxicity in Isolated Rat Hepatocytes.” Archives Toxicology 88 (1): 115–126. https://doi.org/10.1007/s00204-013-1096-3.
- Oka, S., H. Kamata, K. Kamata, H. Yagisawa, and H. Hirata. 2000. “N -Acetylcysteine Suppresses TNF-Induced NF-Κb Activation Through Inhibition of Iκb Kinases.” FEBS Letters 472 (2–3): 196–202. https://doi.org/10.1016/S0014-5793(00)01464-2.
- Omid, K., S. Amirali, and K. Ahmad. 2018. “N‐Acetyl Cysteine Improves Performance, Reproduction, Antioxidant Status, Immunity and Maternal Antibody Transmission in Breeder Japanese Quail Under Heat Stress Condition.” Livestock Science 217:55–64. https://doi.org/10.1016/j.livsci.2018.09.006.
- Oshimura, E., and K. Sakamoto. 2017. “Amino Acids, Peptides, and Proteins. Cosmetic Science and Technology:.” Theoretical Principles and Applications. Elsevier Inc. https://doi.org/10.1016/B978-0-12-802005-0.00019-7.
- Pertiwi, H., M. Y. Nur Mahendra, J. Kamaludeen, and S. Nandi. 2022. “Astaxanthin as a Potential Antioxidant to Improve Health and Production Performance of Broiler Chicken.” Veterinary Medicine International. 2022:1–9. International 4919442. https://doi.org/10.1155/2022/4919442.
- Radtke, K. K., L. D. Coles, U. Mishra, P. J. Orchard, M. Holmay, and J. C. Cloyd. 2012. “Interaction of N-Acetylcysteine and Cysteine in Human Plasma.” Journal of Pharmaceutical Science 101 (12): 4653–4659. https://doi.org/10.1002/jps.23325.
- Rakhshandeh, A., C. F. M. de Lange, J. K. Htoo, A. Gheisari, and A. R. Rakhshandeh. 2019. “Immune System Stimulation Increases the Plasma Cysteine Flux and Whole-Body Glutathione Synthesis Rate in Starter Pigs.” Journal of Animal Science 97 (9): 3871–3881. https://doi.org/10.1093/jas/skz211.
- Rath, P. K., N. C. Behura, S. P. Sahoo, P. Panda, K. D. Mandal, and N. Panighrahi. 2015. “Amelioration of Heat Stress for Poultry Welfare: A Strategic Approach.” International Journal of Livestock Research 5 (3): 1–9. https://doi.org/10.5455/ijlr.20150330093915.
- Rosa, P. S., D. E. D. F. Filho, F. Dahlke, B. S. Vieira, M. Macari, and N. Furlan. 2007. “Performance and Carcass Characteristics of Broiler Chickens with Different Growth Potential and Submitted to Heat Stress.” Revista Brasileira de Ciência Avícola 9 (3): 181–186. https://doi.org/10.1590/S1516-635X2007000300007.
- Samuni, Y., S. Goldstein, O. M. Dean, and M. Berk. 2013. “The Chemistry and Biological Activity of N-Acetylcistein.” Biochimica et Biophysica Acta 1830 (8): 4117–4129. https://doi.org/10.1016/j.bbagen.2013.04.016.
- Sommer, I., H. Schwebel, V. Adamo, P. Bonnabry, L. Bouchoud, and F. Sadeghipour. 2020. “Stability of N-Acetylcysteine (NAC) in Standardized Pediatric Parenteral Nutrition and Evaluation of N,N-Diacetylcystine (DAC) Formation.” Nutrient 12 (6): 1849. https://doi.org/10.3390/nu12061849.
- Suryawan, A., P. M. J. o’connor, J. A. Bush, H. V. Nguyen, and T. A. Davis. 2009. “Differential Regulation of Protein Synthesis by Amino Acids and Insulin in Peripheral and Visceral Tissues of Neonatal Pigs.” Amino Acids 37 (1): 97–104. https://doi.org/10.1007/s00726-008-0149-z.
- Uttamsingh, V., R. B. Baggs, D. M. Krenitsky, and M. W. Anders. 2000. “Immunohistochemical Localization of the Acylases That Catalyze the Deacetylation of N-Acetyl-L-Cysteine and Haloalkene-Derived Mercap- Turates.” Drug Metabolism and Disposition 28 (6): 625–632.
- Valdivia, A. G., A. Martinez, F. J. Damian, T. Quezada, R. Ortiz, C. Martinez, J. Llamas, et al. 2001. “Efficacy of N-Acetylcysteine to Reduce the Effects of Aflatoxin B1 Intoxication in Broiler Chickens1.” Poultry Science 80 (6): 727–734.
- van Nevel, C. J., J. A. Decuypere, N. Dierick, and K. Molly. 2003. “The Influence of Lentinus Edodes (Shiitake Mushroom) Preparations on Bacteriological and Morphological Aspects of the Small Intestine in Piglets.” Archiv fur Tierernahrung 7 (6): 399–412. https://doi.org/10.1080/0003942032000161054.
- Ventura, P., R. Panini, M. C. Pasini, G. Scarpetta, and G. Salvioli. 1999. “N-Acetyl-Cysteine Reduces Homocysteine Plasma Levels After Single Intravenous Administration by Increasing Thiols Urinary Excretion.” Pharmacological Research 40 (4): 345–350. https://doi.org/10.1006/phrs.1999.0519.
- Wang, L., J. Zhou, Y. Hou, D. Yi, B. Ding, J. Xie, Y. Zhang, et al. 2017. “N-Acetylcysteine Supplementation Alleviates Intestinal Injury in Piglet Infected by Porcine Epidemic Diarrhea Virus.” Amino Acid 49 (6): 1–3. https://doi.org/10.1007/s00726-017-2397-2.
- Wang, Q., Y. Hou, D. Yi, L. Wang, B. Ding, X. Chen, M. Long, Y. Liu, and G. Wu. 2013. “Protective Effects of N-Acetylcysteine on Acetic Acid-Induced Colitis in a Porcine Model.” BMC Gastroenterology 13 (1): 133. https://doi.org/10.1186/1471-230X-13-133.
- Wang, Y., X. Yin, D. Yin, Z. Lei, T. Mahmood, and J. Yuan. 2019. “Antioxidant Response and Bioavailability of Methionine Hydroxy Analog Relative to DL-Methionine in Broiler Chickens.” Animal Nutrition 5 (3): 241–247. https://doi.org/10.1016/j.aninu.2019.06.007.
- Werstuck, G. H., S. R. Lentz, S. Dayal, G. S. Hossain, S. K. Sood, Y. Y. Shi, and J. Zhou. 2001. “Homocysteine‐Induced Endoplasmic Reticulum Stress Causes Dysregulation of the Cholesterol and Triglyceride Biosynthetic Pathways.” Journal of Clinical Investigation 107 (10): 1263–1273. https://doi.org/10.1172/JCI11596.
- Willemsen, H., Q. Swennen, N. Everaert, P. A. Geraert, Y. Mercier, A. Stinckens, E. Decuypere, and J. Buyse. 2011. “Effects of Dietary Supplementation of Methionine and Its Hydroxy Analog DL-2-Hydroxy-4-Methylthiobutanoic Acid on Growth Performance, Plasma Hormone Levels, and the Redox Status of Broiler Chickens Exposed to High Temperatures.” Poultry Science 90 (10): 2311–2320. https://doi.org/10.3382/ps.2011-01353.
- Wrotek, S., S. Justina, H. M. Kozłowski, P. Malgorzata, J. Tomasz, and D. Artur. 2020. “New Insight into the Role of Glutathione in the Mechanism of Fever.” International Journal of Molecular Science 21 (4): 1393. https://doi.org/10.3390/ijms21041393.
- Yi, D., Y. Hou, L. Tan, M. Liao, J. Xie, L. Wang, B. Ding, Y. Yang, and J. Gong. 2016. “N‐Acetylcysteine Improves the Growth Performance and Intestinal Function in the Heat‐Stressed Broilers.” Animal Feed Science and Technology 220:83–92. https://doi.org/10.1016/j.anifeedsci.2016.07.014.
- Yi, D., Y. Hou, L. Wang. 2016. “N-acetylcysteine Stimulates Proteinvsynthesis in Enterocytes Independently of Glutathione Synthesis” Amino Acids 48 (2): 523–533. https://doi.org/10.1007/s00726-015-2105-z.
- Yi, D., Y. Q. Hou, L. Wang. 2014. “Dietary N-acetylcysteine supplementation alleviates liver injury in lipopolysaccharide-challengedvpiglets.” British Journal of Nutrition 111 (1): 46–54. https://doi.org/10.1017/S0007114513002171.
- Yoshida, T. 1996. “Determination of Reduced and Oxidized Glutathione in Erythrocytes by High-Performance Liquid Chromatography with Ultraviolet Absorbance Detection.” Journal of Chromatography B: Biomedical Science and Application 678 (2): 678/157–164. https://doi.org/10.1016/0378-4347(95)00489-0.
- Zhang, H., M. Majdeddin, J. Degroote, E. van Liefferinge, N. van Noten, C. van Kerschaver, M. Vandaele, J. Cesar de Paula Dorigam, and J. Michiels. 2023. “Effect of Supplemental Methyl Sulfonyl Methane on Performance, Carcass and Meat Quality and Oxidative Status in Chronic Cyclic Heat-Stressed Finishing Broilers.” Poultry Science 102 (2): 102321. https://doi.org/10.1016/j.psj.2022.102321.
- Zhu, L., X. Cai, Q. Guo, X. Chen, S. Zhu, and J. Xu. 2013. “Effect of N -Acetyl Cysteine on Enterocyte Apoptosis and Intracellular Signalling Pathways’ Response to Oxidative Stress in Weaned Piglets.” British Journal of Nutrition 110 (11): 1938–1947. https://doi.org/10.1017/S0007114513001608.
