ABSTRACT
Water-granulated CaO-modified iron-silicate slags have shown beneficial properties for cement applications. To further evaluate potential applications, the leaching properties must be understood. Therefore, this study aims to characterise and assess the metal leaching of iron-silicate slags (2.6% CaO) modified with lime (CaO, up to 20 wt.%) produced on both laboratory and industrial scales. The granulated samples showed amorphous contents for the studied CaO range. Generally, the metal content of the samples decreased with the increasing CaO content. Batch leaching tests were conducted on the slags, and the metal leaching and CaO content of the slag were strongly correlated. The leaching of Zn and Cu decreased with the increasing CaO content in the slag. Overall, the slags with 12–13% CaO exhibited minimal leaching of Zn, Cu, Ni, and Sb. These findings indicate that CaO influences the properties of the slag and can suppress metal leaching from water-granulated iron-silicate slags.
Les scories de silicate de fer granulées à l’eau et modifiées au CaO ont montré des propriétés bénéfiques pour les applications au ciment. Pour évaluer davantage les applications potentielles, on doit comprendre les propriétés de lixiviation. Par conséquent, cette étude vise à caractériser et évaluer la lixiviation des métaux des scories de silicate de fer (2.6% CaO) modifiées avec de la chaux (CaO, jusqu’à 20% en poids) produites à l’échelle du laboratoire et de l’industrie. Les échantillons granulés ont montré des teneurs amorphes pour la gamme de CaO étudiée. Généralement, la teneur en métal des échantillons diminuait avec l’augmentation de la teneur en CaO. On a effectué des essais de lixiviation par lots sur les scories, et la lixiviation des métaux et la teneur en CaO des scories étaient fortement corrélées. La lixiviation de Zn et du Cu diminuait avec l’augmentation de la teneur en CaO dans les scories. En général, les scories contenant 12 à 13% de CaO exhibaient une lixiviation minimale de Zn, Cu, Ni et Sb. Ces résultats indiquent que le CaO influence les propriétés des scories et peut supprimer la lixiviation des métaux des scories de silicate de fer, granulées à l’eau.
Introduction
The ongoing electrification and digitalisation around the world demand base metals, especially copper. The ore-based pyrometallurgical extraction route for copper produces residues, including slags, dust, and sludges in the copper smelters. These residue products might require extensive treatment before utilisation, internal recycling, or landfilling, to minimise the metal loss and pollution owing to undesired metal leaching. In Europe, stricter environmental regulations have been introduced on waste products regarding their composition and leaching limits, as well as higher requirements on the quality of the residue products. Among these residue products, slag has the highest potential for secondary use owing to its properties and amount.
The optimum technology for improving quality and preventing metal leaching in slag can differ between smelters, depending on the slag processing at each site. The Rönnskär smelter in Sweden operated by Boliden Mineral AB is a pyrometallurgical copper producer. Together with secondary copper-bearing materials, silica, and various recycled materials from the smelter, copper concentrate is smelted in an electrical smelting furnace (ESF) to generate copper matte as the primary product. This copper matte is further converted, refined, and finally subjected to electrorefining to produce cathodes, with a purity of >99.995% Cu. The ESF process also produces a Zn-containing iron-silicate slag that undergoes further processing in a fuming furnace. Secondary materials containing Zn and Pb, e.g. electric arc furnace dust and Pb-slag, are added in this process. The fuming process recovers the Zn and other elements with high vapor pressure such as Pb by blowing pulverised coal and air via tuyeres into the furnace. The injected air (O2) and coal (C) react to form CO/CO2 gas. This gas reduces metal oxides, which mainly come from the Cu ore and secondary materials, into metals such as Cu-metal(loids) containing Cu, Ni, As, and Sb. Further, the iron-silicate slag undergoes a settling process to recover Cu and achieve a cleaner slag. During this step, Cu-sulfide/metal(loid) droplets settle owing to the density difference, with the required settling time decreasing with increasing droplet size [Citation1,Citation2]. The slag is then water-granulated to yield the final by-product [Citation3,Citation4].
Iron silicate-based slag is the most frequently used copper slag worldwide and is also produced at the Rönnskär smelter. It is an acidic slag, with a CaO content of 2–5 wt.%, and hence shows a beneficially low tendency to dissolve acid oxides such as As2O3 and Sb2O3 [Citation5]. However, drawbacks of this slag include magnetite (spinel) formation and a higher viscosity than other slags. In 2008, Jak et al. showed that iron-rich CaO-FeO-SiO2 slags are sensitive to changes in the oxygen partial pressure, PO2 [Citation6]. The spinel phase becomes dominant at PO2 >10−6 atm, and wüstite is dominant at PO2 <10−10 atm.
Calcium ferrite-based slags (CaO between 15 and 20 wt.%) are also used in matte smelting processes [Citation5]. This slag resolves the issue of magnetite formation and has favorable characteristics such as a relatively low viscosity (which increases the settling rates) and a lower risk for slag foaming. Process-wise, however, calcium ferrite slag suffers from disadvantages including a lower solubility of silica and increased wear on MgO-Cr2O3 refractories [Citation5].
To reduce the lining wear, some copper smelters use a mixture of the two slag types [Citation5]. Ferrous calcium silicate, an iron-silicate slag with 5–20 wt.% added CaO, is an alternative to pure iron-silicate and calcium ferrite slags [Citation5]. Modifying this slag with CaO in the copper making processes has been well studied for improving the process control and minimise copper losses to the slag [Citation2,Citation7–10]. This chemical modification makes the slag more basic and decreases its melting temperature and viscosity [Citation7,Citation11,Citation12], which are beneficial from a process point of view. In 2008, Jak et al. showed that a higher CaO content (CaO/SiO2 = 0.1–1.0, Fe/SiO2 = 0.8, PO2 = 10−8 atm.) in a CaO-FeO-SiO2 slag resulted in a change in the primary phase field from spinel to wüstite [Citation6].
The iron-silicate slag may be used in different applications such as ballast, construction material for buildings and roads, as abrasives, and as fillers in cement [Citation13–15]. Moreover, slag modification allows for specific applications, such as its use in cementitious materials. To enhance the cementitious properties, granulating and grinding of the slags are preferred for increasing the reactivity at alkaline pH conditions [Citation14]. Shi et al. in 2008 showed that iron-silicate slags (0.6–10.9 wt.% CaO) could be used as a pozzolanic material and could partially replace Portland cement [Citation14]. In 2019, Feng et al. reported that adding CaO (up to 20 wt.%) to iron-silicate slag further enhanced the cementitious properties [Citation16], and the modified copper slag was suitable for cement applications. However, these studies on adding CaO (up to 20 wt.%) to molten copper slag have not addressed the leaching behavior of metals from such slags.
Several papers have reported on metal leaching from different types of slags, including some copper slags that contain various amounts of CaO (3–22 wt.% CaO) [Citation17–21]. Nevertheless, those studies focused on the slag leaching in general rather than the correlation with CaO addition. Meshram et al. in 2017 found that acid leaching of granulated copper slags is diffusion-controlled [Citation22]. Despite the leaching rate, the chemical composition of the slag surfaces is crucial for the leachability of metals. The current study presents an alternative to some aging methods [Citation23,Citation24] that pre-leach the slag to a minimum steady state before application by modifying slag with CaO, which potentially decreases the undesired leaching of metals from slags.
In the present work, laboratory-scale trials were conducted to examine the leaching behavior of remelted and water-granulated iron-silicate slag samples modified with up to 20 wt.% CaO. Similar industrial-scale trials were also carried out by adding limestone to the slag fuming process. After settling and water granulation, the slags were investigated for their leaching properties. This study aims to (i) characterise the CaO-modified iron-silicate slags according to their mineralogy and microstructure and (ii) assess the metal leaching mechanisms after adding CaO to slag products in both laboratory- and industrial-scale trials to determine the influence of parameters affecting the metal leaching. The results from this study also contribute information about the environmental quality of slags produced within the CaO-FeOx-SiO2 system.
Experimental methodology
Laboratory-scale trials
Granulated iron-silicate slag from Boliden Rönnskär copper smelter in Sweden was used as raw material. The laboratory-scale trials used six different mixtures of slag and CaCO3 (Alfa Aesar, 99.5 wt.%. metals basis) with the compositions shown in , including a reference sample without added CaO. The samples were named by the achieved CaO contents after remelting as RL03 (the reference), L04, L08, L12, L16, and L20 (L = laboratory-scale samples) containing 2.6, 4.2, 7.8, 11.9, 15.7, and 19.8 wt.% CaO, respectively. The range of CaO added (up to 20 wt.%) was chosen to cover the primary phase field of olivine (fayalite) up to the boundary of wüstite in the CaO-FeOx-SiO2 ternary phase diagram at Fe saturation for a slag Fe/SiO2 (w/w) ratio of 0.8 [Citation6]. The samples were homogenised by smelting at Fe saturation in ARMCO pure iron © crucibles in a Tamman furnace at 1300°C for 30 minutes. The temperature was selected to represent the final smelting temperature used in the corresponding industrial process. Argon gas (AGA Gas, Sweden, 99.999%) was used to maintain an inert atmosphere in the crucibles with a flow rate of 2 l min−1. After cooling to room temperature in the furnace, the previously melted homogenised samples were remelted in a muffle furnace (1300°C for 30 minutes in the iron crucibles, air atmosphere) before water granulation (water flow rate: 1.1 l min−1). Finally, the granulated laboratory-scale slags were collected and dried at 105°C for 24 hours before characterisation and analysis.
Table 1. Slag and CaCO3 mixtures used in laboratory-scale trials.
Industrial-scale trials
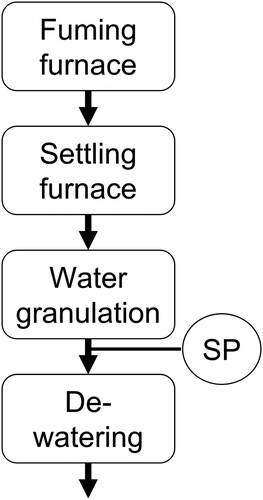
The industrial-scale trials were conducted during normal process conditions except for the limestone addition. The raw material was iron-silicate slags from the ESF at the Boliden Rönnskär copper smelter. shows the process steps for the trials and the sampling point (SP). Limestone (Nordkalk AB, Sweden) was added to the fuming furnace at desired amounts. The fuming process has been described in previous studies in detail [Citation3,Citation4]. The fumed slag was tapped to the settling furnace. The settled slags were water-granulated by water jets to a final product. In total, four batches of slags were produced, a reference batch (RF02, 2.5 wt.% CaO) and three modified slag batches (F06, F10, and F13 containing 5.6, 10.2, and 12.9 wt.% CaO, respectively) F = full-scale trials, R = reference. The weight of each slag batch was in the range of 72–99 ton. The settling time before granulation ranged between 13 and 19 minutes, and the slag temperature at the starting point of granulation was 1220–1250°C, and electrical heating raised it to 1260–1310°C during granulation. Thermocouples measured the temperatures in the molten slag tapped from the settling furnace to the granulation unit.
Sampling of materials
In the industrial-scale trials, samples were collected from the granulated slag products (i.e. at SP) for analysis, as shown in . Specifically, the samples were collected in the middle of the granulation process about 30 minutes after starting. Further, the samples were stored in steel barrels at the smelter for 6–7 months before laboratory work. The water-granulated reference slag and CaO-modified slags were dried and split to proper sample sizes for analysis.
Slag characterisation methods
The chemical composition of laboratory-scale samples and industrial-scale water-granulated slag samples (SP) were determined by the laboratory ALS Scandinavia AB, Sweden, using lithium metaborate digestion and inductively coupled plasma with mass spectrometry (ICP-MS).
The microstructure of slag samples was studied using scanning electron microscopy (SEM) equipped with energy dispersive spectroscopy (EDS) at Luleå University of Technology, and powder X-ray diffraction (XRD) was employed to examine their mineralogy. Carbon-coated and polished cross-sections of bulk subsamples embedded in epoxy molds (Ø = 25 mm) were examined through SEM-EDS on a Zeiss Gemini Merlin instrument, using an accelerating voltage of 20 keV and an emission current of 1.0 nA. Pulverised bulk subsamples were analyzed via XRD using a PANalytical Empyrean X-ray diffractometer equipped with a Cu Kα radiation tube and a monochromator over a 2θ range between 25 and 90° with a step size of 0.026°. The XRD patterns were analyzed using the software Highscore Plus and the Crystallography Open Database (COD) [Citation25].
The particle size distribution was measured using a Ro-Tap sieve shaker and U.S. Standard sieves with openings of 75, 150, 300, 600, 1180, and 2360 µm.
To compare the leachability of different slag samples, one-stage batch leaching tests were conducted according to EN12457-2. The test followed the standard protocol except for the use of 25 grams of solid with a size fraction from 0.30–0.60 mm and 250 ml ultra-pure water (milli-Q). The leachates were filtered (0.45 µm) and acidified with concentrated HNO3 (VWR Chemicals, 68%) using 1 vol.% of the leachate volume. The contents of Al, As, Ba, Ca, Cr, Cu, Fe, K, Mg, Mo, Na, Ni, S, Sb, Si, Pb, Zn, and Ti were analyzed by ICP optical emission spectroscopy (ICP-OES) on a Thermo Scientific iCapTM 7200 duo ICP-OES instrument at Luleå University of Technology using multi external standards. Elemental concentrations in the leachates are presented as the average value (mg kg−1) of two or three samples with the associated standard deviations.
Results
Laboratory-scale trials
Characterisation
presents the chemical composition of the laboratory-scale granulated slags. The main elements were Fe (29.6–38.7 wt.%), Si (11.7–14.9 wt.%), and Ca (1.8–14.2 wt.%). Both Fe and Si showed decreasing trends with increasing CaO content in the samples owing to dilution as the slag volume increased. Sample L20 contained the lowest amounts of Fe and Si, at 29.6 and 11.7 wt.%, respectively. The concentrations of minor elements varied with the addition of CaO. Cu (0.22–0.42 wt.%) and Zn (0.29–0.53 wt.%) decreased with the CaO content, whereas the concentrations of Sb (0.0009–0.0049 wt.%), Ni (0.0053–0.0153 wt.%), As (0.0004–0.0033 wt.%), and Mo (0.156–0.184 wt.%) varied. The six laboratory-scale water-granulated slag samples (RL03 to L20) had d80 values of 1.05, 0.93, 0.82, 0.71, 0.67, and 0.70 mm, respectively. The d80 values show decreasing particle size with the increasing CaO content.
Table 2. Chemical analysis of laboratory- and industrial-scale slag samples.
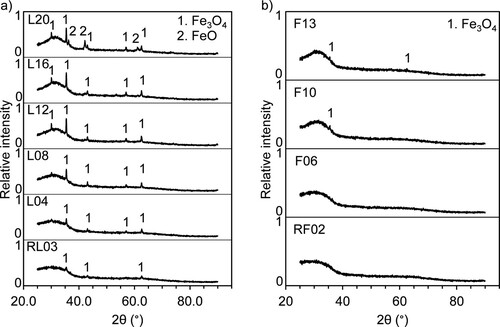
The XRD patterns in (a) show that the slag samples contain an amorphous phase. All patterns displayed minor peaks of Fe3O4 (spinel), the intensity of which increased with the increasing CaO content in the samples. Sample L20 also showed peaks of FeO (wüstite).
Figure 2. XRD patterns of pulverised (a) laboratory-scale slag samples (RL03, L04, L08, L12, L16, and L20) (b) industrial-scale slag samples: RF02, F06, F10, and F13.
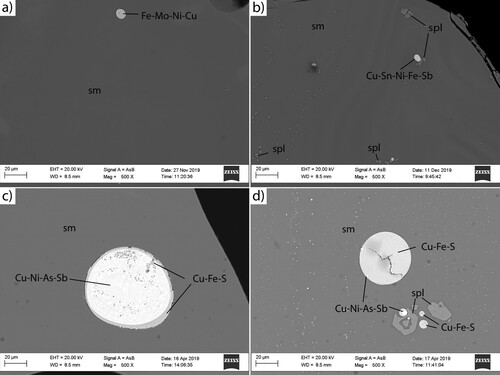
(a)–(b) are backscattered SEM images of samples RL03 and L20, which exhibit three different phases, as determined by EDS analysis: a matrix (Fe-Si-O), Cu-containing inclusions (Cu-Sn-Ni-Fe-Sb), and Fe-Cr containing inclusions (Fe-Cr-O). Zn primarily appeared in the Fe-Si-O phase, with some Zn in the Fe-Cr-O phase.
Leaching behavior
shows the leaching of selected elements from the laboratory-scale CaO-modified slag samples. The pH of the leachates increased in the range of 9.4–11.2 with the increasing CaO content. Sample L20, which had the highest CaO content (19.8 wt.%), showed the highest pH value (11.2), whereas L04 with 4.2 wt.% CaO showed the lowest (9.4). The conductivity of the leachates ranged from 38 µS cm−1 for RL03 to 256 µS cm−1 for L20. From L16 to L20, the conductivity increased from 78 to 256 µS cm−1. Generally, Ca was leached to the greatest degree from all samples, and L20 showed the most severe Ca leaching (288 mg kg−1). When the pH value was higher (9.4–11.2), more Ca, Si, and Al was leached, as shown in .
Table 3. Leaching data for laboratory-scale samples (unit: mg kg−1 sample). Standard deviations of zeros are not shown.
For the leaching of Cu, Zn, Sb, Ni, As, and Mo from the CaO-modified samples, all leaching levels of Cu and Zn were below that of the reference sample RL03, as shown in . Upon CaO modification, the leaching of Cu decreased from 0.71 mg kg−1 for the reference RL03 to <0.05 mg kg−1 for L20 (a relative change of >98%), and Zn leaching decreased in a similar way by >99%. The leaching of Ni and Mo showed decreasing trends from RL03 to their minimum values in sample L12. When even more CaO was added to the samples, the leaching of Ni increased to values similar to those of the reference RL03 (). The leaching of Mo showed an increasing trend from L12 to L20, but the change was much smaller than that for Ni. The leaching values of Sb and As were below their quantification limits.
Industrial-scale trials
Characterisation
The chemical compositions of the granulated industrial-scale slag samples after the settling process are presented in . The concentrations of the main elements Fe (28.5–35.4 wt.%), Si (13.2–15.0 wt.%), and Al (1.7–1.9 wt.%) all decreased with the increasing CaO content in the slags. The concentrations of Zn (0.91–1.00 wt.%), Cu (0.45–0.63 wt.%), As (0.004–0.011 wt.%), and Sb (0.008–0.019 wt.%) varied and showed no clear trends with the increasing CaO addition. By contrast, the concentrations of Ni (0.017–0.042 wt.%) and Mo (0.20–0.23 wt.%) decreased upon CaO addition. The water-granulated slag samples from industrial-scale trials (RF02 to F13) had d80-values of 1.70, 1.60, 1.01, and 1.23 mm, respectively, suggesting a particle size reduction with the increasing CaO content.
(b) presents the XRD patterns of the granulated slag samples (SP), all of which show an amorphous structure. Increasing the CaO content in the samples produced a more distinct ‘halo,’ and peaks matching Fe3O4 (spinel) started to appear. Samples F10 and F13 contained peaks of spinel, and the peak intensity increased with increasing CaO content.
(c)–(d) are typical backscattered SEM images of RF02 and F13. There exist four different phases, as determined by EDS: a matrix (Fe-Si-O), Cu-Fe-S inclusions (Cu-Fe-S), Cu-Ni inclusions (Cu-Ni-As-Sb), and Fe-Cr-Zn inclusions (Fe-Cr-Zn-O). All industrial-scale samples (RF02-F13) contained the three phases of Fe-Si-O, Cu-Fe-S and Cu-Ni-As-Sb, whereas Fe-Cr-Zn-O was found in F10 and F13.
Leaching behavior
shows the leaching results for selected elements from industrial-scale CaO-modified slag samples. The pH values of the leachates were all close to neutral (6.5–7.0). Sample F13 with the highest CaO content (12.9 wt.%) showed the highest leachate pH (7.0), and F10 with the second highest CaO content (10.2 wt.%) showed the lowest (6.5). The conductivity of the leachates was in the range of 15.6–19.1 µS cm−1, being the lowest for F10 and the highest for RF02 ().
Table 4. Leaching data for industrial-scale samples (unit: mg kg−1 sample). Standard deviations of zeros are not shown.
Generally, Na is the most leached element, and its leaching is the highest in sample RF02 (26.0 mg kg−1). Upon CaO addition, the leaching of Ca increased gradually (3.87–7.65 mg kg−1), and that of Si decreased (2.26–1.83 mg kg−1) from RF02 to F13 in . The addition of CaO also suppressed the leaching of Cu, Zn, Sb, and Ni in the modified slag samples (F06–F13). The leaching of Cu, Zn, and Sb decreased up to >45, 92, and 76% from RF02 to F13, from 0.105 to <0.06, 1.38 to 0.11, and 0.46 to <0.11 mg kg−1, respectively. At the same time, the leaching of Ni and Mo decreased from 1.09 to 0.04, and 0.143 to 0.063 mg kg−1, respectively (). The leaching of As was below its quantification limit.
Discussion
Characterisation
According to the chemical analysis (), the levels of main elements (Fe, Si, and Al) decrease in the same manner in the industrial- and the laboratory-scale trials owing to dilution by the added CaO (which increases the slag volume). However, after accounting for the dilution effect from the added CaO, their levels are constant among the slag samples (). Minor elements such as Zn, Cu, and Ni also decrease with increased CaO in the laboratory-scale trials, and the industrial-scale trials confirm the same trends for Cu and Ni. These changes in the minor elements remain after compensating for the dilution effect of CaO, indicating enhanced gravimetric settling and removal of Cu and Ni in the presence of CaO. The Cu content has previously been shown to decrease with increasing CaO in slag [Citation9,Citation26]. This decreasing effect is due to the decreasing viscosity of the slag with increasing CaO at a constant temperature, which promotes the settling of inclusions but also lowers the dissolution of Cu in the slag [Citation26]. Zn does not follow the same decreasing trend in the industrial-scale samples, because the fuming process aims to reduce its content in the liquid slag to a target level [Citation3]. The concentrations of As and Sb fluctuate because of their low concentrations (<190 mg kg−1, ) in the slag matrix both for the laboratory- and industrial-scale trials. Comparing the laboratory- and industrial-scale sample contents of Cu, Ni, As, Sb, and Zn shows that these contents are all lower in the former. Extended time in the molten stage has been reported to settle more Cu inclusions [Citation26,Citation27] and fume Zn [Citation3] from copper smelter slags.
The XRD patterns in (a) show peaks of spinel in all the samples from the laboratory-scale trials, with higher intensity than the corresponding samples from the industrial-scale trials (comparing samples L08–L12 with F10–F13). Therefore, the laboratory-scale samples might contain a relatively larger ratio of crystalline phases, according to the peak intensities. The higher content of crystalline phases in the laboratory-scale samples indicates that the partial pressure of oxygen (PO2) was different between the laboratory-scale and industrial-scale trials. At the Fe-saturation conditions for the laboratory-scale trials, the olivine phase is the primary crystallization phase, according to Jak et al. [Citation6]. However, oxidation at higher PO2 values (>10−6 atm) is most likely the reason for the spinel formation at the surface of the molten slag samples due to the remelting in air atmosphere (PO2 = 0.21 atm) before granulation. Further, the laboratory-scale sample L20 contains wüstite, according to the XRD measurements ((a)). Jak et al. showed that synthetic FeO-CaO-SiO2-Al2O3 slags with a Fe/SiO2 weight ratio of 1.2 and a CaO/SiO2 weight ratio of 0.7–1.0 form wüstite as the primary phase field at Fe saturation [Citation6]. Their findings explain why wüstite was found in L20 in the present study. However, the highest CaO content in the industrial-scale samples was only 12.9 wt.%; therefore, those samples cannot confirm the findings of the laboratory-scale trials.
SEM images and EDS analysis (data not shown) for the industrial-scale samples ((c)–(d)) show Cu, Ni, As, and Sb as Cu-Fe-S and Cu-Ni-As-Sb inclusions in the oxide phase, in bigger droplets (>10 µm) and possibly also micro-sized droplets (<10 µm). These inclusions are commonly found in iron silicate slags [Citation26,Citation28,Citation29]. The Cu sulfide inclusion contains mainly Cu and S, with a minor amount of Fe. The metal(loid) inclusion contains mainly Cu, Ni, As, and Sb according to the SEM-EDS analysis. The chemical analysis () indicates that the laboratory-scale samples contained fewer inclusions with Cu, Ni, As, and Sb than the industrial-scale ones; therefore, the inclusions observed in SEM images ((a)–(d)) and EDS analysis (data not shown) show different compositions. The SEM and EDS analyses confirm that the granulated slags with higher CaO contents (F10 and F13) contain inclusions consisting of Fe and O.
Leaching behavior
The laboratory-scale samples show a general increase in pH (9.4–11.2) in the leachate, with increasing CaO content, which is higher than the pH in common copper smelter slags reported in the literature [Citation18]. L16 and L20 have the highest pH values of 10.5 and 11.2, respectively. When the CaO content is below 13 wt.%, the pH of both the laboratory-scale samples (RL03, L04, L08, and L12, pH = 9.4–10.0) and the industrial-scale ones (RF02, F06, F10, and F13, pH = 6.5–7.0) indicates only minor effects of CaO addition. The industrial-scale sample leachate pH values are in the neutral region, which was also observed in other studies [Citation18,Citation23]. However, if the slag is crushed to liberate fresh surfaces, its pH increases, according to an earlier study [Citation30]. The lower leachate pH for the industrial-scale samples is likely due to pre-leaching during the granulation process and storage at the smelter site. Most of the leachable main elements in the slag (Fe, Si, Ca, and Al) were probably released into the processing water (washed off) during the slag handling process. The industrial slag materials remained in contact with process water for an extended time before dewatering and subsequent laboratory tests. Earlier reports referred to this pre-leaching and weathering effect as ageing [Citation23,Citation24]. The remelting and granulation of the laboratory-scale samples restore the granule surfaces, resulting in higher leaching of Fe, Si, Ca, and Al than those in the corresponding industrial-scale samples, as shown in and . Hence, the general extent of leaching is higher in the laboratory-scale trials than that in the industrial-scale trials; this is corroborated by the conductivity data (38.0–256 and 15.6–19.1 µS cm−1, respectively; see and ). The higher conductivity corresponds to a larger amount of total dissolved solids (TDS) in the leaching liquid [Citation31]. This increased conductivity is mainly caused by Ca leaching (up to 288 mg kg−1). According to the Ca-O-H (10−6 M) Pourbaix diagram, Ca is predominantly in solution at pH <12.6 [Citation24] under the examined conditions.
For the laboratory-scale samples (), the leaching trends for Cu, Zn, and Ni are similar up to 12 wt.% CaO in the slag. At low CaO contents of up to 7.8 wt.%, the leaching decreases slightly, while between 7.8 and 11.9 wt.% CaO, there is a major drop. (Note: Ni was not detected for 11.9 wt.% CaO, i.e. L12.) This decrease in leaching is also confirmed in the industrial-scale samples, as presented in .
Figure 4. Average concentrations (bars represent standard deviations, n = 2) of (a) Zn, (b) Cu, (c) As, (d) Sb, (e) Ni, and (f) Mo in leachates from laboratory- and industrial-scale samples prepared with different CaO contents.
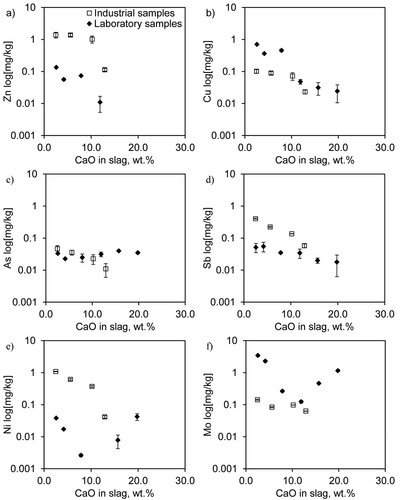
Comparing the leaching of Cu and Zn in this study (<0.05–0.70 and 0.1–1.4 mg kg−1, respectively) with those of granulated copper smelter slags with high CaO contents (20.9 wt.% CaO) without slag treatments (<0.01 and 2.6 mg kg−1, respectively at pH 8.1 [Citation30]) indicates that the slag leaching values in this study are reasonable. Further, similar Cu leaching but lower Zn leaching could be achieved for the slag with 19.8 wt.% CaO in this study compared with previously reported leaching [Citation30].
The respective leaching values of Ni, Mo, and As are minimised when the CaO content is 11.9, 11.9 and 4 wt.%, respectively; these values are restored to the values of the reference sample (2.6 wt.% CaO) when the CaO content is 19.8, 15.7, and 19.8 wt.%, respectively, for the laboratory-scale samples. Jarošíková et al. reported that the leaching of As is likely to increase at alkaline pH for a reverberatory copper smelter slag (6.0 wt.% CaO) because of the pH dependence of arsenates [Citation32].
Based on the experimental findings of this study, to minimise the release of Zn, Cu, and Ni in leaching, the iron-silicate slag modified with CaO should contain a minimum of approximately 12–13 wt.% CaO (L12 and F13). However, the CaO content should not increase beyond approximately 16 wt.% (L16), or else the leaching of Ni, As, and Mo would become significantly worse than that of the reference slag (RL03). In the industrial-scale trial, the optimum modified slag (F13) showed 92%, >45%, >76%, 96%, and 56% lower leaching of Zn, Cu, Sb, Ni, and Mo with respect to that of the reference sample (RF02), according to . These findings agree with the results reported by Reich et al., who investigated the leaching behavior of sintered hazardous waste incineration (HWI) slag with CaO addition. Their study indicated a similar decreasing trend in the leaching of Ni, Cu, and Zn, with a minimum at >15 wt.% CaO in the HWI slag [Citation33].
Parameters influencing leaching
This study aimed to further investigate how the chemical composition, particularly the CaO content, of an iron silicate slag influences metal leaching. The slag leaching caused by CaO addition is affected by dilution (owing to the slag volume increase), but in both laboratory- and industrial-scale trials ( and and ), this effect alone cannot explain the decreased leaching. The increased settling of inclusions and the redistribution of elements into phases present in the slag were also found to decrease leaching. In the industrial-scale trial, the total content of Cu in the slag sample decreased by 26% between RF02 and F13 (), while Cu in the leachate was reduced by >45% (). This large reduction of Cu in the leachate confirms that adding CaO specifically inhibited the leaching instead of merely diluting the metals in the slag.
Further, Huaiwei et al. in 2012, Ducret and Rankin in 2002, and Kowalczyk et al. in 1995 showed that adding an element such as Ca to iron-silicate slags (Fe/SiO2 <1.3) decreases both the molten slag viscosity and liquidus temperature as long as no solids precipitate in the molten slag [Citation7,Citation34,Citation35]. Further, Kim et al. confirmed adding CaO decreases the viscosity, which in turn increases the removal of Cu-containing inclusions from iron-silicate slags [Citation9]. The lower amount of inclusions (Cu, Ni, As, Sb) in a slag partly explains the decreased leaching values in this study. On the other hand, the concentrations of Zn, As, and Sb in the slag () fluctuate without showing a clear correlation with the amount of added CaO. Nonetheless, the amounts of leached Zn, and Sb are decreased (). Therefore, the extent of metal leaching is not simply correlated with their elemental concentrations in the bulk slag. This finding was also observed by Engström et al. in 2014 for steel slags, and in 2015, Piatak et al. reported that leaching of some specific slag elements, such as Ca, correlated with their bulk chemical composition [Citation18,Citation24]. Previous studies have demonstrated that the distribution of Cu (and Sb) in the slag matrix decreases with the increasing CaO content in the slag [Citation9,Citation26,Citation36]. The altered distribution of these elements partly explains the decreased leaching trends. Further, the redistribution of these elements into less-leachable phases supports the findings reported by Engstrom et al. for steel slags, namely, that the overall leaching depends on the leaching of individual slag phases [Citation24]. These results indicate that the decrease in metal leaching is influenced by the added CaO () owing to a combination of dilution, settling, and altered elemental distribution. Further studies are required to distinguish the magnitude of the effect of each parameter on the decreased metal leaching. Other parameters that can influence the leaching include metal solubility and slag viscosity, among others, but these factors fall outside the scope of this study and should be examined in future research.
Conclusions
This study examined the effect of CaO addition on the leaching of metals from iron-silicate slag generated from a copper smelter. The experiments led to the following conclusions:
Modifying molten iron-silicate slag with CaO (up to 20 and 13 wt.% in laboratory- and industrial-scale trials, respectively) resulted in an amorphous slag (except for spinel formation) after water granulation.
The added CaO completely dissolved into the slag matrix in both types of trials. However, precipitation of wüstite was observed in the laboratory-scale sample with 20 wt.% CaO.
CaO addition to iron-silicate slag both in laboratory- and industrial-scale trials inhibited metal leaching to a greater extent than would be expected for dilution of the metals in the slag samples alone, thus demonstrating multiple influences.
The metal concentrations in the samples could not be correlated with their respective leaching values, both in laboratory- and industrial-scale trials.
The leachabilities of Zn, Cu, Ni, and Sb from granulated slags decreased to a minimum when the CaO content was increased to 12 and 13 wt.% in the laboratory- and industrial-scale trials, respectively.
The mechanism underlying the decreased leaching with increased CaO addition requires further investigation, as adding CaO, in addition to influencing the structure of the material, also affects parameters such as the viscosity and smelting temperature.
Acknowledgement
This study was conducted within the strategic innovation program STRIM, a joint investment in strategic innovation areas by Vinnova, Formas and the Swedish Energy Agency and within the Center of Advanced Mining and Metallurgy (CAMM) at Luleå University of Technology.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Schlesinger ME, King MJ, Sole KC, et al. Copper loss in slag. In: Schlesinger ME, King MJ, Sole KC, Davenport WG, editors. Extractive metallurgy of copper (fifth edition). Oxford: Elsevier; 2011. p. 191–203. doi:https://doi.org/10.1016/b978-0-08-096789-9.10011-3.
- Warczok A, Utigard TA. Settling of copper drops in molten slags. Metall Mater Trans B. 1995;26(1):1165–1173. doi:https://doi.org/10.1007/BF02654001.
- Lotfian S, Vikström T, Lennartsson A, et al. Plastic-containing materials as alternative reductants for base metal production. Can Metall Q. 2019;58(2):164–176. doi:https://doi.org/10.1080/00084433.2018.1532951.
- Borell M. Slag—a Resource in the Sustainable Society. In Securing the Future, Proc. Int. Conference on Mining, Environment, Metals, Energy Recovery, Skellefteå, Sweden, 2005, pp 130–138.
- Schlesinger ME, King MJ, Sole KC, et al. Matte smelting fundamentals. In: Schlesinger ME, King MJ, Sole KC, Davenport WG, editors. Extractive metallurgy of copper (fifth edition). Oxford: Elsevier; 2011. p. 73–88. doi:https://doi.org/10.1016/b978-0-08-096789-9.10005-8
- Jak E, Zhao B, Hayes P. Phase equilibria in the system FeO-Fe2O3-Al2O3-CaO-SiO2 with applications to non-ferrous smelting slags. Trans Institutions Min Metall Sect C Miner Process Extr Metall. 2008;117(3):147–152. doi:https://doi.org/10.1179/174328507X198771.
- Ducret AC, Rankin WJ. Liquidus temperatures and viscosities of FeO-Fe2O3-SiO2-CaO-MgO slags at compositions relevant to nickel matte smelting. Scand J Metall. 2002;31(1):59–67. doi:https://doi.org/10.1034/j.1600-0692.2002.310108.x.
- Rusen A, Geveci A, Topkaya YA, et al. Effects of some additives on copper losses to Matte smelting slag. JOM. 2016;68(9):2323–2331. doi:https://doi.org/10.1007/s11837-016-1825-1.
- Kim HG, Sohn HY. Effects of CaO, Al2O3, and MgO additions on the copper solubility, ferric/ferrous ratio, and minor-element behavior of iron-silicate slags. Metall Mater Trans B Process Metall Mater Process Sci. 1998;29(3):583–590. doi:https://doi.org/10.1007/s11663-998-0093-z.
- Živković Ž, Mitevska N, Mihajlović I, et al. The influence of the silicate slag composition on copper losses during smelting of the sulfide concentrates. J Min Metall Sect B Metall. 2009;45(1):23–34. doi:https://doi.org/10.2298/JMMB0901023Z.
- Vartiainen A. Viscosity of iron-silicate slags at copper smelting conditions. In TMS Annual Meeting, 1998, pp 363–371.
- Kaiura GH, Toguri JM, Marchant G. Viscosity of fayalite-based slags. Can Metall Q. 1977;16(1):156–160. doi:https://doi.org/10.1179/cmq.1977.16.1.156.
- Gorai B, Jana RK, Premchand M. Characteristics and utilisation of copper slag – a review. Resour Conserv Recycl. 2003;39(4):299–313. doi:https://doi.org/10.1016/S0921-3449(02)00171-4.
- Shi C, Meyer C, Behnood A. Utilization of copper slag in cement and concrete. Resour Conserv Recycl. 2008;52(10):1115–1120. doi:https://doi.org/10.1016/j.resconrec.2008.06.008.
- Murari K, Siddique R, Jain KK. Use of waste copper slag, a sustainable material. J Mater Cycles Waste Manag. 2014;17(1):13–26. doi:https://doi.org/10.1007/s10163-014-0254-x.
- Feng Y, Yang Q, Chen Q, et al. Characterization and evaluation of the pozzolanic activity of granulated copper slag modified with CaO. J Clean Prod. 2019;232:1112–1120. doi:https://doi.org/10.1016/j.jclepro.2019.06.062.
- Alter H. The composition and environmental hazard of copper slags in the context of the basel convention. Resour Conserv Recycl. 2005;43(4):353–360. doi:https://doi.org/10.1016/j.resconrec.2004.05.005.
- Piatak NM, Parsons MB, Seal RR. Characteristics and environmental aspects of slag: a review. Appl Geochem. 2015;57:236–266. doi:https://doi.org/10.1016/j.apgeochem.2014.04.009.
- Potysz A, van Hullebusch ED, Kierczak J, et al. Copper metallurgical slags – current knowledge and fate: a review. Crit Rev Environ Sci Technol. 2015;45(22):2424–2488. doi:https://doi.org/10.1080/10643389.2015.1046769.
- Das B, Mishra BK, Angadi S, et al. Characterization and recovery of copper values from discarded slag. Waste Manag Res. 2010;28(6):561–567. doi:https://doi.org/10.1177/0734242X09343943.
- Mostaghel S, Samuelsson C. Metallurgical use of glass fractions from waste electric and electronic equipment (WEEE). Waste Manag. 2010;30(1):140–144. doi:https://doi.org/10.1016/j.wasman.2009.09.025.
- Meshram P, Bhagat L, Prakash U, et al. Organic acid leaching of base metals from copper granulated slag and evaluation of mechanism. Can Metall Q. 2017;56(2):168–178. doi:https://doi.org/10.1080/00084433.2017.1293900.
- Lidelöw S, Mácsik J, Carabante I, et al. Leaching behaviour of copper slag, construction and demolition waste and crushed rock used in a full-scale road construction. J Environ Manage. 2017;204:695–703. doi:https://doi.org/10.1016/j.jenvman.2017.09.032.
- Engström F, Larsson ML, Samuelsson C, et al. Leaching behavior of aged steel slags. Steel Res Int. 2014;85(4):607–615. doi:https://doi.org/10.1002/srin.201300119.
- Gražulis S, Daškevič A, Merkys A, et al. Crystallography open database (COD): an open-access collection of crystal structures and platform for world-wide collaboration. Nucleic Acids Res. 2012;40(D1):D420–D427. doi:https://doi.org/10.1093/nar/gkr900.
- Bellemans I, De Wilde E, Moelans N, et al. Metal losses in pyrometallurgical operations – a review. Adv Colloid Interface Sci. 2018;255:47–63. doi:https://doi.org/10.1016/j.cis.2017.08.001.
- Maruyama T, Furui N, Hamamoto M, et al. The copper loss in slag of flash smelting furnace in Tamano Smelter. Yazawa Int Metall Mater Process Princ Technol. 2003;2:337–347.
- Jalkanen H, Vehviläinen J, Poijärvi J. Copper in solidified copper smelter slags. Scand J Metall. 2003;32(2):65–70. doi:https://doi.org/10.1034/j.1600-0692.2003.00536.x.
- Wang X, Geysen D, Padilla Tinoco SV, et al. Characterisation of copper slag in view of metal recovery. Trans Institutions Min Metall Sect C Miner Process Extr Metall. 2015;124(2):83–87. doi:https://doi.org/10.1179/1743285515Y.0000000004.
- Potysz A, Kierczak J, Fuchs Y, et al. Characterization and pH-dependent leaching behaviour of historical and modern copper slags. J Geochemical Explor. 2016;160:1–15. doi:https://doi.org/10.1016/j.gexplo.2015.09.017.
- Corwin DL, Yemoto K. Salinity: electrical conductivity and total dissolved solids. Soil Sci Soc Am J. 2020;84(5):1442–1461. doi:https://doi.org/10.1002/saj2.20154.
- Jarošíková A, Ettler V, Mihaljevič M, et al. The pH-dependent leaching behavior of slags from various stages of a copper smelting process: environmental implications. J Environ Manage. 2017;187:178–186. doi:https://doi.org/10.1016/j.jenvman.2016.11.037.
- Reich J, Pasel C, Herbell J-D, et al. Effects of limestone addition and sintering on heavy metal leaching from hazardous waste incineration slag. Waste Manag. 2002;22(3):315–326. doi:https://doi.org/10.1016/S0956-053X(01)00020-4.
- Huaiwei Z, Fei S, Xiaoyan S, et al. The viscous and conductivity behavior of melts containing iron oxide in the FeOt-SiO2-CaO-Cu2O system for copper smelting slags. Metall Mater Trans B Process Metall Mater Process Sci. 2012;43(5):1046–1053. doi:https://doi.org/10.1007/s11663-012-9698-3.
- Kowalczyk J, Mroz W, Warczok A, et al. Viscosity of copper slags from chalcocite concentrate smelting. Metall Mater Trans B. 1995;26(1):1217–1223. doi:https://doi.org/10.1007/BF02654007.
- Elliot BJ, See JB, Rankin WJ. Effect of slag composition on copper losses to silica-saturated iron silicate slags. Trans Inst Mining Metall C Miner Process Extr Metall. 1978;87:204–211.