?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Bauxite residue characterised as a polymetallic source containing iron, alumina, titanium, calcium, silicon and rare earth elements offers an opportunity for reduced waste volume and the recovery of valuable metals. This study focuses on the agglomeration of bauxite residue with calcite, followed by hydrogen reduction at 1000°C. The resulting iron particles in the reduced pellets were initially very small distributed in a porous oxide matrix, posing challenges for physical separation methods. To address this issue, further heat treatments were conducted on the reduced pellets in an argon atmosphere, ranging from 1100°C to 1550°C. Phase analysis and microstructural examinations were performed using X-ray powder diffraction and scanning electron microscopy, respectively. Experimental findings indicate that the size of iron particles in the reduced pellets increases with higher heat treatment temperatures. Up to 1200°C, the oxide matrix remains a solid state, transitioning to a semi-molten and molten state above this heat treatment temperature and complete separation of iron from the oxide matrix above 1500°C. An alkali-leachable phase is present in the reduced pellets, initially in the form of calcium aluminate (mayenite), while above 1400°C, this phase dissociates to form monocalcium aluminate, and complete conversion at 1500°C. As observed in the microstructural analysis, there is an increase in the particle size of iron in both the solid and semi-molten states with an increasing in temperature from 1100°C up to 1300°C. This comprehensive study sheds light on the transformative effects of varying heat treatment temperatures on the composition and microstructure of bauxite residue-based materials.
Les résidus de bauxite caractérisés comme une source polymétallique contenant du fer, de l’alumine du titane, du calcium, du silicium et des éléments de terre rare, offre une opportunité de réduction du volume de déchets et de récupération des métaux de valeur. Cette étude se concentre sur l’agglomération des résidus de bauxite avec de la calcite, suivie d’une réduction à l’hydrogène à 1000°C. Les particules de fer résultantes dans les boulettes réduites étaient initialement très petites, réparties dans une matrice d’oxyde poreuse, posant des défis pour les méthodes de séparation physique. Pour résoudre ce problème, on a effectué d’autres traitements thermiques sur les boulettes réduites dans une atmosphère d’argon, allant de 1100 à 1550°C. On a effectué une analyse de phase et des examens de microstructure par diffraction des rayons X sur la poudre et par microscopie électronique à balayage, respectivement. Les résultats expérimentaux indiquent que la taille des particules de fer dans les boulettes réduites augmente avec des températures supérieures de traitement thermique. Jusqu’à 1200°C, la matrice d’oxyde reste à l’état solide, passant à un état semi-fondu et fondu au-dessus de cette température de traitement thermique. Notamment, au-dessus de 1500°C, une agglomération significative des particules de fer se produit, conduisant à leur séparation de la matrice d’oxyde. Une phase lixiviable par les alcalis est présente dans les boulettes réduites, initialement sous forme d’aluminate de calcium (mayénite), tandis qu’au-dessus de 1400°C, cette phase se dissocie pour former de l’aluminate monocalcique, et à 1500°C, ceci est complété plus rapidement. Tel qu’observé dans l’analyse microstructurale, il y a une augmentation de la taille de particule de fer à l’état solide et semi-fondu avec une augmentation de la température de 1100°C jusqu’à 1300°C. Cette étude approfondie fait la lumière sur les effets transformateurs des différentes températures de traitement thermique sur la composition et la microstructure des matériaux basés sur les résidus de bauxite.
1. Introduction
Bauxite residue, also known as red mud [Citation1], is a by-product of the Bayer process for alumina production. Red mud is characterised as slurry containing undigested oxides and loaded alkali elements, because of its elevated alkali content, fine particle size and enrichment with heavy metals. Proper handling and disposal methods are essential due to the potential environmental impacts associated with these characteristics [Citation2,Citation3]. Over the past few decades, bauxite residue has predominantly been deposited in storage ponds, resulting in near-zero utilisation. The quantity of bauxite residue generated per ton of alumina typically ranges from 1 to 1.5 tonne, dependent on the processing parameters employed in the Bayer process and the source of the bauxite ore [Citation3–5]. Annually, approximately 150 million tonne of bauxite residue are generated globally, with a staggering 4.5 billion tons stockpiled. This accumulation presents a significant challenge to the industry due to the lack of commercial utility and the escalating environmental concerns associated with storage. Notably, bauxite residue comprises valuable oxides, including iron, alumina, silicon, calcium, sodium and rare earth elements (REEs). Iron and alumina constitute the major components, accounting for approximately 70 wt.% of the residue. A possible solution lies in the recovery of iron and alumina, a process that holds the potential to substantially reduce the volume of bauxite residue. It appears that while there is existing literature on the recovery of metals from bauxite residue using hydrogen, there is a gap in research specifically related to the agglomeration of metallic iron particles at higher heat treatment temperatures [Citation6–8]. This indicates an opportunity for further investigation and exploration in this specific aspect of bauxite residue processing, potentially uncovering new insights and optimising methods for the recovery and utilisation of metallic iron particles.
Barustan and Jung [Citation9] studied the morphology and agglomeration behaviours during the isothermal reduction of iron oxide fines at 800°C in CO and H2 atmosphere. Reduction by CO resulted in increased sticking due to the formation of iron whiskers. The introduction of CaO and MgO during the sintering process improved reduction and reduced sticking by creating calcio/magnesio-wustite on the surface. Despite the addition of CaO and MgO, iron whiskers still formed, but their shape differed from those obtained with pure Fe2O3. The study suggests that a uniform coating layer on particle surfaces may be a crucial factor in minimising sticking between particles.
Du et al. [Citation10] studied in laboratory scale, fine iron ores from Chile undergo reduction in a fluidised bed at temperatures ranging from 973 to 1173 K using CO–H2–CO2 mixtures. The investigation focuses on understanding how varying reduction conditions affect the morphology of newly formed metallic iron and its agglomeration behaviour. The findings suggest that the addition of H2 to CO expedites the movement of the Fe/Fe1–xO interface and increases the iron nucleus formation, leading to a transformation from fibrous to dense iron morphology. The presence of CO2 in CO results in shorter and sparser fibrous iron, with a distinctive ‘cactus-like’ appearance at a 30% CO2 content. Higher reduction temperatures strengthen and activate the fibrous iron. The sticking index reveals that long and robust iron whiskers tend to form stable agglomerates even at low metallisation degrees, while dense iron particles tend to remain separate. Based on the proposed iron precipitation mechanism, the study suggests that the agglomeration behaviour of fine iron ores can be controlled by pre-reduction in a H2-rich reducing gas (H2/(H2 + CO) ≥ 30%, by volume).
Wang et al. [Citation11] studied the agglomeration and recovery of metallic iron and enrichment of titanium in Ti bearing blast furnace slag. In this process, dynamic oxidation process of molten blast furnace slag containing titanium, the reduced components undergo oxidation, generating heat that elevates the slag temperature, thereby reducing viscosity. These effects, combined with increased turbulence, facilitate the agglomeration and recovery of over 85% of metallic iron droplets. Throughout dynamic oxidation, the titanium component exhibits selective enrichment in the perovskite phase. The degree of titanium enrichment reaches 93.2% in oxidised slag without any additive agent and increases to 96.5% when 1.0% steel slag is added during oxidation, compared to a mere 48.0% in the raw slag. The air injection process proves to be a promising method for economically recovering iron and titanium from titanium-bearing blast furnace slag.
The agglomeration of iron particles has not been thoroughly explored in previous studies. Limited literature is available on the subject of iron particle agglomeration. Our earlier research on the hydrogen reduction of bauxite residue calcite mixtures revealed that the reduced iron particles are exceptionally small, measuring approximately 1–2 µm [Citation12]. The challenge arises from the fine size of these reduced iron particles, making the physical separation of metallic iron from the slag matrix a complex and difficult process [Citation7,Citation8,Citation13]. The fine size of iron particles poses a challenge in grinding the reduced pellets to sizes below or close to that of the iron particles. This grinding process is notably energy intensive. Complicating matters, the metallic iron remains embedded within the oxide matrix, making it resistant to complete separation during the physical separation process. Consequently, alongside iron, other oxides are included in the magnetic fraction, leading to a decrease in the iron grade.
This study focuses on subjecting hydrogen-reduced bauxite residue calcite pellets to a series of heat treatments, with temperatures varying from 1100°C to 1550°C. The main objective of this investigation is to monitor the growth of iron particles and their transformation from a solid to a molten state. By employing high-temperature heat treatment, the research aims to enhance the particle size of the metallic iron.
Additionally, the study explores the phase transition of leachable calcium aluminate compounds. Specifically, it examines the shift from mayenite (12CaO.7Al2O3) to the monocalcium aluminate phase (CaO.Al2O3), which occurs simultaneously with the growth of iron particles. Through these detailed examinations, the research endeavours to provide insights into optimising the process conditions for improving the quality and characteristics of the resultant materials.
2. Materials and methods
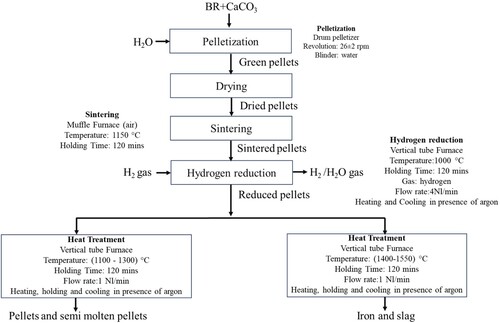
Bauxite residue was provided from Mythellenous SA (formerly known as aluminium of Greece). Residue was received as lumpy form. These residues were dried in air an oven overnight at 80°C to remove excess moisture. The dried residue was deagglomerated and sieved to below 500 µm. Calcite was provided by a paper industry in Norway. Calcite was dried in air an oven for 3 days at 80°C to remove the water and deagglomerated and sieved similar to bauxite residue (BR). After deagglomeration, the bauxite residue and calcite were mixed in appropriate proportions corresponding to CaO.Al2O3, 2CaO.SiO2 and CaO.TiO2 formation during the high-temperature processing. The mixing was carried out in a tabular mixture for 30 min. The experimental flowsheet is presented in .
2.1. Pelletising and hydrogen reduction
The mixture was used for pelletisation by using a disc pelletiser. During pelletisation water was used around 10 wt.% of the dry feed. The generated green pellets were dried in an oven at 80°C overnight to remove excess moisture. The dried pellets were then sintered in air a muffle furnace at 1150°C for 120 min. The optimisation of the sintering parameter was described elsewhere [Citation14]. The sintered pellets were used for hydrogen reduction in a vertical tube furnace. The schematic view and the furnace operation were described in our previous work [Citation8]. For this test, 1000 g of sintered pellets were used for the reduction. The sintered pellets were heated to the targeted temperature under Ar flow and once the targeted temperature was reached the gas was changed to hydrogen. The hydrogen was purged with a flow rate of 4 NL/min for 120 min. After the reduction cycle was over, purging gas was changed to argon. Cooling of the reduced pellets was done in the presence of argon to avoid the oxidation of the metallic iron.
2.2. High-temperature heat treatment
The reduced pellets were heat treated in argon atmosphere (5N purity) to increase the metallic iron particle size. The schematic furnace view and the furnace description are presented here [Citation15]. The purpose of using high-purity argon gas is to avoid reoxidation during the heat treatment. The heat treatment of the reduced pellets was carried out in high-purity alumina crucible. The heat treatment was done with varying temperatures from 1100°C to 1550°C with 2 hours of holding time at the target temperature. All heat treatment cycles were carried out in the presence of argon gas. The heating rate to the target temperature was 15°C/min, and the cooling rate was 25°C/min was observed that during heat treatment of reduced pellets, the pellets were in the solid state for 1100 and 1200°C, however, it was semi-molten at 1300°C. Above 1300°C, all the reduced pellets got molten. At the heat treatment temperatures of 1500 and 1550°C, metallic iron was completely separated from the slag as observed by eye.
2.3. Characterisation methods
The mineralogical phase analysis of the heat-treated pellets was done by using the X-ray powder diffraction (XRD) technique (Bruker D8 A25 DaVinciTM, Karlsruhe, Germany) with the CuKα radiation and 2θ diffraction angle range from 15 to 75° with step size 0.02°. A crystallographic database and EVA software were used for the diffraction analysis of the phases. For XRD analysis, these materials were powdered in a stainless-steel ring mill for 45 s at a rotation speed of 800 rev min–1. The microstructural analysis of the reduced pellets was carried out by a scanning electron microscope (SEM) (Zeiss ultra 55LE, Carl Zeiss, Jena, Germany), and the elemental analysis and mapping was carried out by energy dispersive spectroscopy (EDS) (Bruker, AXS, microanalysis GmbH, Berlin, Germany).
3. Results and discussion
3.1. Phase analysis of the reduced pellets
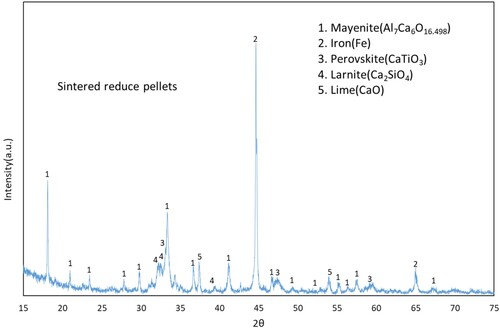
The phases formed during the reduction of bauxite residue calcite pellets are mayenite (Al7Ca6O16.5), iron (Fe), perovskite (CaTiO3), larnite (Ca2SiO4) and lime (CaO) as shown in . Among those phases mayenite and iron are the major phases. The evolution of these phases was observed in our previous study, and the related mechanisms were explained in detail [Citation12].
3.2. Phase analysis of slag of the melted reduced pellets
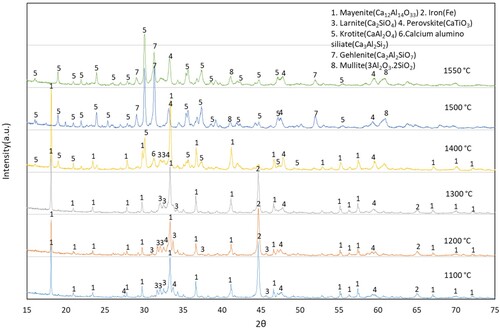
The heat-treated reduced pellets exhibit similarities at 1100°C, 1200°C and 1300°C, and the major phases including mayenite, iron, perovskite and larnite (). However, at 1400°C, there is a notable transition in the calcium aluminate phase from mayenite to a mixture of mayenite and krotite (CaAl2O4). Concurrently, a calcium aluminosilicate phase formed alongside krotite. At 1400°C heat-treated reduced pellets, iron remains integrated with the slag matrix due to the majority of phases existing in semi-liquid form. Above this temperature, there is highly separation of metallic iron from the slag matrix, as evident from XRD analysis, which shows no metallic iron peak in the slag. Additionally, most of the calcium aluminate phase persists in the form of krotite.
For the 1500°C heat-treated reduced pellets, the peaks are similar to those at 1400°C, but they are more intense. At 1500°C and above, a fraction of alumina remains in the form of calcium aluminosilicate, gehlenite and aluminosilicate. This observation indicates further changes in the composition and crystalline structure of the pellets at higher heat treatment temperatures.
3.3 Microstructural analysis
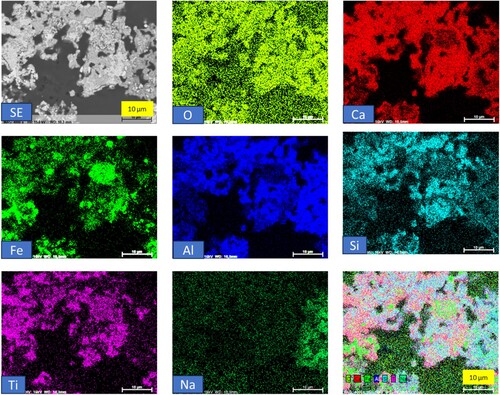
illustrates the elemental mapping of hydrogen-reduced bauxite residue calcite pellets. The smaller bright areas observed correspond to metallic iron, with an average particle size of approximately 1–2 µm. Some of these fine iron particles are very close and aggregated, and not sintered (). The small particle size of iron may be attributed to small iron complexes in the original bauxite residue, which is inherently fine. In the elemental mapping, iron is distinctly separated from the rest without overlapping with other elements. This distinct separation signifies the presence of high-purity metallic iron, indicating successful isolation and purity of the iron component in the reduction process.
The reduced pellets were subjected to heat treatment at 1100°C for 2 h in an argon atmosphere, and displays the elemental mapping of these heat-treated reduced pellets. The mapping reveals that iron does not overlap with other elements in the matrix. The average size of metallic iron is observed to be in the range of 2–3 µm and qualitatively slightly larger than the reduced sample at 1000°C. This increase in iron particle size can be attributed to high-temperature diffusion during the heat treatment process. Notably, the iron particles appear in more spherical shape, in contrast to the more irregular shape observed in the original reduced pellets at 1000°C.
Figure 5. Backscattered electron image and elemental mapping of heat-treated reduced pellets at 1100°C.
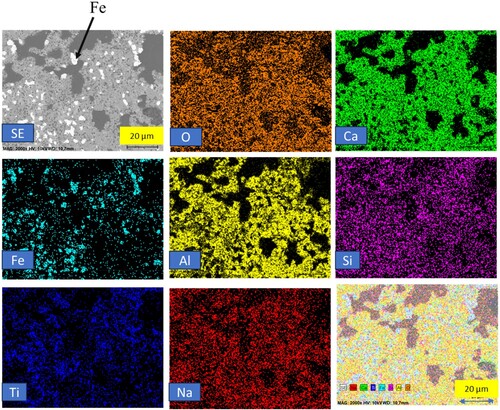
Moving on to , it illustrates the elemental mapping of the reduced pellets heat-treated at 1200°C. In comparison to the 1100°C heat-treated pellets, the iron particle sizes are larger, with an average size of around 4–5 µm. The iron particles are distinctly and individually present in the oxide matrix of the reduced pellets without overlapping with other elements such as oxygen, titanium, silicon and aluminium. This observation underscores the effective isolation and purity of the iron component even at higher heat treatment temperatures.
Figure 6. Backscattered electron image and elemental mapping of heat-treated reduced pellets at 1200°C.
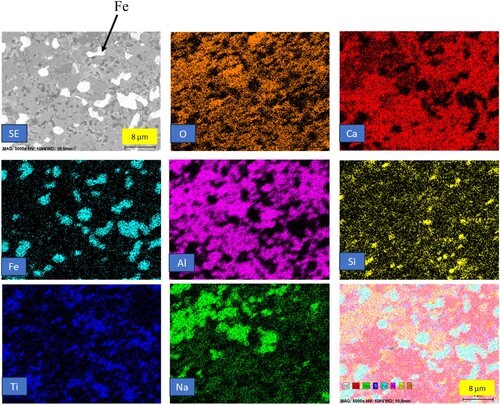
displays the 1300°C heat-treated reduced pellets. The figure reveals that in certain areas, the matrix started to melt, or partially melted. The average size of iron particles in this heat-treated condition is approximately 15–20 µm. Notably, aluminium overlaps with calcium, and oxygen is present, indicating the existence of the krotite phase. Additionally, silicon is observed to combine with alumina and calcium in some areas, suggesting the presence of the gehlenite phase, which is supported by XRD analysis. These observations suggest the occurrence of phase transformations and melting in the matrix at the elevated heat treatment temperature of 1300°C.
Figure 7. Backscattered electron image and elemental mapping of heat-treated reduced pellets at 1300°C.
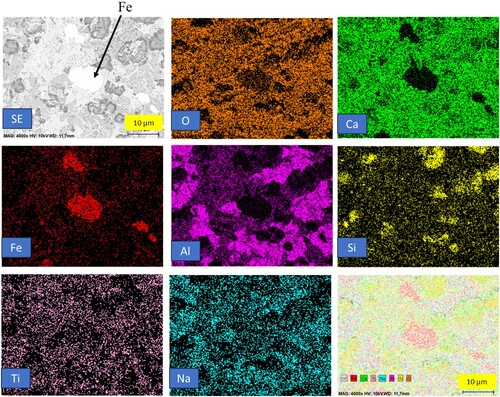
In , the heat treatment of the pre-reduced pellets at 1400°C is depicted, revealing partial separation between iron and the matrix. The pre-reduced pellets exhibit a semi-molten state, and a minor fraction of iron is distinctly separated within the matrix. The matrix is predominantly composed of calcium aluminate slag, identified as krotite. Sodium and silicon are found to be distributed throughout the matrix. This observation indicates a significant transformation in the state of the pellets at the elevated temperature of 1400°C, where partial separation and semi-molten conditions are evident. The back scattered electron image of 1300°C and 1400°C heat-treated pellets are presented in . The elemental mapping of heat-treated reduced pellets at 1400°C is presented in .
Figure 8. Microstructure of 1300°C and 1400°C heat-treated pre-reduced pellets (backscattered electron micrograph).
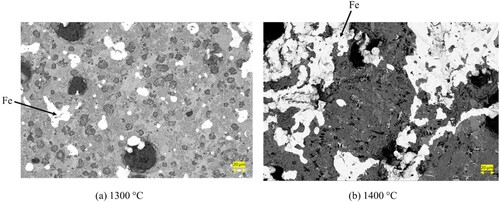
Figure 9. Backscattered electron image and elemental mapping of heat-treated reduced pellets at 1400°C.
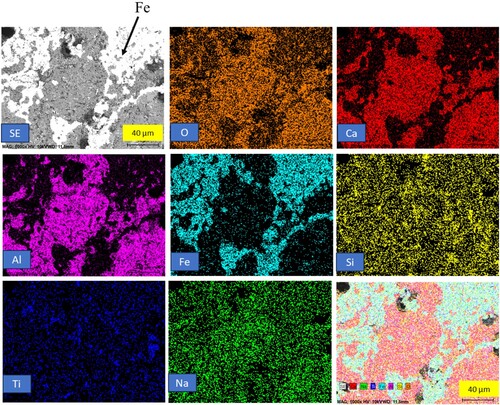
In and , the elemental mapping of the heat-treated pellets at 1500 and 1550°C is presented. In the case of the pellets treated at 1500°C, there is a minor fraction of iron present in the slag matrix. However, at 1550°C, there is complete separation of iron from the rest of the matrix. The presence of iron in the slag matrix at 1500°C may be attributed to the higher viscosity of the slag, which decreases in 1550°C. The slag phases are similar for both 1500°C and 1550°C pellets, with krotite being the major phase in both samples. This observation highlights the temperature-dependent behaviour of the pellet system, affecting the separation and distribution of iron within the matrix.
Figure 10. Backscattered electron image and elemental mapping of heat-treated reduced pellets at 1500°C.
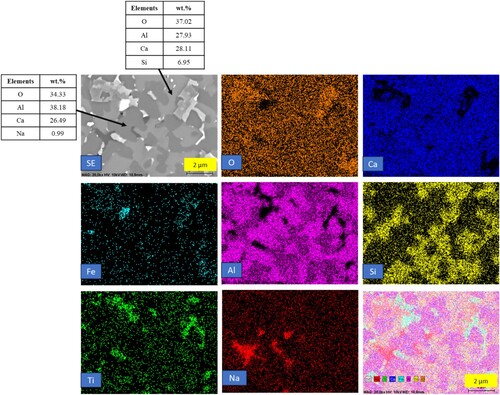
Figure 11. Backscattered electron image and elemental mapping of heat-treated reduced pellets at 1550°C (slag phase).
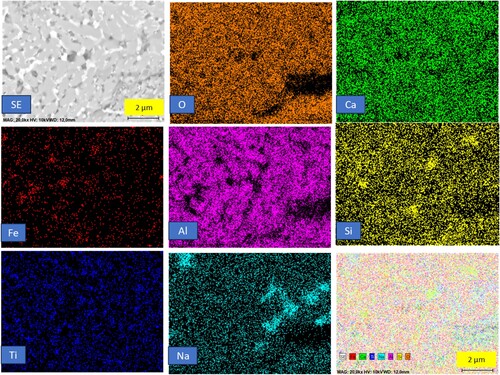
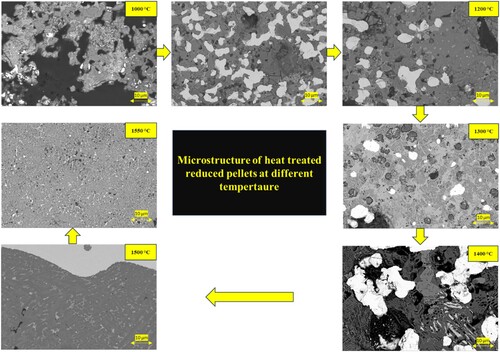
In , a comparison of the microstructure of pre-reduced bauxite residue and calcite pellets subjected to different heat treatments is presented. The micrograph illustrates that with an increase in the heat treatment temperature, the size of metallic particles increases. Up to 1300°C, the pre-reduced pellets maintain to show a solid oxide matrix while the Fe is solid due to its high purity and higher melting point (1535°C), and at higher temperatures, there is evidence of semi-molten slag appearance in the pellets. At heat treatment temperatures above 1500°C and 1550°C, the iron is completely separated from the matrix of calcium aluminate slag. Obviously, the slag and metal are getting molten at these temperatures and the complete separation and forming large iron pieces occurs. This observation reinforces the correlation between heat treatment temperature and the evolving microstructure of the pellets, including changes in particle size and the onset of semi-melting behaviour. Above 1400°C, the iron is separated from the slag matrix, due to that in elemental mapping of 1500 and 1550 iron is not appeared in the mapping. In the 1500°C heat treatment, some iron dots are present in the mapping.
3.4 Porosity loss and iron particles growth
The above microstructures indicate that with increasing the heat treatment temperature the iron particles are getting larger, and they become more spherical. In parallel we see the loss of porosity and getting smaller round pores upon increasing temperature. This change of the pore structure via heat treatment is due to the significant sintering of the particles/phases in the oxide portion. In principle, this phenomenon is due to the tendency of the system to reduce the surface energies. We may claim that this sintering and porosity loss and correspondingly shrinkage of the whole pellet is a major reason for the iron particles growth upon temperature increase (). The microstructural change of the oxide portion of the pellets causes the join of metal particles physically which shows us their further growth by diffusion mechanism. The morphology of the iron particles is changed to more spherical particles as the surface energy of iron phase, or in a better word interfacial energies between the metal and slag tend to be minimised at elevated temperatures. illustrates that with an increase in the heat treatment temperature of the pre-reduced pellets, the porosity decreases. In (a), significant porosity is evident in the pellets at 1000°C. However, this porosity decreases until the temperature reaches 1200°C in the solid state. Up to 1200°C, iron particles are distributed separately, but beyond this temperature, there is agglomeration of metallic iron within the matrix, leading to a substantial loss of porosity at higher temperature.
Figure 13. Microstructure of heat-treated pre-reduced pellets showing the porosity loss with increasing the heat treatment temperature (backscattered electron micrograph).
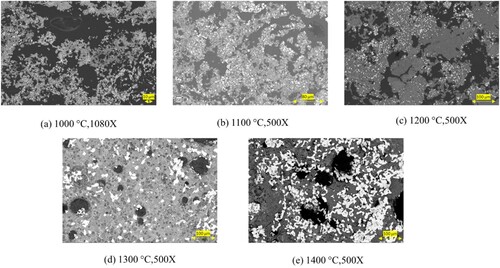
Table 1. Actual particle size increase and molten or completely separated state of iron and slag.
shows the average iron particle size of the heat-treated reduced pellets as compared to the initial reduced pellets.
3.5 Formation of krotite from mayenite
During the heat treatment of pre-reduced bauxite residue CaO pellets, the calcium aluminate predominantly exists in the form of mayenite compound. However, as the heat treatment temperature increases, there is an intensification in the formation of krotite. Beyond 1400°C, at all heat treatment temperatures, the entire mayenite phase undergoes a transformation into krotite. This transformation signifies a significant structural and compositional change in the calcium aluminate during the heat treatment process.
(1)
(1) The produced CaO in the above reaction may incorporate with the adjacent silica-containing phases in the semi-molten slag phase.
4. Conclusion
The following conclusion were drawn from the high-temperature heat treatment of reduced bauxite residue calcite pellets:
Complete reduction of iron oxides in bauxite residue calcite pellets to metallic iron is achieved at a reduction temperature of 1000°C with a reduction time of 90 min, yielding iron particles with 1–2 µm diameter.
Upon increasing the heat treatment temperature, the iron particles undergo growth in the solid state until reaching 1200°C. Beyond this temperature, the matrix begins to exhibit semi-molten (1300°C) and molten characteristics.
At temperatures exceeding 1500°C, there is a complete separation of iron particles from the surrounding oxide matrix, yielding large lumpy iron particle.
The alkali-leachable phase mayenite in the reduced pellets undergoes a conversion to the monocalcium aluminate phase above 1400°C during heat treatment.
The maximum observed growth of iron particles occurs at 1300°C, reaching a size of 15–20 micrometres within the solid-state matrix. Subsequently, as the temperature rises, the matrix transitions to a semi-molten state.
The change in the microstructure of the oxide portion of the pellet and porosity loss is the main reason of iron particles growth at the temperature ranges below the slag melting point.
This study’s theoretical contribution is the preservation of leachable calcium aluminate (monocalcium aluminate or mayenite phase) as the iron particle size increases with higher heat treatment temperatures. From a practical standpoint, the growth in iron particle size is vital for effective magnetic separation from non-metallic components, as demonstrated by other researchers in the Harare project. However, a limitation of this process is that both the reduction and heat treatment require high temperatures, potentially leading to increased energy costs.
Acknowledgement
This project has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No. 958307. This publication represents only the authors’ views, exempting the Community from any liability.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Habashi, F. Karl Josef Bayer and his time — part 1. On the occasion of the hundredth anniversary of his death. CIM Bull. 2004, 97, 61–64.
- Evans K. The history, challenges, and new developments in the management and use of bauxite residue. J Sustain Metall. 2016;2:316–331. doi:10.1007/s40831-016-0060-x
- Kar MK, Önal MAR, Borra CR. Alumina recovery from bauxite residue: a concise review. Resour Conserv Recycl. 2023;198:107158, doi:10.1016/j.resconrec.2023.107158
- Lazou A, Van Der Eijk C, Tang K, et al. The utilization of bauxite residue with a calcite-rich bauxite ore in the Pedersen process for iron and alumina extraction. Metall Mater Trans B. 2021: 1–12.
- Borra CR, Blanpain B, Pontikes Y, et al. Smelting of bauxite residue (red mud) in view of iron and selective rare earths recovery. J Sustain Metall. 2016;2:28–37. doi:10.1007/s40831-015-0026-4
- Pilla G, Hertel T, Blanpain B, et al. Influence of H2 content on Fe, Al, and Na recovery during low-temperature reduction of bauxite residue. In Proceedings of the 8th international slag valorisation symposium. No publisher, 2023; pp. 146–149.
- Kar MK, Hassanzadeh A, van der Eijk C, et al. Comparative study of hydrogen reduction of bauxite residue-calcium sintered and self-hardened pellets followed by magnetic separation for iron recovery. Mining, Metall Explor. 2023: 1–14.
- Kar MK, Hassanzadeh A, van der Eijk C, et al. Properties of self-hardened CaO-added bauxite residue pellets, and their behavior in hydrogen reduction followed by leaching and magnetic separation for iron and alumina recovery. Int J Hydrogen Energy. 2023.
- Barustan MIA, Jung S-M. Morphology of iron and agglomeration behaviour during reduction of iron oxide fines. Met Mater Int. 2019;25:1083–1097. doi:10.1007/s12540-019-00259-6
- Du Z, Zhu Q, Fan C, et al. Influence of reduction condition on the morphology of newly formed metallic iron during the fluidized bed reduction of fine iron ores and its corresponding agglomeration behavior. Steel Res Int. 2016;87:789–797.
- Wang MY, Zhang L, Sui ZT, et al. Study on recovery of metallic Fe and enrichment behaviour of titanium in Ti bearing blast furnace slag. Ironmak Steelmak. 2009;36:388–392. doi:10.1179/174328108X393867
- Kar MK, Eijk C, van der; Safarian J. Kinetics study on the hydrogen reduction of bauxite residue-calcite sintered pellets at elevated temperature. Metals (Basel). 2023;13:644, doi:10.3390/met13040644
- Hassanzadeh A, Kar MK, Safarian J, et al. An investigation on reduction of calcium added bauxite residue pellets by hydrogen and iron recovery through physical separation methods. Metals (Basel). 2023;13:946, doi:10.3390/met13050946
- Kar MK, Safarian J. Characteristics of bauxite residue–limestone pellets as feedstock for Fe and Al2O3 recovery. Processes. 2023;11:137, doi:10.3390/pr11010137
- Rasouli A, Herstad KE, Safarian J, et al. Magnesiothermic reduction of natural quartz. Metall Mater Trans B. 2022: 2132–2142.