Abstract
The present study reports the chromosome number and meiotic behavior of 25 populations belonging to 10 species of Onobrychis sect. Hymenobrychis from Iran. Most populations showed the chromosome number 2n = 2x = 14. However, the chromosome number of two populations belonging to O. michauxii and O. subnitens was 2n = 2x = 16. It seems that the species with x = 7 are derived through aneuploid loss. In addition, intra- and interspecific variations in the chromosome number within O. sect. Hymenobrychis, aneuploidy and polyploidy in Onobrychis with special reference to the section Hymenobrychis and also the evolution of the chromosome number throughout the genus are discussed here. As the result of the present study and by reviewing the chromosome number within the genus, it can be concluded that O. sect. Hymenobrychis includes comparatively highly derived taxa and can be considered as a heterogeneous unit within the genus Onobrychis. Almost all the studied taxa displayed regular bivalent pairing and chromosome segregation at meiosis. However, some meiotic abnormalities observed in different taxa are discussed here.
Introduction
Onobrychis Miller (Hedysareae, Fabaceae) comprises about 170 species under 12 higher taxa mainly distributed in southwest Asia, the Mediterranean region, temperate Europe and Asia, a few of which are cultivated as fodder or as ornamentals (Lock and Simpson Citation1991; Yakovlev et al. Citation1996; Mabberley Citation1997; Ranjbar et al. 2009a). In Flora Iranica (Rechinger Citation1984) 54 species from Iran were treated under eight sections: Dendrobrychis, Lophobrychis, Onobrychis, Laxiflorae, Anthyllium, Afghanicae, Heliobrychis, and Hymenobrychis. However, the number of species of this genus in Iranian flora is about 65 (Ranjbar et al. Citation2004, Citation2007; Ranjbar Citation2009; Ranjbar et al. Citation2010a., Ranjbar et al. Citation2010c). The taxonomy of the genus continues to be subject of much confusion, mainly because of the different approaches to species delimitation, resulting in varying numbers of recognized species (Boissier Citation1872; Sirjaev Citation1925; Hedge Citation1970; Ball Citation1978; Duman and Vural Citation1990; Aktoklu Citation2001; Ranjbar et al. Citation2004, 2007, Citation2011, Citation2012; Ranjbar Citation2009; Ranjbar et al. Citation2009f; Ranjbar et al. Citation2010a, Citation2010b; Toluei et al. Citation2012, Citation2013).
Most cytological studies in the genus have concentrated on the chromosome count (Baltisberger Citation1991; Karshibaev Citation1992; Slavivk et al. Citation1993), with little work focused on detailed karyological criteria for taxonomic purposes (e.g. Khatoon and Ali 1991; Mesicek and Sojak Citation1992). From these and other reports (e.g. Fedorov Citation1969; Goldblatt Citation1981a, Citation1981b, Citation1984, Citation1985, Citation1988; Romano et al. Citation1987; Goldblatt and Johnson Citation1991; Diaz-Lifante et al. Citation1992; Tamas Citation2006; Ranjbar et al. Citation2009a, Citation2010; Hesamzadeh Hejazi and Ziaei Nasab Citation2010; Ranjbar et al. Citation2010c; Ranjbar et al. Citation2010d; Ranjbar et al. 2010f; Arslan et al. 2012) it is evident that the chromosome count is known for just over a quarter of the species. Two basic chromosome numbers (x = 7 and x = 8) and three ploidy levels (2n = 2x = 14, 2n = 4x = 28, 2n = 8x = 56 and 2n = 2x = 16, 2n = 4x = 32) are present in the genus Onobrychis. Ashurmetov and Normatov (Citation1998) assumed that the primary centre of genetic diversity of Onobrychis is in Mediterranean region, while Yildiz et al. (Citation1999) and Ranjbar et al. (Citation2009c, 2010e) argued that it is in north and southwest Asia.
O. sect. Hymenobrychis, with nearly 14 species in Iran, is one of the most important sections of the genus. Studies on the impact of karyotypic and meiotic behavior data on the interspecific and phylogenetic relationships in the section are still limited (Ranjbar et al. 2010f; Ranjbar et al. Citation2010c). The present study reports meiotic chromosome number and behavior of 25 populations belonging to 10 species of O. sect. Hymenobrychis and tries to increase basic cytogenetical knowledge of the section.
Materials and methods
For cytogenetic study 15 flower buds from at least five plants at an appropriate stage of development were fixed in 96% ethanol, chloroform and propionic acid (6:3:2) for 24 h at room temperature and then stored in 70% alcohol at 4°C until used. Anthers were squashed and stained with 2% acetocarmine. All slides were made permanent using Venetian turpentine. Photographs of chromosomes were taken on an Olympus BX-41 photomicroscope at initial magnification of × 1000. Chromosome counts were made from well-spread metaphases in intact cells, by direct observation and from photomicrographs. Voucher specimens are kept at BASU, Hamedan, Iran (Table ).
Table 1. Taxa studied of O. sect. Hymenobrychis.
Results and discussion
Chromosome numbers and meiotic behavior were determined in 125 individuals belonging to 25 populations of 10 species. A summary of their cytological features is given in Table , and the chromosomes are illustrated in Figures -27. A total of 9808 diakinesis/metaphase I (D/MI), 7715 anaphase I/telophase I (AI/TI), 2650 metaphase II (MII) and 6390 anaphase II/telophase II (AII/MII) cells were analyzed. The meiotic irregularities observed in different populations included chromosome stickiness, B-chromosomes, precocious division of centromeres, chromosome bridges resulting from stickiness, the occurrence of laggard chromosomes, formation of micronuclei in tetrad cells, formation of multipolar cells, desynapsis, asynchronous nucleus and cytomixis, which have been discussed below (Table ). Such irregularities also have been reported previously for O. chorassanica of this section and also in O. viciifolia and O. altissima of O. sect. Onobrychis (Ranjbar et al. 2010c; Ranjbar et al. 2010d; Ranjbar et al. 2009a, 2010e)
Desynapsis
Desynapsis is considered as the precocious separation of bivalents in metaphase of meiosis I leading to the formation of varied degree of univalents. Desynapsis occurs either due to the action of recessive ds genes in a homozygous situation or early chiasma terminalization which may lead to the formation of meiocytes with double normal chromosome number. In several cases such univalents may have difficulty during anaphase I movement and become lagged, therefore producing aneuploid gametes causing reduction in pollen fertility of plants. However they may skip the first anaphase and form restitution nuclei resulting in the formation of unreduced gametes as reported in some other species (Veilleux Citation1985; Sheidai et al. Citation2007). Desynapsis was observed in one population from each of O. michauxii, O. sintenisii, O. kuchanensis, O. meshhedensis, O. ptolemaica, O. chorassanica and two populations of O. oshnaviyehensis and O. subnitens (Figures 16 and 20).
Table 2. Number of pollen mother cells (PMCs) analyzed and percentage of PMCs meiotic behavior in O. sect. Hymenobrychis.
Figures 1–9 Representative meiotic cells in different species of O. sect. Hymenobrychis: 1, 2. diakinesis and cytomixis in O. ptolemaica; 3. diakinesis in O. schahuensis; 4. diakinesis in O. kuchanensis; 5. diakinesis in O. meshhedensis; 6–9. diakinesis, bridge, tripolar cell and cytomixis in Mashhad population of O. chorassanica. Scale bar: 3 μm.
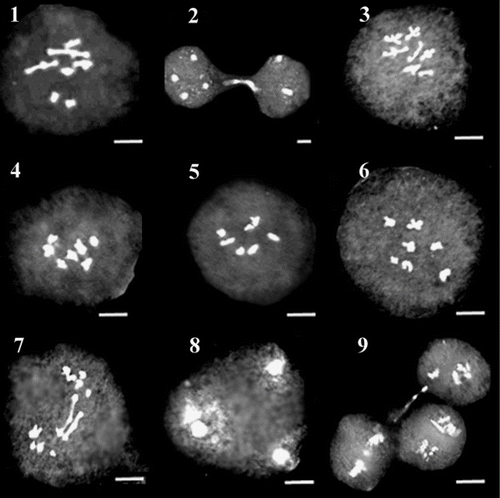
Figures 10–18 Representative meiotic cells in different species of O. sect. Hymenobrychis: 10, 11. diakinesis and precocious ascension in metaphase I cells in Quchan population of O. chorassanica; 12. diakinesis in Qidar population of O. subnitens; 13–16. diakinesis, stickiness, cytomixis and desynapsis in Kalibar population of O. subnitens; 17, 18. diakinesis and bridge in O. mazanderanica. Scale bar: 3 μm.
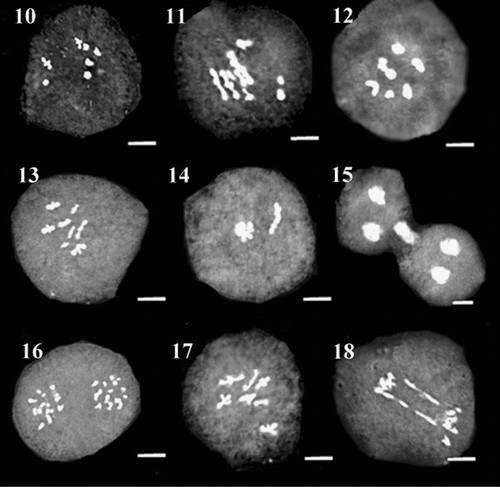
Figures 19–27 Representative meiotic cells in different species of O. sect. Hymenobrychis: 19, 20. diakinesis and desynapsis in Qotur population of O. michauxii; 21, 22. diakinesis and unreduced gamete in Ardebil population of O. michauxii; 23. diakinesis in O. sintenisii; 24–27. diakinesis, laggard, B-chromosome and micronucleus in O. oshnaviyehensis. Scale bar: 3 μm.
Micronucleus formation
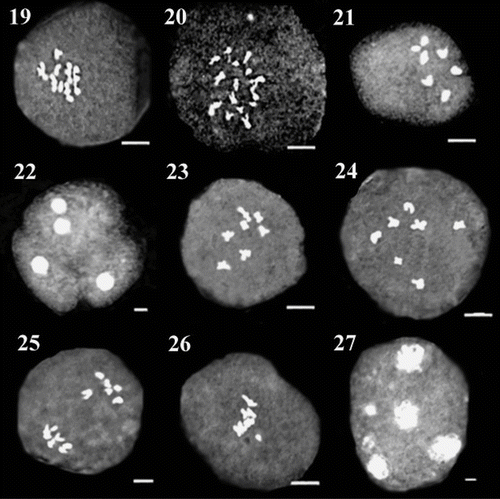
Micronucleus formation is another abnormality that was found in all populations of O. chorassanica, O. ptolemaica and O. sintenisii, three populations of O. subnitens and one population from each of O. oshnaviyehensis, O. schahuensis and O. meshhedensis (Figure 27). Chromosomes that produced micronuclei during meiosis were eliminated from microspores as microcytes. The micronucleus reached the microspore wall and formed a kind of bud, separated from the microspore. The eliminated microcytes gave origin to small and sterile pollen grains (Baptists-Giacomoelli et al. Citation2000).
B-chromosomes
B-chromosomes or accessory chromosomes, which occur in addition to the standard or A-chromosomes in some of the plants, are smaller than other chromosomes and do not form any association with them. B-chromosomes, when present in high numbers affect negatively the growth and vigor of the plants, while in low numbers may benefit the plant possessing them (Jones and Houben Citation2003). The evolution of tolerance could depend on several factors, including the fitness cost of tolerance itself. The evolution of incomplete tolerance would imply that B chromosome effects on fitness would be manifested only in individuals with high number of Bs. If drive is not suppressed, incomplete tolerance would simply imply a new equilibrium with higher number of B chromosomes per individual (Camacho et al. 2002). B-chromosomes were observed in all populations of O. oshnaviyehensis, O. ptolemaica and O. subnitens (except for one population), two populations of O. sintenisii and one population from each of O. meshhedensis, O. chorassanica and O. michauxii (Figure 26).
Chromosome stickiness and bridges
Sticky chromosomes were observed from early stages of prophase till the final stages of meiosis in all populations studied (Figure 14). Chromosome bridges resulting from stickiness were observed in most populations of O. oshnaviyehensis, O. ptolemaica and O. subnitens, two populations of O. michauxii and one population of other species (Figures 7 and 18). The thickness of bridges observed and the number of chromosomes involved in their formation varied among different meiocytes. Genetic as well as environmental factors have been considered as the reason for chromosome stickiness in different plant species (Nirmala and Rao Citation1996).
Laggard chromosomes
According to Nicklas and Ward (Citation1994), non-oriented bivalents may be related to impaired attachment of kinetochores to the spindle fibers. Pagliarini (Citation1990) reported that laggards may result from late chiasma terminalization. Ascending chromosomes are the result of precocious migration and, according to Utsunomiya et al. (Citation2002), generally consist of univalent chromosomes formed during late prophase stages by precocious chiasma terminalization in early metaphase I or may even result from low chiasma frequency or from the presence of asynaptic or desynaptic genes (Pagliarini Citation2000). Laggards and non-oriented chromosomes may produce micronuclei, if they fail to reach the poles in time to be included in the main telophase nucleus (Koduru and Rao Citation1981; Utsunomiya et al. 2002), leading to the formation of micro-pollen, and probably to gametes with unbalanced chromosome numbers (Mansuelli et al. Citation1995), such as aneuploids (Defani-Scoarize et al. Citation1995). Most populations of O. oshnaviyehensis and O. subnitens and only one population from each of O. meshhedensis, O. chorassanica, O. sintenisii and O. kuchanensis showed this abnormality (Figure 25). The laggards at this phase of division might have degenerated or may have resulted in the formation of polyads, particularly at the resting phase (Basi et al. Citation2006).
Precocious division of centromeres
During metaphase I the number of cells with univalents presenting precocious migration to the poles in one population of O. oshnaviyehensis, O. meshhedensis, O. subnitens, O. ptolemaica and O. schahuensis was high (Figure 11), while other populations did not show this abnormality. Because univalents usually do not suffer regular segregation in the first division, the frequency of univalents in diakinesis/metaphase I has been used as a standard measure of meiotic disturbances in plant species (Scoles and Kaltsikes Citation1974).
Cytomixis
The phenomenon of cytomixis consists in the migration of chromosomes between meiocytes through cytoplasmic connection. Since cytomixis creates variation in the chromosome number of the gametes, it could be considered a mechanism of evolutionary significance (Ghaffari Citation2006). This phenomenon was observed in some populations of O. oshnaviyehensis, O. meshhedensis, O. subnitens, O. ptolemaica, O. chorassanica, O. sintenisii, O. michauxii, O. kuchanensis and O. schahuensis in metaphase I, II, anaphase I and telophase II cells (Figures 2, 9 and 15).
Multipolar cells
Failure of chromosome movement occurred in one of the poles of cells in anaphase, leading to the formation of tripolar cells. Such cells produce normal reduced and unreduced daughter cells. This phenomenon was found in most populations (Figure 8). Such unreduced meiocytes may lead to the information of 2n pollen grains (Sheidai et al. Citation2007). The spindle apparatus is normally bipolar and acts as a single unit, playing a crucial role in chromosome alignment during metaphase. Any distortion or breakage in the spindle may result in random sub-grouping of the chromosome (Nirmala and Rao Citation1996). Pentapolar and hexapolar cells were observed in some populations of O. oshnaviyehensis, O. meshhedensis, O. subnitens, O. sintenisii, O. kuchanensis and O. schahuensis. Such cells may lead to the formation of abnormal tetrads and infertile pollen grains.
Unreduced gamete
Failure of chromosome movement occurred in one of the poles of cells in anaphase, leading to the formation of normal reduced and unreduced daughter cells. This phenomenon was found in some populations of O. sintenisii, O. kuchanensis, O. ptolemaica and O. michauxii in telophase II cells (Figure 22). Such unreduced meiocytes may lead to the formation of 2n pollen grains.
Intra- and interspecific variations of the chromosome number in O. sect. Hymenobrychis
The basic chromosome number in O. sect. Hymenobrychis is either x = 7 or x = 8 (Table ). The majority of the species studied here showed 2n = 14, while one population from each of O. michauxii and O. subnitens represented 2n = 16. Most of these recorded chromosome counts are the first published counts or confirmations for these species. With exception of the new counts, chromosome numbers represented here are in agreement with those from earlier reports on the section. However, differences in chromosome counts, or even in the basic chromosome numbers, have been reported earlier in O. sect. Hymenobrychis by other authors (Abou-el-Enain Citation2002; Hesamzadeh Hejazi and Ziaei Nasab 2010). Counts of 2n = 2x = 14 and 2n = 2x = 16 for O. hohenackeriana have been also recorded by Ranjbar et al. (2010c) and Hesamzadeh Hejazi and Ziaei Nasab (2010), respectively. The chromosome number 2n = 2x = 14 for four populations of O. sintenisii are consistent with previously published counts for this species (Hesamzadeh Hejazi and Ziaei Nasab 2010; Ranjbar et al. 2010c). Abou-el-Enain (2002) recorded 2n = 2x = 16 for O. ptolemaica, while we counted 2n = 2x = 14 in two populations of this species. Despite the chromosome number 2n = 2x = 14 for different populations of O. michauxii and O. subnitens, the Qidar population of O. michauxii and the Kalibar population of O. subnitens showed 2n = 2x = 16. According to Hughes (Citation1998), Cardoso et al. (Citation2000) and Abou-el-Enain (2002), and also from observations of the present study, it can be concluded that the recorded variation in the chromosome numbers in each of O. hohenackeriana (2n = 16 reported by Hesamzadeh Hejazi and Ziaei Nasab (2010) and 2n = 14 recorded in the present study), O. ptolemaica (2n = 16 reported by Abou-el-Enain (2002) and 2n = 14 recorded in the present study), O. michauxii and O. subnitens (2n = 14, 16 in the present study) can be referred to taxonomic differences within the section. On the other hand, such variation can demonstrate a complex evolutionary pattern within the section. Although a part of variation can be attributed to technical difficulties or even misidentifications, resulting in errors of counting or interpretation, nonetheless much of this variation appears to be real. The present study recorded 2n = 2x = 14 for different populations of O. kuchanensis, O. meshhedensis, O. oshnaviyehensis, O. chorassanica, O. mazanderanica and O. schahuensis (Figure 28).
Aneuploidy and polyploidy in the genus Onobrychis with special reference to O. sect. Hymenobrychis
Speciation by aneuploidy and polyploidy changes in the chromosome numbers is common in flowering plants and is an almost characteristic occurrence in angiosperms. Elegant examples of such evolutionary patterns have been exhibited in the genus Onobrychis. Chromosome numbers reveal intriguing evolutionary patterns that have occurred by aneuploidy and polyploidy. Although there are numerous examples of progressive loss of chromosomes in plants, the factors driving aneuploidy have been far less investigated than those of polyploidy. It is very likely that different degrees of polyploidy and aneuploidy in the genus Onobrychis are caused by various meiotic irregularities. For example, the origin of aneuploid cells is due to lagging chromosomes, unequal disjunction, chromatin bridges and multipolar anaphase spindles. Levin (Citation2002) stated that aneuploidy is generated by the addition or subtraction of a single chromosome, mostly by translocation. As was similarly considered by Grant (Citation1981), the aneuploidy in Onobrychis may be hypothesized as a series of unequal reciprocal translocations followed by the elimination of a chromosome without important genes. It seems that Onobrychis species experienced descending aneuploidy during their evolutionary history, especially within O. sect. Hymenobrychis. Increasingly, chromosome abnormalities such as desynapsis and laggard chromosome functions are indeed expected to accompany a change in chromosome number, and the number may have evolved by relatively drastic aneuploid reduction. Stebbins (Citation1950) reported that the presence of aneuploidy is related to the ancestors occupying and adapting to pioneer habitats in open or semiarid situations.
Contrary to the sections Onobrychis and Lophobrychis, polyploidy was not found in O. sect. Hymenobrychis, with exception of one count of O. bobrovii (2n = 4x = 28), which has been reported by Abou-el-Enain (2002). This is not surprising given the low percentage of polyploids (18%) in the Fabaceae (Goldblatt Citation1981b) and polyploidy apparently was early established in the evolution of the genus Onobrychis.
Chromosome number evolution
Chromosome numbers in the genus Onobrychis as a whole show considerable variation (Table ). The commonly reported haploid numbers are 7 and 8, with a widespread polyploid series of tetraploid being developed on x = 7 and rarely x = 8. There are only three previous reports on tetraploid numbers based on x = 8: the record of 2n = 4x = 32 in O. megataphros reported by Hosgoren (Citation2006) and those of 2n = 4x = 32 in O. caput-galli and O. pulchella by Abou-el-Enain (2002).
Table 3. List of previous chromosome counts made for the genus Onobrychis.
The observation of the present study as well as the available data on chromosome number in the genus Onobrychis indicates that, among the approximately 77 species with known chromosome counts, the diploid species, either annual or perennial, represent 49% of the whole, while the polyploids represent 51%. However, as pointed out above, polyploidy is probably an infrequent phenomenon in O. sect. Hymenobrychis. It should be pointed out that the highest percentage of polyploidy can be related to the sections Onobrychis (Hosgoren Citation2006; Ozturk et al. Citation2009; Ranjbar et al. 2009a, 2010e; Hesamzadeh Hejazi and Ziaei Nasab 2010) and Lophobrychis (Abou-el-Enain 2002), while the sections Heliobrychis and Hymenobrychis do not show polyploid series. As mention above, polyploidy was established early in the evolution of the genus Onobrychis. Moreover, by reviewing the chromosome numbers of four main sections of the genus (Table ), it can be concluded that the sections Hymenobrychis and Onobrychis have base numbers of x = 7 and x = 8, while the sections Heliobrychis and Lophobrychis (except one count for O. caput-galli (x = 7) reported by Abou-el-Enain (2002)) have base number of x = 8. The data summarized in Table certainly indicate that aneuploidy is a much more widespread occurrence than was earlier believed. Basic chromosome numbers and presence of polyploidy within the sections especially in O. sect. Onobrychis may suggest the occurrence of an extensive evolution by aneuploidy and polyploidy. So, it seems that natural hybridization probably plays a significant role in the evolution of this section, while in O. sect. Hymenobrychis, speciation mostly takes place by aneuploidy. Finding occasional plants in various populations with aneuploid chromosome numbers belonging to this section indicates that aneuploid gametes not only are produced, but actually functioned.
Although Abou-el-Enain (2002) in agreement with other authors related a decrease in chromosome number to a shift from perennial to annual habit, they revealed that x = 8 is associated with the annual species, while x = 7 is in species that are perennials. However, results from our study showed that perennial taxa represent more reduction in chromosome number annual ones. As mentioned above, the basic chromosome number of O. sect. Heliobrychis, as the largest section of the genus that possesses perennial species, is x = 8. On the other hand, a great number of species in this section and others show a basic chromosome number of x = 8 as perennial taxa.
If the variation in chromosome number is projected onto the cladogram recognized by Ahangarian et al. (Citation2007), it becomes clear that higher basic chromosome numbers (x = 8) are maintained in basal taxa of tribe Hedysareae such as Alhaji and Taverniera (Khatoon and Ali 2006), whereas smaller numbers (x = 7) are found in terminal genera such as Onobrychis. In this genus basal sections such as Dendrobrychis and Lophobrychis have x = 8 as the basic chromosome number, followed by section Onobrychis which has two basic chromosome number (x = 7 and x = 8) and polyploidy. Finally, infrequent polyploidy and x = 7 as the basic chromosome number of a great number of species of O. sect. Hymenobrychis confirm this section as a more progressive section in the genus Onobrychis. However, O. sect. Heliobrychis, as a sister group of O. sect. Hymenobrychis, is one of the evolved sections and represents x = 8, which we assume to be due to reversal evolution. As a result, in contrast with studies by Falistocco (1991) and Gomurgen (Citation1996) that argued evolution within the genus has occurred when the basic number increases from x = 7 to x = 8, results from our study are in agreement with Goldblatt (1981a), in which it was suggested that x = 8 is ancestral in the genus and the species with x = 7 are derived through aneuploid loss. Like polyploidy, aneuploidy is an evolutionary process producing variation in plants, and hence new and adaptable species that can colonize changing habitats, and new environments which may arise.
In general, cytological studies of the O. sect. Hymenobrychis species growing in Iran indicate that polyploidy is not a frequent phenomenon within the section and most species around two populations of O. michauxii (14494) and O. subnitens (13629) with 2n = 2x = 16 have the chromosome number 2n = 2x = 14, so species with x = 7 are derived through aneuploid loss. It can be assumed that these two taxa are likely less highly evolved and other taxa that possess x = 7 are the end results of a decreasing aneuploidy from an ancestral x = 8 (i.e. the more highly evolved ones). According to the result from this study and by reviewing the chromosome numbers in Onobrychis, we found that O. sect. Hymenobrychis has comparatively highly derived taxa and can be considered as a heterogeneous unit in the genus Onobrychis. Such data may be used in the taxonomy and phylogenetic consideration of the genus. It seems that, to shed further light on the origin of polyploid Onobrychis species, more complete cytogenetic and also molecular evidence will be necessary. In addition, more accessions should be cytogenetically studied and a directed analysis through genomic (GISH) and fluorescent in situ hybridization (FISH) would supply interesting and important information.
Acknowledgments
The great help of Dr. Vitek, Dr. Wallnofer, and Dr. Till during the first author’s visit to Vienna University of Economics and Business is much appreciated. This research has received financial support from the Bu-Ali-Sina University. We thank the Director of the Herbarium of Ferdowsi University of Mashhad (FUMH), and the Herbarium Research Centers of Natural Research and Animal Affairs of Isfahan, Kashan, Kerman, Mashhad, Semnan, Shiraz, Isfahan, Tabriz and Zahedan for making the herbarium facilities available for our study.
References
- Abou-el-Enian , MM . 2002 . Chromosomal criteria and their phylogenetic implications in the genus Onobrychis Mill. sect. Lophobrychis (Leguminosae), with special reference to Egyptian species . Bot J Linn Soc. , 139 ( 4 ) : 409 – 414 .
- Ahangarian , S , Kazempour Osaloo , SH and Maassoumi , AA . 2007 . Molecular phylogeny of the Hrdysareae with special reference to Onobrychis (Fabaceae) as inferred from nr DNA ITS sequences . Iran J Bot , 13 ( 2 ) : 64 – 74 .
- Aktoklu , E . 2001 . Two new varieties and a new record in Onobrychis from Turkey . Turk J Bot , 25 ( 5 ) : 359 – 363 .
- Arslan , E , Ertuğrul , K , Tugay , O and Dural , H . 2012 . Karyological studies of the genus Onobrychis Mill. and the related genera Hedysarum L and Sartoria Boiss. & Heldr. (Fabaceae, Hedysareae) from Turkey . Caryologia , 65 ( 1 ) : 11 – 17 .
- Ashurmetov , OA and Normatov , BA . 1998 . Embryology of annual species of the genus Onobrychis Mill . Flora , 193 ( 3 ) : 259 – 267 .
- Ball , PW . 1978 . “ Onobrychis Mill ” . In Flora Europaea , Edited by: Tutin , TG , Heywood , VH , Burges , NA , Moore , DM , Valentine , SM and Webb , DA . Vol. 2 , 187 – 191 . Cambridge , , (UK) : Cambridge University Press .
- Baltisberger , M . 1991 . IOPB chromosome data 3 . Int Organ Plant Bios Newsletter. , 17 : 5 – 7 .
- Baptists-Giacomoelli , FR , Pagliarini , MS and Almeida , JL . 2000 . Elimination of micronuclei from microspores in a Brazilian oat (Avena sativa L.) variety . Genet Mol Biol , 23 ( 3 ) : 681 – 684 .
- Basi , SL , Subedi , P and Adhikari , NR . 2006 . Cytogenetic effects of gamma rays on India rice radha-4 . J Inst Agric Anim Sci. , 27 : 25 – 36 .
- Boissier , E . 1872 . “ Onobrychis ” . In Flora Orientalis , Edited by: Boissier , E . Vol. 2 , 545 – 553 . Geneva : Basilease and Lugundi .
- Camacho , JPM , Bakkali , M. , Corral , JM , Cabrero , J , López-León , MD , Aranda , I , Martín-Alganza , A and Perfectti , F . 2002 . Host recombination is dependent on the degree of parasitism . Proc Biol Sci. , 269 ( 1505 ) : 2173 – 2177 .
- Cardoso , MB . 2000 . Taxonomic and evolutionary implications of intraspecific variability in chromosome numbers of species of Leucaena Benth. (Leguminosae) . Bot J Linn Soc. , 134 ( 4 ) : 549 – 556 .
- Defani-Scoarize , MA , Pagliarini , SM and Aguiar , CG . 1995 . Evaluation of meiotic behavior in double-cross maize hybrids and their parents . Maydica , 40 : 319 – 324 .
- Diaz-Lifante , Z , Luque , T and Santa-Barbara , C . 1992 . Chromosome numbers of plants collected during Iter Meditranium II in Israel . Bocconea , 3 : 229 – 250 .
- Duman , H and Vural , M . 1990 . New taxa from south Anatolia . Turk J Bot , 14 : 45 – 48 .
- Falistocco , E. 1991 . Chromosome study and genome relationships in perennial species of Onobrychis . J Genet Breed , 45 : 25 – 31 .
- Fedorov AA 1969. Chromosome numbers of flowering plants. In: Komarov VL, editor. Leningrad: Botanical Institute.
- Ghaffari , SM . 2006 . Occurrence of diploid and polyploidy microspores in Sorghum bicolor (Poaceae) is the result of cytomixis . Afr J Biotechnol. , 5 ( 16 ) : 1450 – 1453 .
- Goldblatt , P . 1981a . “ Cytology and the phylogeny of Leguminosae ” . In Advances in legume systematics, Part 2 , Edited by: Polhill , RM and Raven , PH . 427 – 463 . Kew , , (UK) : Royal Botanic Gardens .
- Goldblatt P 1981b. Index to plant chromosome numbers 1975–78. Monographs in Systematic Botany, Vol. 5. Saint Louis: Missouri Botanical Garden.
- Goldblatt P 1984. Index to plant chromosome numbers 1979–81. Monographs in Systematic Botany, Vol. 8. Saint Louis: Missouri Botanical Garden.
- Goldblatt P 1985. Index to plant chromosome numbers 1982–83. Monographs in Systematic Botany, Vol. 13. Saint Louis: Missouri Botanical Garden.
- Goldblatt P 1988. Index to plant chromosome numbers 1984–85. Monographs in Systematic Botany, Vol. 23. Saint Louis: Missouri Botanical Garden.
- Goldblatt P, Johnson DE 1991. Index to plant chromosome numbers 1988–89. Monographs in Systematic Botany, Vol. 40. Saint Louis: Missouri Botanical Garden.
- Gomurgen , AN . 1996 . Meiotic analysis of selected material of sainfoin and its progeny with branched and unbranched peduncles . Turk J Bot , 20 ( 5 ) : 399 – 411 .
- Grant , V . 1981 . Plant speciation , 2nd ed , New York , , (NY) : Columbia University Press .
- Hedge , IC . 1970 . “ Onobrychis ” . In Flora of Turkey and the Aegean Islands , Edited by: Davis , PH . Vol. 3 , 584 – 589 . Edinburgh : Edinburgh University Press .
- Hesamzadeh Hejazi , SM and Ziaei , Nasab M . 2010 . Cytotaxonomy of some Onobrychis (Fabaceae) species and populations in Iran . Caryologia , 63 ( 1 ) : 18 – 31 .
- Hosgoren , H. 2006 . Total numbers of chromosome number in species of Onobrychis Miller (Fabaceae) in southeastern Anatholia region . Biotechnol Biotec Eq , 20 ( 2 ) : 57 – 61 .
- Hughes C 1998. Monograph of Leucaena (Leguminosae-Mimosoideae). Systematic Botany Monographs, 55. The American Society of Plant Taxonomists. 244 pp, hardbound,
- Jones , N and Houben , A . 2003 . B-chromosomes in plant: escapees from the A-chromosome genome? . Trends Plant Sci , 8 ( 9 ) : 417 – 423 .
- Karshibaev HK 1992. Chromosome numbers of some Fabaceae in Uzbekistan. Tezisy 3 Soveshchanie Po Kariologii Rastenii. 27:1–2.
- Khatoon , S and Ali , S . 1991 . Chromosome numbers in subfamily Papilionoideae (Leguminosae) from Pakistan . Willdenowia , 20 ( 1–2 ) : 159 – 165 .
- Koduru , PRK and Rao , MK . 1981 . Cytogenetics of synaptic mutants in higher plants . Theor Appl Genet. , 59 ( 4 ) : 197 – 214 .
- Levin , DA . 2002 . The role of chromosomal change in plant evolution , Oxford , , (UK) : Oxford University Press .
- Lock , JM and Simpson , K . 1991 . Legumes of West Asia , Kew , , (UK) : Royal Botanic Gardens .
- Mabberley , DJ . 1997 . The plant book: a portable dictionary of the vascular plants , 2nd ed , Cambridge , , (UK) : Cambridge University Press .
- Mansuelli , RW , Tanimoto , EY , Brown , C and Comai , L . 1995 . Irregular meiosis in a somatic hybrid between Solanum bulbocastanum and S. tuberosum detected by species-specific PCR markers and cytological analysis . Theor Appl Genet. , 91 ( 3 ) : 491 – 408 .
- Mesicek , J and Sojak , J . 1992 . Chromosome numbers of Mongolia angiosperms . Preslia , 64 : 193 – 206 .
- Nicklas , RB and Ward , SC . 1994 . Elements of error correction in mitosis: microtubule capture, release and tension . Cell Biology , 126 ( 5 ) : 1241 – 1253 .
- Nirmala , A and Rao , PN . 1996 . Genetics of chromosome numerical mosaism in higher plants . The Nucleus , 39 : 151 – 175 .
- Ozturk , M , Martin , E , Dinc , M , Duran , A , Ozdemir , A and Cetin , O . 2009 . A cytogenetical study on some plants taxa in Nizip region (Aksaray, Turkey) . Turk J Biol. , 33 ( 1 ) : 35 – 44 .
- Pagliarini , MS . 1990 . Meiotic behavior and pollen fertility in Aptenia cordifolia (Aizoaceae) . Caryologia , 43 ( 2 ) : 157 – 162 .
- Pagliarini , MS . 2000 . Meiotic behavior of economically important plant species: the relationship between fertility and male sterility . Genet Mol Biol. , 23 ( 4 ) : 997 – 1002 .
- Ranjbar , M . 2009 . Onobrychis oshnaviyehensis sp. nov. (sect. Hymenobrychis, Fabaceae) from Iran . Nord J Bot , 27 : 1 – 5 .
- Ranjbar , M , Amirabadizadeh , H , Karamian , R and Ghahremani , MA . 2004 . Notes on Onobrychis sect. Heliobrychis (Fabaceae) in Iran . Willdenowia , 34 : 187 – 190 .
- Ranjbar , M , Hajmoradi , F and Karamain , R . 2011 . Meiotic chromosome number and behaviour of Onobrychis alborzensis (Fabaceae): a new species from northern Iran . Feddes Repertorium , 122 ( 7–8 ) : 1 – 12 .
- Ranjbar , M , Karamian , R , Hadadi , A and Joharchi , MR . 2012 . Taxonomic notes on Onobrychis sect. Onobrychis subsect. Macropterae (Fabaceae) from Iran . Phytotaxa , 39 : 51 – 60 .
- Ranjbar , M , Karamian , R and Hadadi , A . 2009a . Biosystematic study of Onobrychis vicifolia Scop. and Onobrychis altissima Grossh. (Fabaceae) in Iran . Iran J Bot. , 15 ( 1 ) : 85 – 95 .
- Ranjbar , M , Karamian , R and Hajmoradi , F . 2009b . Taxonomic notes on Onobrychis sect. Hymenobrychis (Fabaceae, Hedysareae) in Iran . Novon , 19 ( 2 ) : 215 – 218 .
- Ranjbar , M , Tolui Z , Karamian R and Amirabadizadeh , H . 2007 . Onobrychis assadii (Fabaceae), a new species from Iran . Ann Bot Fenn. , 44 ( 6 ) : 481 – 484 .
- Ranjbar , M , Karamian , R and Vitek , E . 2010a . Onobrychis bakuensis (Fabaceae), a new species from Azerbaijan . Ann Bot Fenn. , 47 ( 3 ) : 233 – 236 .
- Ranjbar , M , Karamian , R and Vitek , E . 2010b . Notes on Onobrychis sect. Hymenobrychis (Fabaceae) in Tajikistan, with the description of a new species . Nord J Bot , 28 ( 2 ) : 182 – 185 .
- Ranjbar , M , Hajmoradi , F and Karamian , R . 2010c . Mitotic study of some species of Onobrychis sect. Hymenobrychis DC. in Iran . Iran J Plant Biol , 1 ( 1–2 ) : 47 – 54 .
- Ranjbar , M , Karamian , R and Afsari , S . 2010d . Meiotic chromosome number and behaviour of Onobrychis avajensis (Fabaceae): a new species from western Iran . Plant Ecol Evol. , 143 ( 2 ) : 170 – 175 .
- Ranjbar , M , Karamian , R and Hadadi , A . 2010e . Cytosysatematics of three Onobrychis species (Fabaceae) in Iran . Caryologia , 63 ( 3 ) : 237 – 249 .
- Ranjbar M, Karamian R, Hajmoradi F. 2010f. Chromosome number and meiotic behaviour of two populations of Onobrychis chorassanica Bunge (O. sect. Hymenobrychis) in Iran. J Cell Mol Res. 2(1):49–55.
- Rechinger KH 1984. Onobrychis. In: Rechinger KH, editor. Flora Iranica, Vol. 157. Akademische Druck-u.-Verlagsanstalt, Graz. p. 389–459.
- Romano , S , Mazzola , P and Raimondo , FM . 1987 . Numeri cromosomici per la flora Italiana . Inform Bot Italiano , 19 : 173 – 180 .
- Scoles , GJ and Kaltsikes , PJ . 1974 . The cytology and cytogenetics of Triticale . Z Pflanzenzuchtg , 73 : 13 – 43 .
- Sheidai , M , Sottodeh , M and Akbarei , B . 2007 . Cytogenetic variability in several oil seed rape cultivars . Pakistan J Biol Sci. , 10 ( 4 ) : 553 – 560 .
- Sirjaev , G . 1925 . Onobrychis generis revisio critica. Publications of the Faculty of Science . University of Masaryk , 56 : 96 – 97 .
- Slavivk , B , Jarolivmovav , V and Chrtek , J . 1993 . Chromosome counts of some plants from Cyprus . Candolle , 48 : 221 – 230 .
- Stebbins , GL . 1950 . Variation and evolution in plants , New York , (NY) : Columbia University Press .
- Tamas , E . 2006 . Cytological aspects of the Onobrychis genus . Bul USAMV , 62 : 154 – 158 .
- Toluei Z, Atri M, Ranjbar M, Wink M 2012. Morphological, genetical and ecogeographical characterization of long-winged species of Onobrychis sect. Onobrychis (Fabaceae) in Iran. Iran J Bot. 18(1):31–41.
- Toluei , Z , Atri , M , Ranjbar , M and Wink , M . 2013 . Iranian Onobrychis carduchorum (Fabaceae) populations: morphology, ecology and phylogeography . Plant Ecol Evol. , 146 ( 1 ) : 1 – 15 .
- Utsunomiya KS, Bione NCP, Pagliarini MS 2002. How many different kinds of meiotic abnormalities could be found in a unique endogamous maize plant? Cytologia. 67(2):169–176.
- Veilleux , RE . 1985 . Diploid and polyploid gametes in crop plants: Mechanisms of formation and utilization in plant breeding . Plant Breed Rev. , 3 : 253 – 288 .
- Yakovlev , GP , Sytin , AK and Roskov , YR . 1996 . Legumes of Northern Eurasia, a check-list , Kew , , (UK) : Royal Botanic Gardens .
- Yildiz , B , Ciplak , B and Aktoklu , E . 1999 . Fruit morphology of sections of the genus Onobrychis Miller (Fabaceae) and its phylogenetic implications . Israel J Plant Sci. , 47 ( 4 ) : 269 – 282 .