Abstract
Polysomaty is the occurrence of cells with different ploidy levels in the same organ or tissue and there are records of this in root-tip cells of some Mimosa species. The objective of the present work was to verify the occurrence, possible cause and significance of polysomaty in a range of species of this genus. In root-tip cells of 68 accessions of 43 diploid and tetraploid Mimosa species, the percentage of polysomatic cells ranged from 5% to 87%. Pretreatment with the antimitotic paradichlorobenzene did not cause polysomaty but increased it in some accessions. When relating seedling root size and percentage of polysomatic cells, for most of the species a higher percentage of polysomatic cells was found in roots from 6 to 10 cm length. For M. scabrella (“bracatinga”), a comparative analysis of seedling root-tip cells and root-tip cells from well-developed plants kept in pots in a greenhouse showed that polysomaty occurred only in the seedling root-tips. The data suggest that, in Mimosa, polysomaty occurs only for a short period of the seedling development, probably as a natural mechanism to accelerate its development and establishment.
Introduction
Mimosa L. (Fabaceae-Mimosoideae) comprises around 540 species, 500 of which are distributed in the Neotropics and circa 40 in the Old World (Simon et al. Citation2011). In the Americas there around 461 species distributed from the USA to Argentina and Uruguay (Barneby Citation1991). The Brazilian “cerrado” is a biodiversity spot where a quarter of all Mimosa species are found (Simon & Proença Citation2000). Many species are economically important, such as M. scabrella Benth. (“bracatinga”) (Figure 1), cultivated for several purposes as building, carpentry, cellulose, fuel and shade in coffee plantations. Other species are used as living fences, in the cosmetic industry and traditional folk medicine (Barneby Citation1991).
Polysomaty refers to the occurrence of cells with different ploidy levels in the same organ or tissue (Smulders et al. Citation1994) and it is the consequence of repeated DNA synthesis cycles but no cell division, resulting in cells with higher chromosome numbers (Nagl Citation1976). The term endoreduplication is used as a general term encompassing all kinds of cell cycles without division, one of them being endopolyploidy (Lee et al. Citation2010). Endoreduplication may lead to chromosomes with multiple chromatids or to cells with higher chromosome numbers (D’Amato Citation1984; Joubes & Chevalier Citation2000), which may lead to polysomaty. Endoreduplication is the most common mode of polyploidization in many plant tissues and is estimated to occur in more than 90% of angiosperms in different tissues and organs (D’Amato Citation1984), but endopolyploidization ratio may vary among species and even among individuals of the same species or ecotype.
During differentiation most active cells need a certain amount of DNA, therefore endoreduplication is an evolutionary strategy to replace a lack of phylogenetic increase in nuclear DNA (Nagl Citation1976). It is generally assumed that polyploidization amplifies desirable genes that are necessary to synthesize products in specific tissues, without spending time and energy in cell division (Galbraith et al. Citation1991; Leitch Citation2000). Polysomaty may be important in accelerating plant growth and also in the physiological functions of a given cell, as cells with a larger volume would save time during tissue development, (Castro et al. Citation2007), as well as presenting a way for organisms to raise functional gene copy number within each cell (Galbraith et al. Citation1991).
It is known that endoreduplication results in bigger cells but the molecular basis of the process is still not clearly understood. Despite several studies, the mechanisms and signals that are necessary to change from a classic cell cycle to endoreduplication, and to initiate and maintain the endocycles, as well as the physiological significance of endoreduplication are not well known. It is not clear if endoreduplication is genetically programmed or a consequence of differentiation (Kondorosi & Kondorosi Citation2004; Chevalier et al. Citation2011). Environmental factors such as light, nutrition, temperature and hormones may influence endoreduplication and the degree of endopolyploidization and should be further studied (Barow Citation2006; Jovtchev et al. Citation2007; Chen et al. Citation2011).
According to Nagl (Citation1976), there is a negative correlation between genome size and polysomaty, to maintain the functional and regulatory state of certain cells of the species. More recent studies have shown that polysomaty has a strong correlation with phylogenetic position, a weaker one with life strategy and a slightly negative correlation with genome size. Natural polyploids have higher endopolyploidy levels than artificial ones (Jovtchev et al. Citation2007).
Polysomaty in plants is a phenomenon that occurs mainly during cell differentiation and expansion, chiefly, but not only, in highly specialized cell types such as vascular elements, endosperm storage cells and embryo suspensors (Nagl Citation1976; Joubes & Chevalier Citation2000). In several species polysomaty has been described in several organs and different developmental stages, e.g. in Arabidopsis (DC.) Heynh epidermal tissue (Melaragno et al. Citation1993), Anchusa capensis Thunb. and Spinacia oleracea L. primary roots (Nero-Buffalino & Witkus Citation1984); Cucumis sativus seedlings and plants (Gilissen et al. Citation1993) and seeds (Rewers et al. Citation2009).; Brassica oleracea L. seedling development (Kudo & Kimura Citation2001); Polygala vayredae Costa and P. calcarea F. W. Schultz endosperm, leaves and petals (Castro et al. Citation2007); Beta vulgaris L. in vitro culture seedlings (Sliwinska & Lukaszewska Citation2005; Lukaszewska & Sliwinska Citation2007); Sorghum bicolor L. endosperm (Kladnik et al. Citation2006); Solanum tuberosum L. callus from leaf in vitro culture (Pijnacker Citation1989); Bulbophyllum auricomum Lindl root-tips (Than et al. Citation2011) and Phalaenopsis aphrodite subsp. formosana protocorms, leaves, roots and flowers (Chen et al. Citation2011), among others.
Berger et al. (Citation1958), during cytotaxonomic studies in several Leguminosae, even proposed a characterization of the subfamilies based on the frequency of polysomatic cells compared to the usual ones. According to this study, in the Papilionoideae this phenomenon would be less frequent than in the Mimosoideae and the Caesalpinoideae.
In Mimosa, polysomaty has been reported only in root-tip cells of some species by Witkus & Berger (Citation1947), Seijo (Citation1993, Citation1999), and Olkoski & Schifino-Wittmann (2011). Dahmer et al. (Citation2011) reported root-tip polysomaty in 26.5% of the 125 Mimosa accessions analyzed.
Given the complexity of the subject, this study was not designed to investigate the mechanisms or exact role of polysomaty, but to report its occurrence among a large number of Mimosa species and accessions, and also to verify a possible influence of antimitotics, root size and plant development stage on this phenomenon.
Material and methods
Plant material
A list of all Mimosa species and accessions (seed lots) examined are listed in Table . Original chromosome numbers of these accessions had been determined by Dahmer et al. (Citation2011). Taxonomic vouchers are kept at the following herbaria: FHO (Oxford University, UK), K (Kew – Royal Botanic Gardens, UK), CEN (Embrapa Recursos Genéticos e Biotecnologia, Brazil), UB (Universidade de Brasília, Brazil), RB (Jardim Botânico do Rio de Janeiro, Brazil), UAMIZ (Universidad Autónoma Metropolitana, México) and ICN (Departamento de Botânica Universidade Federal do Rio Grande do Sul, Brazil).
Table 1. Identification of 68 Mimosa species and accessions analyzed, place of collection, chromosome number (after Dahmer et al. Citation2011) and percentages of polysomatic cells after PDB treatment.
Cytological studies
Chromosome counts were performed in root-tip cells as described in Dahmer et al. (Citation2011): seeds were scarified by a small cut in the testa and germinated in Petri dishes with moist filter paper (Figure 2). When the roots were 1 cm long they were pretreated with a saturated solution of paradichlorobenzene for 24 h at 4°C, fixed in 6:3:1 (ethanol: chloroform: acetic acid) for 24 h and stored in 70% alcohol below 0°C until required. Slides were prepared by hydrolyzing the roots with 1 N HCl at 60°C for 8–10 min, which were then stained by Feulgen for 2–3 h (sometimes followed by a 2% pectinase treatment for 2 min) and squashed in propionic carmine. Cells with good chromosome spreading (generally over 50 per plant, with few exceptions) and at equivalent contraction were analyzed per plant. The best cells were photographed and/or registered by a digital image capturing system.
To verify if pretreatment could cause or influence the occurrence of polysomatic cells, 10 species were randomly selected (Table ) and chromosome number was determined in root-tip cells with and without pretreatment with Paradichlorobenzene (PDB). As in slides without pretreatment, the exact number of chromosomes could not be determined as they were not completely separated, the percentage of polysomatic cells was determined visually by the size of the chromosome clusters in the metaphase nuclei, as studies show that there was a positive correlation between nuclei size and ploidy level (or DNA content) (D’Amato Citation1984; Jovtchev et al. Citation2006).
To verify a possible relation between seedling root size and polysomaty, for 23 seed-lots of 22 randomly selected species (Table ), chromosome numbers in cells of root tips of 1–5 mm, 6–10 mm and 11–15 mm were determined.
To verify if polysomaty also occurred in roots of adult plants, a comparison of chromosome numbers in seedling root-tip cells and root-tip cells of well-grown potted plants of five M. scabrella accessions, kept in a greenhouse, was performed.
Results and discussion
Polysomaty ranged from 5& to 87% among the 68 accessions of 43 Mimosa species, 34 of which were diploid (2n = 2x = 26), seven tetraploid (2n = 4x = 52) and two with both diploid and tetraploid accessions (Table , ).
Figure 2 Mitotic metaphases of Mimosa paposa (a diploid species) with (A) 26 chromosomes; (B) 52 chromosomes; (C) 104 chromosomes. Scale bar 10 μm.
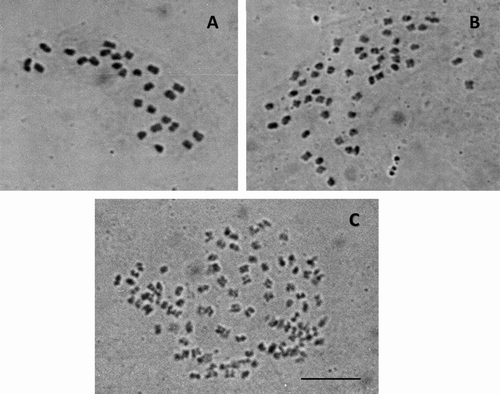
Figure 3 Mitotic metaphases in Mimosa biucifera (a tetraploid species) with (A) 52 chromosomes and (B) 104 chromosomes. Scale bar 10 μm.
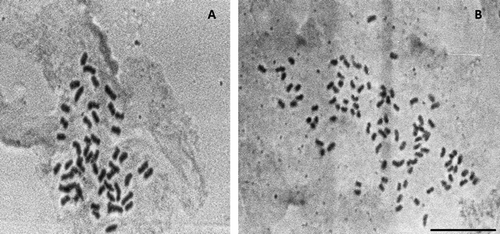
Figure 4 Mitotic metaphases in Mimosa incana (a tetraploid species) showing a 52-chromosome cell and a polysomatic 104-chromosome cell side-by-side in the same slide. Scale bar 10 μm.
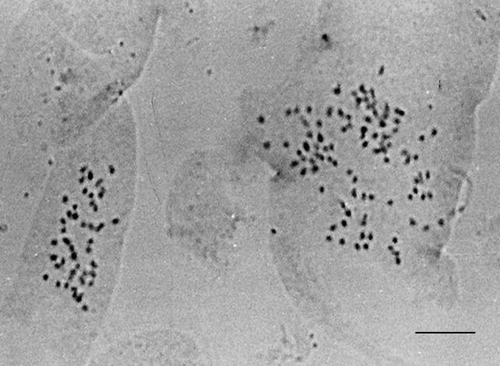
In the naturally diploid species and accessions, most of the polysomatic cells were tetraploid, with 2n = 52 chromosomes, but hexaploid and octaploid cells (with 2n = 78 and 2n = 104 chromosomes), and in three accessions cells with 2n = 39 chromosomes were also found (Table , Figure ) in varying percentages. In the naturally tetraploid species and accessions, the polysomatic cells were generally octaploid (with 2n = 104 chromosomes) but also hexaploid (2n = 78 chromosomes) (Table , ) in varying percentages.
No relation was found between a lower or higher degree of polysomaty and the natural ploidy level of the species (Table ), but in some cases different accessions of the same species had different percentages of polysomatic cells, e.g. M. foliolosa (Simon, M.F. 663) (15% of tetraploid cells and 18% of octaploid cells) and M. foliolosa (Simon, M.F. 321) (41% of tetraploid cells); M. diplotricha (Simon, M.F. 304) (7% of tetraploid cells) and M. diplotricha (Simon, M.F. 877) (28% of tetraploid cells); and M. orthocarpa (Simon, M.F. 855) (9% of tetraploid cells) and M. orthocarpa (Grether, R. 2907) (18% of tetraploid cells).
A very interesting point is that, for some accessions of some species, diploid or tetraploid, such as M. borealis, two accessions of M. dutrae, M. dysocarpa, M. lacerata, one accession of M. scabrella and one of M. somnians, the percentage of polysomatic cells was higher than the percentage of cells with the normal ploidy level (Table ). Seijo (Citation1999) also reported, for some diploid Mimosa species, a higher number of polysomatic than diploid cells.
Antimitotics, e.g. colchicine, paradichlorobenzene and hydroxyquinolein, are normally used to impair the achromatic spindle function, therefore spreading the chromosomes in the cells, making chromosome counting and karyotyping possible.
In the present work we compared root-tip cells of untreated seedlings and root-tip cells of seedlings treated with paradichlorobenzene, and found that PDB did not cause polysomaty (Table ) but increased the frequency of polysomatic cells in 80% of the accessions analyzed.
Table 2. Percentage of polysomatic cells with and without PDB pretreatment in Mimosa species.
There were differences in percentages of polysomatic cells in seedling roots of different sizes (Table ). For M. adenocarpa, M. diplotricha, M. foliolosa, M. orthocarpa, M. polyantha, M. polycarpa var. subandina, M. pudica, M. pudica var. hispida and M. setosissima polysomatic cells were found only in roots of 1–5 mm. Seijo (Citation1993), for M. glanduliseta, M. diplotricha var diplotricha, M somnians subsp. somnians, M. polycarpa var. spegazzini, M paupera, M. dolens subsp. rigida, M dolens subsp. acerba and M. debilis reported polysomaty in seedling roots of 4 mm. In M. pudica, Witkus & Berger (Citation1947) observed only diploid cells when the seedling roots were 1 mm in length, diploid and tetraploid cells in roots of 4 mm and again only diploid cells in roots of 10 mm, showing that in M. pudica polysomaty is restricted to a specific root size, probably reflecting a specific developmental stage.
Table 3. Percentage of polysomatic cells in different seedling root sizes of Mimosa species.
For M. apodocarpa, M. artemisiana, M. borealis, M. claussenii, M. echinocaula, M. goldmanii, M. heringeri, M. lacerata, M. luisana, M. monancistra, M. papposa var. papposa, M. pigra var. dehiscens, M. scabrella and M. somnians var. viscida (Table ) higher percentages of polysomatic cells were found in seedling roots of 6–10 mm, smaller varying percentages of polysomatic cells in roots of 11–15 mm and no polysomatic cells in roots of 1–5 mm.
When comparing occurrence of polysomaty in seedlings and grown plants of M. scabrella (a tetraploid species with 2n = 4x = 52) (Figure. ), it was verified that polysomatic cells with 2n = 78 and 2n = 104 occurred only in seedling roots. Polysomaty was totally absent in roots of well-grown plants. Similar results were found by Olkoski & Schifino-Wittmann (2011) in M. bimucronata Kuntze, in which polysomaty was restricted to seedling roots, being absent in roots of grown plants and in pollen mother cells.
Figure 1 (A) Grown plants of Mimosa scabrella in the greenhouse; (B) detail of a grown plant with well-developed roots and (C) seedlings.

Therefore, the results presented here clearly suggest that in Mimosa polysomaty is restricted to a short period of seedling development, immediately after seed germination, and is related to cell growth and elongation, probably as a mechanism to accelerate seedling growth and establishment. Increasing DNA content could be an advantage for the plant’s establishment in an unfavorable environment. Many of the species studied in this paper are from the Brazilian cerrado, a habitat with long dry periods (four to five months per year), and others inhabit stony and sandy habitats (Simon & Proença Citation2000).
Acknowledgments
Thanks to Dr. Marcelo Simon, EMBRAPA, Brazil, for providing most of the seed-lots. Also to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil, for financial support.
References
- Barneby , R . 1991 . Sensitivae censitae: a description of the genus Mimosa Linnaeus (Mimosaceae) in the New World . Mem New York Botan G , 65 : 1 – 835 .
- Barow , M . 2006 . Endopolyploidy in seed plants . BioEssays , 28 : 271 – 281 .
- Berger , CA , Witkus , ER and McMahon , RM . 1958 . Cytotaxonomic studies in Leguminosae . B Torrey Bot Club , 85 : 405 – 410 .
- Castro , S , Loureiro , J , Rodrigues , E , Silveira , P , Navarro , L and Santos , C . 2007 . Evaluation of polysomaty and estimation of genome size in Polygala vayredae and P. calcarea using flow cytometry . Plant Sci , 172 : 1131 – 1137 .
- Chen , WH , Tang , CY , Lin , TY , Weng , YC and Kao , YL . 2011 . Changes in the endopolyploidy pattern of different tissues in diploid and tetraploid Phalaenopsis aphrodite subsp. formosana (Orchidaceae) . Plant Sci , 181 : 31 – 38 .
- Chevalier , C , Nafati , M , Mathieu-Rivet , E , Bourdon , M , Frangne , N , Cheniclet , C , Renaundin , JP , Gevaudant , F and Hermould , M . 2011 . Elucidating the functional role of endoreduplication in tomato fruit development . Ann Bot , 107 : 1159 – 1169 .
- Dahmer , N , Simon , MF , Schifino-Wittmann , MT , Hughes , CE , Miotto , STS and Giuliani , JC . 2011 . Chromosome numbers in the genus Mimosa L.: Cytotaxonomic and evolutionary implications . Plant Syst Evol , 291 : 211 – 220 .
- D’Amato , F . 1984 . “ Role of polyploidy in reproductive organs and tissues ” . In Embriology of endosperms , Edited by: Johri , BM . 519 – 566 . New York : Springer .
- Galbraith , DW , Harkins , KR and Knapp , S . 1991 . Systemic endopolyploidy in Arabidopsis thaliana . Plant Physiol , 96 : 985 – 989 .
- Gilissen , LW , Van , MJS , Creemers-Molenaar , J and Verhoeven , HA . 1993 . Development of polysomaty in seedlings and plants of Cucumis sativus L . Plant Sci , 91 : 171 – 179 .
- Joubes , J and Chevalier , C . 2000 . Endoreduplication in higher plants . Plant Mol Biol , 43 : 735 – 745 .
- Jovtchev , G , Barow , M , Meister , A and Schubert , I . 2007 . Impact of environmental and endogenous factors on endopolyploidization in angiosperms . Env Exp Bot , 60 : 404 – 411 .
- Jovtchev , G , Schubert , V , Meister , A , Barow , M and Schubert , I . 2006 . Nuclear DNA content and nuclear and cell volume are positively correlated in angiosperms . Cytogenet Genome Res , 114 : 77 – 82 .
- Kladnik , A , Chourey , PS , Pring , DR and Dermastia , M . 2006 . Development of the endosperm of Sorghum bicolor during the endoreduplication-associated growth phase . J Cereal Sci , 43 : 209 – 215 .
- Kondorosi , E and Kondorosi , A . 2004 . Endoreduplication and activation of the anaphase-promoting complex during symbiotic cell development . FEBS Lett , 567 : 152 – 157 .
- Kudo , N and Kimura , Y . 2001 . Patterns of endopolyploidy during seedling development in cabbage (Brassica oleracea L.) . Ann Bot , 87 : 275 – 281 .
- Lee , HO , Davidson , JM and Duronio , RJ . 2010 . Endoreduplication: polyploidy with purpose . Genes Dev , 23 : 2461 – 2477 .
- Leitch , AR . 2000 . Higher levels of organization in the interphase nucleos of cycling and differentiated cells . Microbiol Mol Biol Rev , 64 : 138 – 152 .
- Lukaszewska , E and Sliwinska , E . 2007 . Most organs of sugar-beet (Beta vulgaris L.) plants at the vegetative and reproductive stages of development are polysomatic . Sex Plant Reprod , 20 : 99 – 107 .
- Melaragno , JE , Mehrotra , B and Coleman , AW . 1993 . Relationship between endopolyploidy and cell size in epidermal tissue of Arabidopsis . Plant Cell , 5 : 1661 – 1668 .
- Nagl , W . 1976 . DNA endoreduplication and polyteny understood as evolutionary strategies . Nature , 261 : 614 – 615 .
- Nero-Buffalino , DL and Witkus , R . 1984 . The first appearance of polyploidy nuclei in primary roots of two diploid angiosperms . Ann Bot , 53 : 53 – 58 .
- Olkoski , D and Schifino-Wittmann , MT . 2011 . Cytogenetics of Mimosa bimucronata (DC.) O. Kuntze (Mimosoideae, Leguminosae): chromosome number, polysomaty and meiosis . Crop Breed Appl Biot , 11 : 27 – 35 .
- Pijnacker , LP . 1989 . Flow cytometry and karyological analysis of polysomaty and polyploidization during cell callus formation from leaf segments of various potato genotypes . Theor Appl Genet , 77 : 102 – 110 .
- Rewers , M , Sadowski , J and Sliwnska , E . 2009 . Endoreduplication in cucumber (Cucumis sativus) seeds during development, after processing and storage, and during germination . Ann Appl Biol , 155 : 431 – 438 .
- Seijo , G . 1993 . Números cromosómicos en especies argentinas del género Mimosa (Leguminosae) . Bol Soc Argentina Bot , 29 : 219 – 223 .
- Seijo , G . 1999 . Chromosome studies in Argentinean species of Mimosa . Cytologia , 64 : 241 – 246 .
- Simon , MF , Grether , R , Queiroz , LP , Särkinen , TE , Dutra , VF and Hughes , CE . 2011 . The evolutionary history of Mimosa (Leguminosae): toward a phylogeny of the sensitive plants . Am J Bot , 98 : 1201 – 1221 .
- Simon , MF and Proença , C . 2000 . Phytogeographic patterns of Mimosa (Mimosoidae, Leguminosae) in the Cerrado biome of Brazil: an indicator genus of high-altitude centers of endemism? . Biol Cons , 96 : 279 – 296 .
- Sliwinska , E and Lukaszewska , E . 2005 . Polysomaty in growing in vitro sugar-beet (Beta vulgaris L.) seedlings of different ploidy level . Plant Sci , 168 : 1067 – 1074 .
- Smulders , MJM , Rus-Kortekaas , W and Gilissen , LJW . 1994 . Development of polysomaty during differentiation in diploid and tetraploid tomato (Lycopersicon esculentum) plants . Plant Sci , 97 : 53 – 60 .
- Than , MMM , Pal , A and Jha , S . 2011 . Chromosome number and modal karyotype in a polysomatic endangered orchid, Bulbophyllum auricomum Lindl., the Royal Flower of Myanmar . Plant Syst Evol , 294 : 167 – 175 .
- Witkus , ER and Berger , CA . 1947 . Polyploid mitosis in the normal development of Mimosa pudica . Bull Torrey Bot Club , 74 : 179 – 182 .