Abstract
Individuals of Astyanax bockmanni from one population belonging to the Paranapanema River basin, southeastern Brazil, were cytogenetically examined. In the present paper, we report a diploid number of 50 chromosomes, with some individuals bearing additional chromosomes, characterized as different variants of B chromosomes. The B microchromosome of acrocentric morphology (Ba) was the most frequent type in this population (77.7%). Two other variants were identified as metacentric B chromosomes, but of distinct sizes, characterized as macrochromosome (BM) and microchromosome (Bm). Conspicuous heterochromatic blocks were observed in the terminal regions of some chromosomes of the standard karyotype and partially heterochromatic blocks were detected in all B chromosomes. 18S ribosomal RNA genes were distributed in the terminal region of several chromosomes, but were not located on the B chromosomes.
Introduction
Astyanax comprises more than 100 valid species and is one of the most specious incertae sedis genera recognized within the family Characidae (Marinho and Lima Citation2009). Several species of the genus Astyanax have already been genetically studied, mainly with a cytogenetic focus. Astyanax species show a high level of chromosomal polymorphism, with a great variety of diploid and triploid karyotypes, polymorphism of heterochromatin, nucleolus organizer region (NOR) diversity, and location of histone and ribosomal genes (Moreira-Filho and Bertollo Citation1991; Oliveira et al. Citation2009; Hashimoto et al. Citation2011; Machado et al. Citation2012).
Moreover, Astyanax species show a high variation of B chromosomes, which is the core issue of several studies in this fish group. B chromosomes are characterized by great diversity in Astyanax scabripinnis, with variations in size, morphology, DNA composition, and frequency, which can be related to altitude, sex, or environmental pollution (Porto-Foresti et al. Citation1997; Mestriner et al. Citation2000; Néo et al. Citation2000). Several approaches have been undertaken to make sense of the complex and variable nature of A. scabripinnis, which is characterized as a “species complex” (Moreira-Filho and Bertollo 1991), and is currently composed of 15 valid species (Bertaco and Lucena Citation2006).
Some studies have shown a stable diploid number of 50 chromosomes for individuals of Astyanax bockmanni. However, some populations of A. bockmanni can demonstrate a high inter- and intra-individual variability of the NOR phenotypes, as well as an extensive polymorphism in the heterochromatin distribution (Kavalco et al. Citation2009; Fernandes et al. Citation2010; Hashimoto and Porto-Foresti Citation2010).
The present study reports the cytogenetic characterization of A. bockmanni individuals bearing B chromosome variants, which yielded data for the inference of the possible origin of these extra chromosomes in the genome of A. bockmanni.
Materials and methods
Cytogenetic analyses were performed on chromosome preparations obtained from 46 individuals (22 females and 24 males) of A. bockmanni belonging to the population from the Alambari River (22°27′ 6.12′′ S, 49°14′ 25.76′′ W), Paranapanema River basin, southeastern Brazil. The fishes were identified according to Vari and Castro (Citation2007), and the voucher specimens were stored in the fish collection of the Laboratório de Genética de Peixes, UNESP, Bauru, SP, Brazil. Chromosome preparations were obtained from gill and kidney tissues using the technique described by Foresti et al. (Citation1981). Silver staining followed the technique of Howell and Black (Citation1980), and C-banding was performed according to Sumner (Citation1972).
Fluorescent in situ hybridization (FISH) was performed using the method described by Yang et al. (Citation1999). Sequences of the 18S ribosomal DNA (rDNA) were isolated according to Hatanaka and Galetti (Citation2004). For the preparation of the probes, PCR products of 18S rDNA were labeled during the secondary PCR by incorporating the nucleotide tetramethyl-rhodamine-5-dUTP (Roche).Chromosomes were counterstained with DAPI(4′,6-diamidino-2-phenylindole, Vector Laboratories). FISH images were captured and processed using the CytoVision Genus system (Applied Imaging, USA) and a Cohu CCD camera mounted on an Olympus BX-60 microscope.
Results and discussion
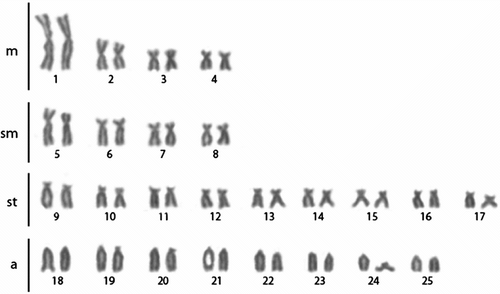
Our results showed a diploid number equal to 50 chromosomes and a karyotype formula composed of 8m + 8sm + 18st + 16a (fundamental number = 84), in both sexes (Figure ). These data corroborate previous information about the diploid chromosome number and karyotype characteristics reported by Hashimoto et al. (Citation2011). In contrast to the species complexes Astyanax fasciatus and Astyanax scabripinnis, which show variable diploid chromosome numbers in different individuals living in sympatry (Moreira-Filho and Bertollo 1991; Artoni, Shibatta et al. Citation2006, Machado et al. Citation2012), cytogenetic studies in some populations of A. bockmanni demonstrated a stable 2n (Kavalco et al. Citation2009; Fernandes et al. Citation2010; Hashimoto and Porto-Foresti Citation2010).
Figure 1 Giemsa-stained karyotype showing 2n = 50 chromosomes of one individual of Astyanax bockmanni from the Alambari River, southeastern Brazil.
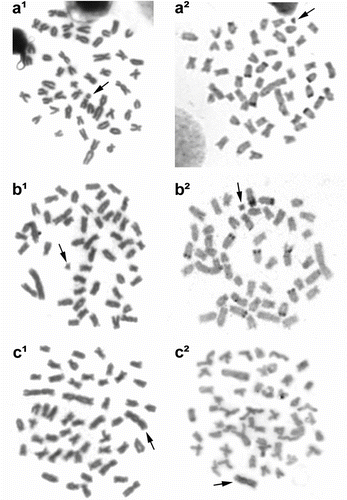
Our data indicate the occurrence of nine individuals bearing extra chromosomes, characterized as B chromosomes, which demonstrated variations in morphology, size, and frequency. Three different B chromosomes were observed (Table and Figure ). The most frequent B chromosome variant is an acrocentric chromosome (Ba) of small size, similar to the smallest chromosomes belonging to the complement A (Table ). Two other B chromosome variants were classified as metacentric, but showed distinct sizes, i.e. a macrochromosome (BM) similar in size to chromosome pair 1 and a microchromosome (Bm) similar in size to Ba (Table and Figure ).
Table 1. Relation of different individuals bearing B chromosome variants in Astyanax bockmanni from the population of the Alambari River, southeastern Brazil.
Figure 2 Metaphases from different specimens bearing B chromosome variants in Astyanax bockmanni. (a) Individual bearing the Ba chromosome; (b) specimen carrying the Bm chromosome; and (c) individual bearing the BM chromosome. (1) Metaphases by conventional Giemsa staining; and (2) metaphases after C-banding technique. Arrows indicate the B chromosomes.
Distinct B chromosomes have been described in several species of the genus Astyanax, and the most frequent variant is the metacentric macrochromosome (BM) similar to chromosome pair 1, which might be considered the ancestral B chromosome with probably an early origin preceding the species differentiation (Moreira-Filho et al. Citation2001; Vicari et al. Citation2011; Machado et al. Citation2012).
Other variants represent rare occurrences, such as a submetacentric similar in size to the BM, a medium metacentric and an acrocentric smaller than the BM, and B microchromosomes (for a review, see Moreira-Filho et al. Citation2004). The secondary forms of B chromosomes probably originated from chromosome rearrangements, such as pericentric inversion and deletion (Moreira-Filho et al. Citation2004). In our study, a BM variant similar to those described for Astyanax species was also observed, but its frequency was lower than that found in the Ba variant. Similar data exhibiting the occasional presence of B chromosomes of different heterochromatin morphologies and patterns were analyzed by Machado et al. (Citation2012).
In A. scabripinnis, structural and functional evidence suggest that BM corresponds to an isochromosome derived from an acrocentric chromosome of the standard karyotype (Mestriner et al. Citation2000; Vicari et al. Citation2011; Machado et al. Citation2012). However, according to the isochromosomes origin, the centromere misdivision of an acrocentric chromosome ancestor and subsequent chromatid non-disjunction may simultaneously originate B microchromosomes and BM variants, an event that was not found in any population of A. scabripinnis (Moreira-Filho et al. Citation2004).
Conversely, our results showed the occurrence of BM and B microchromosome variants together in the same population, which supports the isochromosome origin of the B chromosomes in A. bockmanni. The distinct morphologies between the microchromosomes (Ba and Bm) found herein may be ascribable to structural rearrangements, as it occurs in the B chromosome variants of the fish Prochilodus lineatus (Artoni, Vicari et al. Citation2006).
The analysis of constitutive heterochromatin patterns showed heterochromatic blocks in the pericentromeric regions of most chromosomes and evident signals in the terminal regions of some chromosomes (Figure ), similar to those found in other populations of A. bockmanni (Kavalco et al. Citation2009; Hashimoto and Porto-Foresti Citation2010). In relation to the B chromosomes, all three morphological variants (BM, Bm, and Ba) were identified as heterochromatic (Figure ), in accordance with a common characteristic reported for B chromosomes in the genus Astyanax (Néo et al. Citation2000; Moreira-Filho et al. Citation2001; Hashimoto et al. Citation2008; Machado et al. Citation2012).
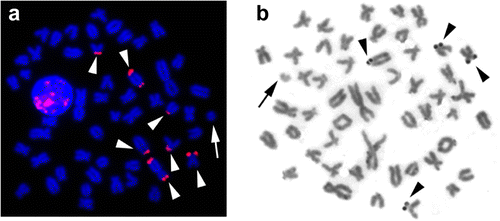
FISH signals of the 18S rDNA probe were detected in the terminal regions of several chromosomes, while the silver staining showed active NORs in only four chromosomes (Figure ), according to the polymorphism frequently observed in different species of Astyanax (Kavalco et al. Citation2009). B chromosomes in several species of plants (Camacho Citation2005) and animals, including fish species (Poletto et al. Citation2010), carry rRNA genes. However, in the present study, nucleolar activity or rRNA genes were not found in the B chromosome variants of A. bockmanni.
Figure 3 (Colour online) Metaphases showing the chromosome location of (a) 18S rRNA genes by FISH; and (b) nucleolar regions detected by silver staining. Arrows indicate the B chromosomes. Arrowheads show chromosomes bearing (a) 18S rDNA clusters and (b) NORs.
Several species of Astyanax present populations bearing B chromosomes in the genome of particular individuals, but most of them represent sporadic cases (Moreira-Filho et al. Citation2004). According to Hashimoto and Porto-Foresti (Citation2010), A. bockmanni shows a high degree of chromosome similarity to the species complex A. scabripinnis. Thus, B chromosomes also appear as an interesting feature that can be fixed in other populations of A. bockmanni, not representing a sporadic event. Although few populations of A. bockmanni have been cytogenetically analyzed, the occurrence of three morphologically variant B chromosomes demonstrates that extensive studies in this species are essential to improve the knowledge of the chromosome diversification in this fish group.
Acknowledgments
The authors wish to thank Fernando C.P. D’Agosta and Ricardo M.C. Castro for the taxonomical identification. This work was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP (Proc. 2010/06919-8, 2011/15007-5) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
References
- Artoni , RF , Shibatta , OA , Gross , MC , Schneider , CH , Almeida , MC , Vicari , MR and Bertollo , LAC . 2006 . Astyanax aff. fasciatus Cuvier, 1819 (Teleostei; Characidae): evidences of a species complex in the upper rio Tibagi Basin (Paraná, Brazil) . Neotrop Ichthyol , 4 : 197 – 202 .
- Artoni , RF , Vicari , MR , Endler , AL , Cavallaro , ZI , Jesus , CM , Almeida , MC , Moreira-Filho , O and Bertollo , LAC . 2006 . Banding pattern of A and B chromosomes of Prochilodus lineatus (Characiformes, Prochilodontidae), with comments on B chromosomes evolution . Genetica , 127 : 277 – 284 .
- Bertaco , VA and Lucena , CAS . 2006 . Two new species of Astyanax (Ostariophysi, Characiformes, Characidae) from eastern Brazil, with a synopsis of the Astyanax scabripinniso species complex . Neotrop Ichthyol , 4 : 53 – 60 .
- Camacho JPM. 2005. B chromosomes. In Gregory TR, editor. The evolution of the genome. San Diego: Elsevier.
- Fernandes CA, Martins-Santos IC. 2005. Sympatric occurrence of three cytotypes and four morphological types of B chromosomes of Astyanax scabripinnis (Pisces, Characiformes) in the River Ivaí Basin, state of Parana, Brazil. Genetica. 124:301–306.
- Fernandes , CA , Piscor , D , Bailly , D , Silva , VFB and Martins-Santos , IC . 2010 . Cytogenetic studies comparing three Characidae fish species from the Iguatemi River Basin Brazil . Cytologia , 75 : 329 – 333 .
- Foresti , F , Almeida-Toledo , LF and Toledo-Filho , SA . 1981 . Polymorphic nature of nucleolus organizer regions in fishes . Cytogenet Cell Genet , 31 : 137 – 144 .
- Hashimoto , DT , Ferguson-Smith , M , Rens , W , Foresti , F and Porto-Foresti , F . 2011 . Chromosome mapping of H1 histone and 5S rRNA gene clusters in three species of Astyanax (Teleostei, Characiformes) . Cytogenet Genome Rese , 134 : 64 – 71 .
- Hashimoto , DT , Gonçalves , VR , Bortolozzi , J , Foresti , F and Porto-Foresti , F . 2008 . First report of a B chromosome in a natural population of Astyanax altiparanae (Characiformes, Characidae) . Genet Mol Biol , 31 : 275 – 278 .
- Hashimoto , DT and Porto-Foresti , F . 2010 . Chromosome polymorphism of heterochromatin and nucleolar regions in two populations of the fish Astyanax bockmanni (Teleostei: Characiformes) . Neotrop Ichthyol , 8 : 861 – 866 .
- Hatanaka , T and Galetti , PM Jr . 2004 . Mapping of the 18S and 5S ribosomal RNA genes in the fish Prochilodus argenteus Agassiz, 1829 (Characiformes, Prochilodontidae) . Genetica , 122 : 239 – 244 .
- Howell , WM and Black , DA . 1980 . Controlled silver-staining of nucleolus organizer regions with a protective colloidal developer: a 1-step method . Experientia , 36 : 1014 – 1015 .
- Kavalco , KF , Pazza , R and Almeida-Toledo , LF . 2009 . Astyanax bockmanni Vari and Castro, 2007, an ambiguous karyotype in the Astyanax genus . Genetica , 136 : 135 – 139 .
- Machado NS, Ferreira Neto M, Bakkali M, Vicari MR, Artoni RF, Oliveira C, Foresti F 2012. Natural triploidy and B chromosomes in Astyanax scabripinnis (Characiformes, Characidae): a new occurrence, Caryologia. Int J Cytol, Cytosyst Cytogenet. 65(1):40–46. doi: 10.1590/S1984-46702012000200008.
- Marinho , MMF and Lima , FCT . 2009 . Astyanax ajuricaba: a new species from the Amazon basin in Brazil (Characiformes: Characidae) . Neotrop Ichthyol , 7 : 169 – 174 .
- Mestriner , CA , Galetti , PM Jr , Valentini , SR , Ruiz , IRG , Abel , LDS , Moreira-Filho , O and Camacho , JPM . 2000 . Structural and functional evidence that a B chromosome in the characid fish Astyanax scabripinnis is an isochromosome . Heredity , 85 : 1 – 9 .
- Moreira-Filho , O and Bertolo , LAC . 1991 . Astyanax scabripinnis (Pisces Characidade): a species complex Brazil . J Genet , 14 : 331 – 357 .
- Moreira-Filho , O , Fenochio , AS , Pastori , MC and Bertollo , LAC . 2001 . Occurrence of a metacentric macrochromosome B in different species of the genus Astyanax (Pisces, Characidae, Tetragonopterinae) . Cytologia , 66 : 59 – 64 .
- Moreira-Filho , O , Galetti , PM Jr and Bertollo , LAC . 2004 . B chromosomes in the fish Astyanax scabripinnis (Characidae, Tetragonopterinae): An overview in natural populations . Cytogenet Genome Res , 106 : 230 – 234 .
- Néo , DM , Bertollo , LAC and Moreira-Filho , O . 2000 . Morphological differentiation and possible origin of B chromosomes in natural Brazilian population of Astyanax scabripinnis (Pisces, Characidae) . Genetica , 108 : 211 – 215 .
- Oliveira , C , Foresti , F and Hilsdorf , AWS . 2009 . Genetics of neotropical fish: from chromosomes to populations . Fish Physiol Biochemist , 35 : 81 – 100 .
- Poletto AB, Ferreira IA, Martins C 2010. The B chromosomes of the African cichlid fish Haplochromis obliquidens harbor 18S rRNA gene copies. BMC Genet. 11. doi: 10.1186/1471-2156-11-1.
- Porto-Foresti , F , Oliveira , C , Maistro , EL and Foresti , F . 1997 . Estimated frequency of B-chromosomes and population density of Astyanax scabripinnis paranae in a small stream Brazil . J Genet , 20 : 377 – 380 .
- Sumner , AT . 1972 . A simple technique for demonstrating centromeric heterochromatin . Exp Cell Res , 74 : 304 – 306 .
- Vari , RP and Castro , RMC . 2007 . New species of Astyanax (Ostariophysi: Characiformes: Characidae) from the Upper Rio Paraná System Brazil . Copeia , 1 : 150 – 162 .
- Vicari , MR , Pistune , HFM , Castro , JP , Almeida , MC , Bertollo , LAC , Moreira-Filho , O , Camacho , JPM and Artoni , RF . 2011 . New insights on the origin of B chromosomes in Astyanax scabripinnis obtained by chromosome painting and FISH . Genetica. , 139 : 1073 – 1081 . doi: 10.1007/s10709-011-9611-z
- Yang , F , O’Brien , PC , Milne , BS , Graphodatsky , AS , Solanky , N , Trifonov , V , Rens , W , Sargan , D and Ferguson-Smith , MA . 1999 . A complete comparative chromosome map for the dog, red fox, and human and its integration with canine genetic maps . Genomics , 62 : 189 – 202 .