Abstract
This study aimed at comparative karyotype analysis and measurement of the nuclear DNA amount in giant miscanthus, Miscanthus × giganteus, and its hypothetical ancestors: eulalia grass (M. sacchariflorus) and porcupine grass (M. sinensis). The triploid chromosome number 2n = 57 in M. × giganteus and the diploid chromosome number 2n = 38 in the other species were confirmed. In the karyotype of giant miscanthus three satellite chromosomes were observed, whereas in M. sinensis there were two and in M. sacchariflorus probably four chromosomes of this type. In the first species 1–4 B-chromosomes were evidenced. The highest proportion of the C-banding/DAPI (4’,6-diamidino-2-phenylindole) positive heterochromatin was found in M. sinensis cv. Gracillimus, and the least in the M. sinensis M07 genome. The banding patterns observed in M. × giganteus resembled those observed in M. sacchariflorus. The 2C nuclear DNA content in M. × giganteus was 7.47 pg and in M. sacchariflorus it was 5.14 pg; two analysed lines of M. sinensis differed in 2C DNA value (5.18 pg and 5.49 pg).
Introduction
It has been more than half a century since the first attempts to use giant miscanthus as a bioenergy plant were made in Denmark. Even earlier, in the 1930s the first species from the genus Miscanthus was imported to Europe as ornamental plants. Giant miscanthus – Miscanthus × giganteus Greef and Deuter ex Hod., Renvoize (Hodkinson and Renvoize Citation2001) is a triploid species 2n = 3x = 57. Nowadays it is considered one of the most important bioenergy crops (Chou Citation2009). Its advantages include fast growth, a natural ability to condense vegetative shoots, which reduces agrotechnical works to minimum, and sterility, which guarantees maintenance of crops within areas designated for them. Furthermore, it is characterised by the ability to reallocate minerals and other nutrients. This is why during the period of the most effective exploitation of a plantation, lasting up to 20 years, only minimum doses of fertilisation are used. Biomass production in giant miscanthus is close to the theoretical maximum value for accessible solar energy (Beale et al. Citation1996). For a plant in which C4 photosynthesis occurs, giant miscanthus grows excellently in a moderate climate (Papini and Simeone Citation2010), maintaining photosynthetic activity in temperatures 6°C lower than the minimum temperature for maize (Wang et al. Citation2008). Because reproduction of this form is not efficient at temperatures below 20°C, studies on its micropropagation have been conducted (Płażek and Dubert Citation2010). More recently, detailed embryological studies aimed at finding the barriers that prevent sexual seed production in M. × giganteus have been presented by Słomka et al. (Citation2012). Disturbances affecting pollen grain viability observed by authors were similar to these observed in Pennisetum hybrids (Paiva et al. Citation2012).
The most often recorded basic chromosome number in the genus Miscanthus is 19 (Adati and Shiotani Citation1962). From the area of Africa and the Himalayas species possessing a different basic chromosome number (x = 15 and x = 10) have been reported, although, due to morphological and molecular distinction, their affiliation to the genus Miscanthus has been questioned (Hodkinson, Chase, Lledó et al. Citation2002). Chromosomes in this genus, like in other taxa from the tribe Andropogoneae, are small in comparison with the majority of other grasses – they are 1–2 μm long.
High basic chromosome number (x = 19) in Miscanthus has most probably a secondary origin: it is a result of hybridisation between ancestors possessing x = 9 and x = 10 chromosomes (Adati and Shiotani Citation1962). Previous cytotaxonomic and morphological studies of M. × giganteus and other representatives of this genus suggest that the species derives from eulalia grass (Miscanthus sacchariflorus (Maxim.) Hack) and porcupine grass (Miscanthus sinensis Anderss.) (Adati Citation1958; Linde-Laursen Citation1993). The two species coexist in Japan, which is probably the country where giant miscanthus originated (Nishiwaki et al. Citation2011). The discussed genus groups species of different ploidy levels (from 2x to 6x); however, the majority of them are diploid or tetraploid plants (Clifton-Brown and Lewandowski Citation2000). M. sinensis is assumed to be generally diploid (Lafferty and Lelley Citation1994), yet within the taxon polyploid cytotypes have been recorded – both natural and artificially induced (Clifton-Brown and Lewandowski Citation2000), for example, the triploid form Miscanthus sinensis cv. Goliath. M. sacchariflorus is usually tetraploid, although the whole range of ploidy characteristic of the genus Miscanthus has been found in it (Hodkinson et al. Citation1997). According to most publications, the chromosome number M. × giganteus is 2n = 57 (Deuter [date unknown] [Internet]). It was only Linde-Laursen (Citation1993) who reported 2n = 58 chromosomes. This author also described the occurrence of 1–4 B-chromosomes, the number of which was variable within specimen. Chromosomes B were also observed in Miscanthus floridulus by Price (Citation1963).
For determination of the origin of triploid giant miscanthus, comparative studies of meiotic chromosomes proved useful. In meiosis, in the autotriploid cytotype Miscanthus sinensis var. condensatus a high number of trivalents was observed, whereas in M. × giganteus almost equal numbers of bivalents, trivalents and univalents were recorded. During anaphase the occurrence of numerous delayed chromosomes was detected. This suggests that it is an allotriploid with two genomes of high homology and one genome showing a lower level of homology in comparison with the two others (Linde-Laursen Citation1993; Lafferty and Lelley Citation1994).
Recently, some research on giant miscanthus and other related species was carried out by molecular methods (Hodkinson, Chase and Renvoize Citation2002; Hodkinson, Chase, Takahashi et al. Citation2002). The studies confirmed the previous suppositions suggesting that Miscanthus × giganteus was an allotriploid derived from M. sinensis and M. saccharifolius and showed that its mother form was M. saccharifolius. However, DNA analyses did not allow establishment of which of the parent genomes occurred in a single and which in a double dose. Neither was the problem solved with the use of in situ hybridization (fluorescence in situ hybridization – FISH with rDNA probe, genomic in situ hybridization – GISH), which proved ineffective in differentiating the genomes which constitute the karyotype of M. × giganteus (Hodkinson, Chase, Takahashi et al. Citation2002).
The data concerning the karyotype structure of giant miscanthus and contribution of parent genomes are vital in the context of attempts at its resynthesis made in order to create improved cultivars. The research aimed at comparison of the karyotype structure, amount of heterochromatin and the genome size in this species and hypothetical parent species: eulalia grass (M. sacchariflorus) and porcupine grass (M. sinensis).
Materials and methods
We studied one genotype of M. × giganteus, one genotype of M. sacchariflorus and two genotypes of M. sinensis. Three of the genotypes coming from Polish husbandry had been cultivated in the Department of Plant Physiology, University of Agriculture in Krakow. The fourth genotype was obtained from a seed bank in the USA (Table ).
Table 1. Sources of the research material.
During cytological and cytometric analyses 11 specimens of each genotype were investigated. Chromosomes were conventionally stained with acetic orceine (Golczyk and Joachimiak Citation2003) and differentially by the C-banding/DAPI method (Grabowska-Joachimiak et al. Citation2011). For each genotype 10 orceine-stained and five C-banded metaphase plates were analysed. The conventional karyotype analysis in the three studied species was based on the following parametres: karyotype length, monoploid genome length, mean chromosome length and karyotype formula. Additionally, we used the two coefficients proposed by Paszko (Citation2006) and Peruzzi et al. (Citation2009, Citation2011) in order to compare the karyotype asymmetry (AI) among the studied genotypes: coefficient of variation of chromosome length (CVCL) and coefficient of variation of centromeric index (CVCI).
Chromosome observations were made using a Nikon Eclipse E800 microscope and the images were captured and processed with a Nikon DS-2MBWc camera and the NIS Elements software.
Flow cytometric measurements of the nuclear DNA content were performed in nuclei of juvenile leaves stained with propidium iodide (PI; 50 μg ml–1), using a CyFlow Green flow cytometer (Partec GmbH, Münster, Germany). Pisum sativum cv. Set (2C = 9.11 pg; Sliwinska et al. Citation2005) was used as an internal standard. The samples were prepared and analysed according to previously described methodology (Klos et al. Citation2009). Nuclear DNA content was calculated using the linear relationship between the ratios of the 2C peak position of Miscanthus/Pisum on the histogram of fluorescence intensities.
Results
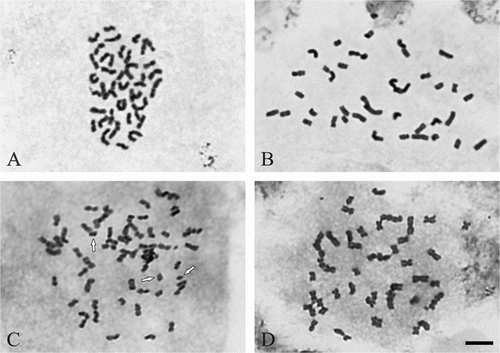
In M. × giganteus triploid chromosome number 2n = 57 was established (Figure C). Moreover, numerous metaphases with incomplete chromosome number (ranging from 31 to 56) were noted (Figure D). In some metaphase plates 1–4 B-chromosomes were detected (Figure C), as well as smaller objects (chromosomal bodies), which were probably acentric fragments of chromosomes. The genotypes of the other forms, i.e. M. sacchariflurus M115, M. sinensis M07 and M. sinensis cv. Gracillimus were characterised by the diploid chromosome number 2n = 38 (Figure A–B). In the case of M. sinensis M07 the diploid chromosome number did not agree with the information from the cultivator, according to which it was supposed to be a triploid with the chromosome number 2n = 57. In all the analysed forms the mitotic aberrations (chromosome bridges) were observed.
Figure 1 Metaphase chromosomes in three Miscanthus genotypes, staining with acetic orceine. (A) M. sinensis M07 (2n = 2x = 38). (B) M. sacchariflorus M115 (2n = 2x = 38). (C) M. × giganteus (2n = 3x = 57 + 3B). (D) M × giganteus 2n = 46. B-chromosomes arrowed. Scale bar = 5 μm.
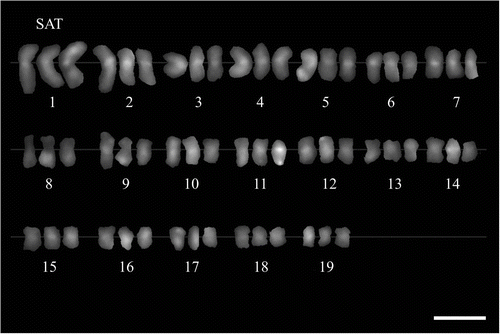
M. × giganteus showed a symmetrical karyotype containing metacentric and submetacentric chromosomes in the ratio c.5:1. In M. sacchariflorus M115, M. sinensis M07 and M. sinensis cv. Gracillimus metacentric and submetacentric chromosomes occurred in the ratio close to 3:1. The karyotype asymmetry in M. sacchariflorus was significantly higher than in the other forms (Table ). The mean karyotype length in M. × giganteus was 126.21 μm, in M. sacchariflorus M115 40.04 μm, in M. sinensis cv. Gracillimus 39.46 μm, and in M. sinensis M07 54.40 μm (Table ). In the chromosome complement of giant miscanthus three satellite (SAT) chromosomes were detected (Figures 2D, 3); two of them had the secondary constriction on the long arm, and one had it on the short arm. In both forms of M. sinensis the occurrence of two SAT chromosomes was recorded (Figures 2A–B, 4). In M. sacchariflorus probably two pairs of morphologically different satellite chromosomes occurred (Figures 2C, 5).
Table 2. Karyotype structure in four studied Miscanthus genotypes.
Figure 2 Metaphase chromosomes in four Miscanthus genotypes, C-banding/DAPI staining. (A) M. sinensis M07 (2n = 38). (B) M. sinensis cv. Gracillimus (2n = 38). (C) M. sacchariflorus M115 (2n = 38). (D) M. × giganteus (2n = 57). Satellite chromosomes (SAT) arrowed. Scale bar = 5 μm.
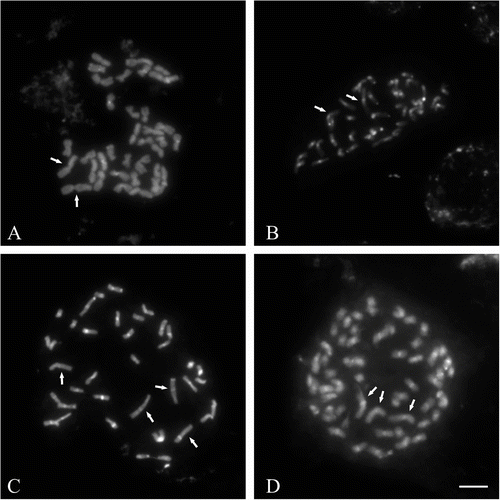
Figure 4 Karyograms of two Miscanthus sinensis genotypes (2n = 38), C-banding/DAPI staining. (A) M. sinensis M07. (B) M. sinensis cv. Gracillimus. Satellite chromosomes (SAT) indicated. Scale bar = 5 μm.
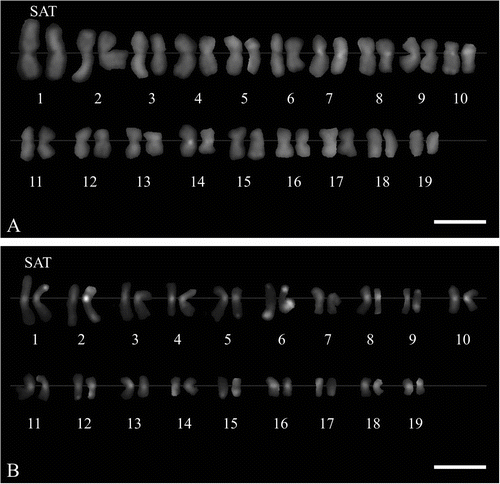
Figure 5 Karyogram of Miscanthus sacchariflorus M115 genotype (2n = 38), C-banding/DAPI staining. Satellite chromosomes (SAT) indicated. Scale bar = 5 μm.
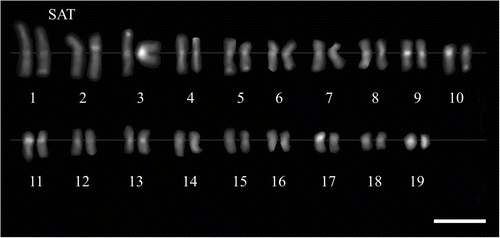
Figure 3 Karyogram of Miscanthus × giganteus (2n = 57), C-banding/DAPI staining. Satellite chromosomes (SAT) indicated. Scale bar = 5 μm.
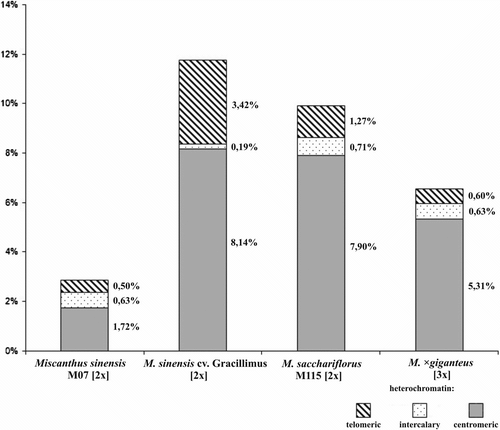
The analysis made on the basis of C-banding/DAPI method revealed mainly centromeric type of heterochromatin distribution in all the species (Figure 3–5). Contribution of heterochromatin to the karyotype of M. sacchariflorus was 9.98%, whereas in M. × giganteus it was 6.54%. A particularly large difference in heterochromatin content was recorded between M. sinensis cv. Gracillimus and M. sinensis M07 (11.75% and 2.85%, respectively) (Figure ). M. sacchariflorus and M. sinensis var Gracillimus, which possess the largest amount of heterochromatin, showed a similar amount of centromeric heterochromatin (7.90% and 8.14%); they differed, however, with respect to the amount of heterochromatin located in other areas (1.98% versus 3.61%). As far as the number and location of DAPI-positive bands are concerned, the karyotype of M. × giganteus was the most similar to the karyotype of M. sacchariflorus.
The DNA measurements confirmed the ploidy levels of the analysed species established in karyological studies (Figure , Table ). Interestingly, there was 8% difference in the nuclear DNA amount in two analysed varieties of porcupine grass (M. sinensis M07, 2C = 5.18 pg and M. sinensis var. Gracillimus, 2C = 5.49 pg). Statistical analysis revealed significant differences in the mean genome size between these two forms and lack of such differences between genotypes M07 of M. sinensis and M115 of M. sacchariflorus (Table ).
Figure 7 DNA histograms of nuclear preparation from leaves of four Miscanthus genotypes. (A) M. sinensis M07 (2n = 38). (B) M. sinensis cv. Gracillimus (2n = 38). (C) M. sacchariflorus M115 (2n = 38). (D) M. × giganteus (2n = 57). 1: peak G0/G1 of Miscanthus; 2: peak G0/G1 of internal standard (P. sativum, 9.11 pg/2C).
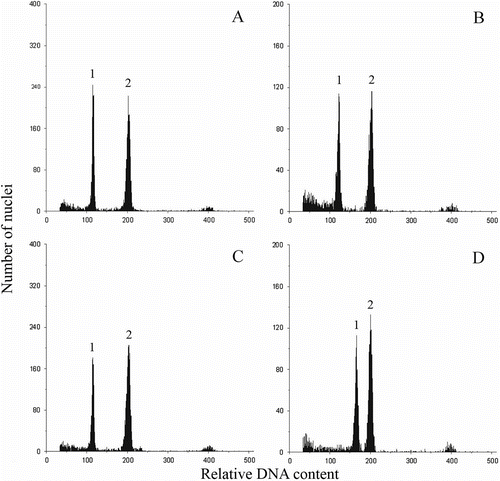
Table 3. Statistical analysis of the nuclear DNA amount of four Miscanthus genotypes.
Discussion
The results of karyotype analysis after conventional chromosome staining point to a close relation between giant miscanthus and its hypothetical parent species and a lack of major chromosome rearrangements in the analysed hybrid. Obtained data concerning the morphology of chromosomes in M. × giganteus are in agreement with the results presented by Lafferty and Lelley (Citation1994) who established that only meta- and submetacentrics occurred in its chromosome complement. The occurrence of acrocentric chromosomes, described for this species by Linde-Laursen (Citation1993), was not confirmed. The presence of aneuploid metaphase plates may indicate chromosome instability in this triploid, which occurs at least in the cells of root apical meristem. A similar phenomenon was also observed in some other polyploid species (Cieślak et al. Citation2000; Joachimiak et al. Citation2001).
The presence of small chromosomal bodies in giant miscanthus was reported only by Linde-Laursen (Citation1993). These Feulgen-positive structures were interpreted by this author as B-chromosomes, although their size (0.7 μm) “prevented a safe identification of a centromeric constriction”. During the present research the occurrence of both typical B-chromosomes (with well identifiable centromeres) and small chromosomal bodies was recorded. The latter were most probably artefacts which appeared as a result of mechanical fragmentation of chromosomes or cell nuclei. The origin of B-chromosomes is still unresolved question, but they must originate from chromosomes/sequences existing within the basic A chromosome set (Jones Citation2012). In the other three studied Miscanthus genotypes belonging to hypothetical parent species, stable diploid chromosome number was established, although even in them occasional aberrations of mitotic divisions in form of chromosome bridges were detected.
The observations revealing the occurrence of three satellite chromosomes in the karyotype of M. × giganteus are in agreement with research carried out by the FISH technique, which showed the presence of three rDNA loci in this species (Hodkinson, Chase, Takahashi et al. Citation2002) A lower number of such chromosomes was established by Linde-Laursen (Citation1993) and Lafferty and Lelley (Citation1994) who used a conventional method of staining. Furthermore, the probable presence of an extra pair of satellite chromosomes established by us in the diploid M. sacchariflorus M115 genotype may be an effect of its origin. According to the cultivator, this line represents a hybrid between Miscanthus sacchariflorus cv. Robustus and M. sinensis. On the other hand, the presence of two pairs of morphologically different SAT chromosomes in the M115 genotype may also be connected with the hybrid origin of the basic species M. saccharifolius. Some authors suggest that many plants described under this name are diploids with one genome from M. sinensis and one from an unidentified species (see discussion in Hodkinson et al. Citation2002c).
GISH technique used by some researchers in order to investigate parent genomes of giant miscanthus did not bring the expected results (Hodkinson, Chase, Takahashi et al. Citation2002). Although this technique seems to be optimal for detection of parent genomes in hybrid forms, it does not always prove effective for species with very closely related genomes, in which repetitive sequences have only slightly evolved. Our banding studies did not bring an explicit answer to the question which of the hypothetical parent species contributed two genomes and which contributed one. However, comparison of heterochromatin distribution in M. × giganteus, in M. saccharifolius and M. sinensis provides such an answer indirectly. The C-banding/DAPI banding pattern in M. × giganteus is the most similar to M. saccharifolius and probably it was this species which contributed two genomes to the karyotype of the triploid hybrid. This could be a further confirmation of the hypothesis adopted by the majority of previous authors (Linde-Laursen Citation1993; Rayburn et al. Citation2009, and references therein).
Generally, 2C DNA values estimated by us are similar to the results of analogous measurements taken by other authors (Rayburn et al. Citation2009; Swaminathan et al. Citation2010). Clearly lower DNA values in M. sinensis (3.94–4.08 pg) have been recently reported by Rounsaville et al. (Citation2011), but the authors obtained them with the use of base-specific fluorochrome DAPI (4’,6-diamidino-2-phenylindole). A significant difference in the genome size of two M. sinensis genotypes points to a difference which has occurred between the young variety of this species (M. sinensis M07) and probably the oldest one (M. sinensis cv. Gracillimus). This might be a result of variation in the amount of heterochromatin in the karyotype, a phenomenon often observed in grasses (Joachimiak et al. Citation1997). The correlation between genome size and heterochromatin amount was indicated recently in Bromus (Klos et al. Citation2009). The other widespread phenomenon is the change in DNA content following hybrid and/or polyploid formation (Baack et al. Citation2005; Leitch and Bennett Citation2004). It was demonstrated, however, that some recently formed hybrids and triploids may show additivity in DNA amount in comparison to their parents (Grabowska-Joachimiak et al. Citation2006; Bory et al. Citation2008; Zhou et al. Citation2010). Decreased 1Cx DNA content in triploid miscanthus studied here suggests rather genome downsizing.
Successful re-synthesis of M. × giganteus can bring new cultivars which can be used as both bioenergy and ornamental plants. It seems that for such experiments the most convenient are tetraploid ecotypes of M. saccharifolius and diploid ecotypes of M. sinensis which occur naturally in Japan (Nishiwaki et al. Citation2011). The obtained triploid hybrids, like M. × giganteus cultivated nowadays, are sterile. Some reports about formation of hexaploid (therefore potentially fertile) genotypes of giant miscanthus have aroused fears connected with uncontrolled spread on the natural habitat by seeds (Głowacka et al. Citation2009). Triploid hybrids are also formed between other grass species, e.g. Pennisetum purpureum (2n = 4x = 28) and P. glaucum (2n = 2x = 14). The resulting hybrids as well as hexaploids produced by chromosome duplication showed meiotic abnormalities related to chromosome segregation (Paiva et al. Citation2012).
Acknowledgements
We would like to show our gratitude to Prof. Agnieszka Płażek from the Department of Plant Physiology, University of Agriculture in Krakow and Ms Wilhelmina Wasik from National Clonal Germplasm Repository in Miami (USA) for the plant material for our research.
References
- Adati , S . 1958 . Studies on the genus Miscanthus with special reference to the Japanese species suitable for breeding purposes as fodder crops . Bull Fac Agric Mie Univ , 17 : 1 – 112 .
- Adati , S and Shiotani , I . 1962 . The cytotaxonomy of the genus Miscanthus and its phylogenic status . Bull Fac Agric Mie Univ , 25 : 1 – 14 .
- Baack , EJ , Whitney , KD and Rieseberg , LH . 2005 . Hybridization and genome size evolution: timing and magnitude of nuclear DNA content increases in Helianthus homoploid hybrid species . New Phytol , 167 ( 2 ) : 623 – 630 .
- Beale , CV , Bint , DA and Long , SP . 1996 . Leaf photosynthesis in the C-4 grass Miscanthus × giganteus, growing in the cool temperate climate of southern England . J Exp Bot , 47 ( 2 ) : 267 – 273 .
- Bory , S , Catrice , O , Brown , S , Leitch , IJ , Gigant , R , Chiroleu , F , Grisoni , M. , Duval , M-F and Besse , P . 2008 . Natural polyploidy in Vanilla planifolia (Orchidaceae) . Genome , 51 ( 10 ) : 816 – 826 .
- Chou , Ch-H . 2009 . Miscanthus plants used as alternative biofuel material: The basic studies on ecology and molecular evolution . Renew Energ , 24 : 1908 – 1912 .
- Cieślak , E , Ilnicki , T and Flis , M . 2000 . Cytotaxonomical studies on the Caltha palustris complex (Ranunculaceae) in Poland . Preliminary Report. Acta Biol Cracov Bot , 42 ( 1 ) : 121 – 129 .
- Clifton-Brown , J and Lewandowski , I . 2000 . Overwintering problems of newly established Miscanthus plantations can be overcome by identifying genotypes with improved rhizome cold tolerance . New Phytol , 148 ( 2 ) : 287 – 294 .
- Deuter M [date unknown] [Internet]. Breeding approaches to improvement of yield and quality in Miscanthus grown in Europe. Available from: http://www.cantusbiopower.com/research.html
- Głowacka , K , Jeżowski , S and Kaczmarek , Z . 2009 . Polyploidization of Miscanthus sinensis and Miscanthus × giganteus by plant colchicine treatment . Ind Crop Prod , 30 ( 3 ) : 444 – 446 .
- Golczyk , H and Joachimiak , A . 2003 . NORs in Rhoeo (Commelinaceae) revisited . Caryologia , 56 ( 1 ) : 31 – 35 .
- Grabowska-Joachimiak A, Mosiolek M, Lech A, Góralski G. 2011. C-Banding/DAPI and in situ hybridization reflect karyotype structure and sex chromosome differentiation in Humulus japonicus Siebold & Zucc. Cytogenet Genome Res. 132(3):203–211.
- Grabowska-Joachimiak , A , Sliwinska , E , Piguła , M , Skomra , U and Joachimiak , AJ . 2006 . Genome size in Humulus lupulus L. and H. japonicus Siebold & Zucc. (Cannabaceae) . Acta Soc Bot Pol , 75 ( 3 ) : 207 – 214 .
- Hodkinson , TR , Chase , MW , Lledó , MD , Salamin , N and Renvoize , SA . 2002 . Phylogenetics of Miscanthus, Saccharum and related genera (Saccharinae, Andropogoneae, Poaceae) based on DNA sequences from ITS nuclear ribosomal DNA and plastid trnL intron and trnL-F intergenic spacers . J Plant Res , 115 ( 5 ) : 381 – 392 .
- Hodkinson , TR , Chase , MW and Renvoize , SA . 2002 . Characterization of a genetic resource collection for Miscanthus (Saccharinae, Andropogoneae, Poaceae) using AFLP and ISSR PCR . Ann Bot , 89 ( 5 ) : 627 – 636 .
- Hodkinson , TR , Chase , MW , Takahashi , C , Leitch , IJ , Bennet , MD and Renvoize , SA . 2002 . The use of DNA sequencing (ITS and trnL-F), AFLP, and fluorescent in situ hybridization to study allopolyploid Miscanthus (Poaceae) . Am J Bot , 89 ( 2 ) : 279 – 286 .
- Hodkinson , TR and Reinvoze , S . 2001 . Nomenclature of Miscanthus × giganteus (Poaceae) . Kew Bull , 56 ( 3 ) : 759 – 760 .
- Hodkinson , TR , Renvoize , SA and Chase , MW . 1997 . Systematics of Miscanthus. Asp Appl Biol , 49 : 189 – 198 .
- Joachimiak , A , Kula , A and Grabowska-Joachimiak , A . 1997 . On heterochromatin in karyosystematic studies . Acta Biol Cracov Bot , 39 : 69 – 77 .
- Joachimiak , A , Kula , A , Śliwińska , E and Sobieszczańska , A . 2001 . C-banding and nuclear DNA amount in six Bromus species . Acta Biol Cracov Bot , 43 : 105 – 115 .
- Jones , NR . 2012 . B chromosomes in plants. Plant Biosyst , 146 ( 3 ) : 727 – 737 .
- Klos , J , Sliwinska , E , Kula , A , Golczyk , H , Grabowska-Joachimiak , A , Ilnicki , T , Szostek , K , Stewart , A and Joachimiak , AJ . 2009 . Karyotype and nuclear DNA content of hexa-, octo-, and duodecaploid lines of Bromus subgen . Ceratochloa. Genet Mol Biol , 32 ( 3 ) : 528 – 537 .
- Lafferty , J and Lelley , T . 1994 . Cytogenetic studies of different Miscanthus species with potential for agricultural use . Plant Breed , 113 ( 3 ) : 246 – 249 .
- Leitch , IJ and Bennett , MD . 2004 . Genome downsizing in polyploid plants . Biol J Linn Soc , 82 ( 4 ) : 651 – 663 .
- Linde-Laursen , IB . 1993 . Cytogenetic analysis of Miscanthus ‘Giganteus’, an interspecific hybrid . Hereditas , 119 ( 3 ) : 297 – 300 .
- Nishiwaki , A , Mizuguti , A , Kuwabara , Sh , Toma , Y , Ishigaki , G , Miyashita , T , Yamada , T , Matuura , H , Yamaguchi , S , Rayburn , AL , Akashi , R and Stewart , JR . 2011 . Discovery of natural Miscanthus (Poaceae) triploid plants in sympatric populations of Miscanthus sacchariflorus and Miscanthus sinensis in southern Japan . Am J Bot , 98 ( 1 ) : 154 – 159 .
- Paiva , EAA , Bustamante , FO , Barbosa , S , Pereira , AV and Davide , LC . 2012 . Meiotic behavior in early and recent duplicated hexaploid hybrids of napier grass (Pennisetum purpureum) and pearl millet (Pennisetum glaucum) . Caryologia , 65 ( 2 ) : 114 – 120 .
- Papini , A and Simeone , MC . 2010 . Forest resources for second generation biofuels production . Scand J Forest Res , 25 ( suppl. 8 ) : 125 – 133 .
- Paszko , B . 2006 . A critical review and a new proposal of karyotype asymmetry indices . Plant Syst Evol , 258 ( 2 ) : 39 – 48 .
- Peruzzi , L , Bedini , G and Andreucci , A . 2011 . Homoploid hybrid speciation in Doronicum (Asteraceae)? Morphological, karyological and molecular evidences . Plant Biosyst , 146 ( 1 ) : 1 – 11 .
- Peruzzi , L , Leitch , IJ and Caparelli , KF . 2009 . Chromosome diversity and evolution in Liliaceae . Ann Bot , 103 ( 3 ) : 459 – 475 .
- Płażek , A and Dubert , F . 2010 . Improvement of medium for Miscanthus × giganteus callus induction and plant regeneration . Acta Biol Cracov Bot , 52 ( 1 ) : 105 – 110 .
- Price , S . 1963 . Accessory chromosomes in Miscanthus floridulus . J Hered , 54 ( 1 ) : 13 – 16 .
- Rayburn , AL , Crawford , J , Rayburn , CM and Juvik , JA . 2009 . Genome size of three Miscanthus species . Plant Mol Biol Rep , 27 ( 2 ) : 184 – 188 .
- Rounsaville , TJ , Touchell , DH and Ranney , TG . 2011 . Fertility and reproductive pathways in diploid and triploid Miscanthus sinensis . HortScience , 46 ( 10 ) : 1353 – 1357 .
- Sliwinska , E , Zielinska , E and Jedrzejczyk , I . 2005 . Are seeds suitable for flow cytometric estimation of plant genome? . Cytometry , 64A ( 2 ) : 72 – 79 .
- Słomka , A , Kuta , E , Płażek , A , Dubert , F , Żur , I , Dubas , E , Kopeć , P and Żurek , G . 2012 . Sterility of Miscanthus × giganteus results from hybrid incompatibility . Acta Biol Cracov Bot , 54 ( 1 ) : 1 – 8 .
- Swaminathan , K , Alabady , MS , Varala , K , De Paoli , E , Ho , I , Rokhsar , DS , Arumuganathan , AK , Ming , R , Green , PJ Meyers , BC . 2010 . Genomic and small RNA sequencing of Miscanthus × giganteus shows the utility of sorghum as a reference genome sequence for Andropogoneae grasses . Genome Biol , 11 ( 2 ) : R12
- Wang , DF , Portis , AR , Moose , SP and Long , SP . 2008 . Cool C4 photosynthesis – pyruvate pi dikinase expression and activity corresponds to the exceptional cold tolerance of carbon assimilation in Miscanthus × giganteus . Plant Physiol , 148 ( 1 ) : 557 – 567 .
- Zhou X, Ma J, Wang W, Gong N, Zhang Y, Liu J. 2010. Genome size of the diploid hybrid species Hippophae goniocarpa and its parental species, H. rhamnoides ssp . sinensis and H. neurocarpa ssp . neurocarpa (Elaeagnaceae). Acta Biol Cracov Bot. 52(2):12–16.