Abstract
In this study, conventional Giemsa staining, Silver-Nucleolar Organizer Region (Ag-NOR) and C-banding were applied to eight specimens of Tench, Tinca tinca, of Lake Beyşehir, and their detailed cytogenetic properties were investigated. It was determined that all of the chromosomes in karyotypes of the samples investigated were bi-armed (metacentric, submetacentric, subtelocentric). Also a secondary constriction was observed on the short arm of the third pair of chromosomes. No morphological difference was identified between sex chromosomes of male and female specimens. All chromosomes of the specimens had centromeric and pericentromeric C-bands. There was an interstitial band on the short arm of a chromosome pair, while all the short arms of three chromosome pairs were C-positive. Heteromorph C-bands were detected in two chromosome pairs. By Ag-nitrate staining, active NOR was identified on the short arm of the number three metacentric chromosome pair of all specimens. However, this active NOR was not related to C-heterochromatin. All the active NORs determined in the studied specimens were homomorphisms.
Keywords:
Introduction
Turkey is very rich in inland waters, and about 226 fish species and subspecies belonging to 27 families are naturally found in Turkey. Cyprinidae is widely distributed throughout the world and in Turkey. This family is represented by 33 genera and 116 species and subspecies (Kuru Citation2004). Tench, Tinca tinca, is widely distributed in Europe and is found also in the near-east and western Siberia (Geldiay and Balık Citation2007).
Cytogenetically, fishes have been the least studied of all vertebrates. Due to their fairly large numbers of relatively small chromosomes, good metaphase spreads are difficult to obtain (Padilla et al. Citation1993). The conventionally stained karyotype of T. tinca was described by Cataudella et al. (Citation1977) and Sola et al. (Citation1983) from Italy and by Hamalosmanoglu and Kuru (Citation2004) from Lake Mogan (Ankara) in Turkey. However, information on differentially stained chromosomes and detailed structure of the karyotype is still lacking in this species for populations from Turkey. The aim of this paper is to perform a chromosomal banding analysis of the karyotype of T. tinca with the use of C-banding and Silver-Nucleolar Organizer Region (Ag-NOR) staining and to compare the findings with those obtained in a previous studies of T. tinca.
Materials and methods
Eight specimens of T. tinca were collected from Lake Beyşehir (37°46′ N, 31°31′ E), Konya, Turkey. The fish specimens were transported live to the laboratory, and kept in well aerated aquaria until analysis. Chromosomes were prepared directly from the head kidney according to the method of Collares-Pereira (Citation1992). Colchicine solution was prepared with 0.005 g in 20 ml of physiological serum. The fish were injected intraperitoneally with colchicine at a dose of 0.02 ml of body weight using an insulin syringe, and then were placed in the aquarium for 4–5 hours. The kidneys of these specimens were then removed and placed in hypotonic 0.36% KCl solution for 45 minutes at room temperature (25°C). Thereafter, the solutions were centrifuged for 10 minutes at 1000 rpm, adding 2–3 drops of fresh cold Carnoy’s fixative (1:3, acetic acid:methanol) before centrifugation. The supernatants were then discarded and 5 ml of fresh cold fixative was added to the sediments, mixed thoroughly, and then left for 1 hour. The fixation and centrifugation stages were repeated twice. The suspensions were then trickled onto cold slides. Air-dried slides were stained conventionally by 10% Giemsa for 10 minutes. Constitutive heterochromatin and nucleolar organizer regions (NORs) were detected by the techniques of C-banding (Sumner Citation1972) and Ag-NOR staining (Howell and Black Citation1980), respectively. For C-banding, slides were aged at 37°C for 7–10 days, treated with 0.2 N HCl at room temperature for 20 minutes, rinsed, dried, and then incubated at 60°C for 20 minutes. They were then covered with saturated Ba(OH)2 for 10 minutes at room temperature, followed by rinsing and drying. Slides were subsequently flooded with 2 × SSC, incubated at 60°C for 50 minutes, rinsed, and dehydrated in an ethanol series. Staining was 4% Giemsa for 30 min. For Ag-NOR staining, slides were then mounted in a 50% solution of silver nitrate with coverslip, placed in a humid chamber and incubated in a water bath at 65°C for 1 hour. From each specimen, 10 to 20 slides were prepared, and at least 20 well-spread metaphase plates were analysed. Definition of the shapes of the chromosomes was established according to Levan et al. (Citation1964).
Results
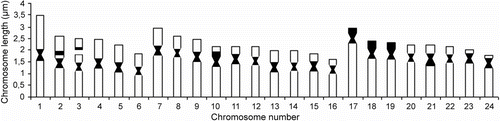
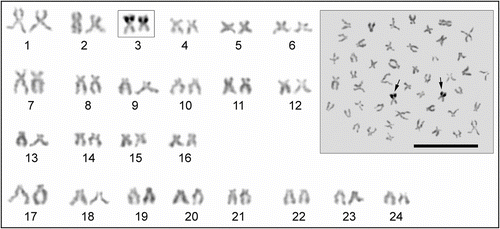
The karyotype of tench from Lake Beyşehir consists of 48 chromosomes including six metacentric pairs (nos. 1–6), 10 submetacentric pairs (nos. 7–16) and eight subtelocentric pairs (nos. 17–24) of autosomes (number of chromosomal arms (NF) = 80). Secondary constriction was observed in the short arm of the autosomal pair no. 3. The sex chromosomes were not determined in the set (Figure ). Chromosome shapes are determined in Table and an ideogram is given Figure .
Figure 1 Metaphase spread and karyotype of Tinca tinca. Arrows indicate the secondary constrictions (scale bar = 10 μm).
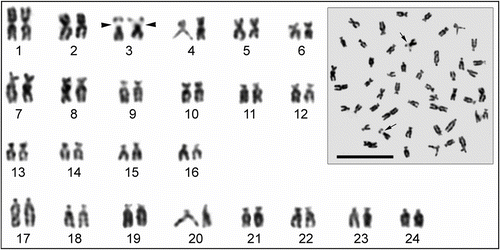
Table 1. Chromosome classification (μm) of Tinca tinca from Lake Beyşehir according to Levan et al. (Citation1964).
The C-banded karyotype of T. tinca is illustrated in Figure . All the autosomes have centromeric or pericentromeric C-bands. Pericentromeric blocks of heterochromatin in T. tinca have different size arrangements with respect to the centromere (no. 10) and one chromosome pair (no. 2) has an interstitial C-band. C-heterochromatic short arms were found in three autosomal pairs (nos. 17–19) in the complements of the specimens. The size of the C-positive region on pairs 7 and 8 were heteromorphic (a homologous chromosome has variable heterochromatin morphology).
Figure 3 Metaphase spread and C-banded karyotype of Tinca tinca. Arrows indicate the secondary constrictions (scale bar = 10 μm).
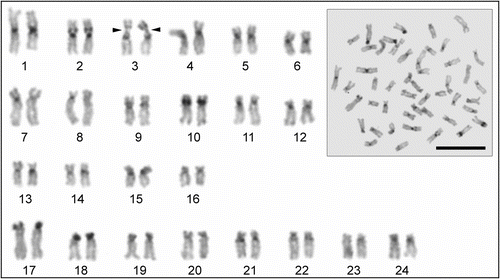
The active Ag-NOR regions were found in one biarmed autosomal pair (no. 3) in complements of all the specimens. The NORs were observed on the short arms of metacentric autosomes (Figure ).
Discussion
In all cytogenetic studies carried out to date, the diploid chromosome number of T. tinca was found to be 48 (Cataudella et al. Citation1977; Sola et al. Citation1983; Padilla et al. Citation1993; Hamalosmanoglu and Kuru Citation2004). Although Padilla et al. (Citation1993) detected secondary constriction on the metacentric third chromosome by conventional Giemsa staining, Hamalosmanoglu and Kuru (Citation2004) could not determine this in samples from Turkey. In this study, in accordance with the study of Padilla et al. (Citation1993), a secondary constriction was detected on a pair of metacentric chromosome by conventional Giemsa staining, and the result was confirmed by Ag-NOR staining. No variation has been determined related to this result in our specimens. Although the chromosomes of our samples are mostly similar to those in the study of Padilla et al. (Citation1993) as well as the other studies carried out in Turkey, no acrocentric chromosome was identified. This difference is probably due to methodology.
Hafez (Citation1979) reported that C-bands of this species were generally in the centromeric region of the chromosomes and the bands showed polymorphism even between the chromosomes. Padilla et al. (Citation1993) found similar results, and they also found that secondary constriction was related to the C-heterochromatin region. However, in this study, it was determined that the region of secondary constriction was not related to C-heterochromatin (Figure ).
The presence of a pair of active NORs with Ag staining was identified in all samples. No variation in the size of NORs was also observed. The results of this research are quite similar to the results of Hafez (Citation1979) and Padilla et al. (Citation1993), although there are some differences; and they show that karyological properties of this species are protected in different geographical regions.
As a result, when the chromosome measurements were taken into consideration, it was determined that all the chromosomes in the karyotype of our samples are two-armed according to the terminology, unlike earlier studies conducted both in Turkey and in Europe (Hafez Citation1979; Padilla et al. Citation1993). Although chromosome numbers and morphology change in different populations of some Cyprinidae species (Cataudella et al. Citation1977; Sola et al. Citation1983; Padilla et al. Citation1993; Hamalosmanoglu and Kuru Citation2004), the karyotype of T. tinca was observed to be preserved among populations, except for the number of chromosomes and the presence or absence of one-armed chromosomes. Banding methods for differentiation of species populations, other than conventional Giemsa staining, are not often used in fish karyosystematics (Cataudella et al. Citation1977; Sola et al. Citation1983; Padilla et al. Citation1993; Hamalosmanoglu and Kuru Citation2004). Banding studies on the chromosomes of T. tinca have shown similarities between Turkish and European populations both in terms of heterochromatin and number and distribution of active NORs.
Karahan and Ergene (Citation2009) described the karyotype analysis of Garra rufa samples with G-, C- banding and Ag-NOR staining techniques from four distinct localities in Turkey. Using comparative analysis they revealed intraspecific variability of NORs and fixed differences in their number in the four populations.
This study emphasizes the importance of banding studies in karyotype analysis. In further studies of the karyosystematics of fish, clearer results will be provided by using C- and Ag-NOR banding as well as conventional Giemsa staining for detection of heterochromatin distribution and NOR differences in both inter-species and intra-species populations. In addition, it is advocated that the phylogeographic relation of the populations of this species within Turkey, Asia and Europe should be revealed by the mtDNA sequence analysis technique, which has recently been used to determine the phylogenetic relationship between species and populations.
Acknowledgements
The paper was supported by grants from the Coordination Committee of Scientific Research Projects of Selçuk University (No. BAP 11201005).
References
- Cataudella , S , Sola , L , Accame Muratori , R and Capanna , E . 1977 . The chromosomes of 11 species of cyprinidae and one cobitidae from Italy, with some remarks on the problem of the polyploidy in the cypriniformes . Genetica , 47 : 161 – 171 .
- Collares-Pereira MJ. 1992. In vivo direct chromosome preparation (protocol for air drying technique). First International Workshop on Fish Cytogenetic Techniques. 14–24 September, Concarneau, France.
- Geldiay , R and Balık , S . 2007 . Türkiye tatli su baliklari , Bornova-İzmir , Turkey : Ege Üniversitesi Basım Evi .
- Hafez , R . 1979 . Analysis du caryotype de la tanche (Tinca tinca L.) par l’obtention des bandes C et G . Cybium , 3 : 15 – 26 .
- Hamalosmanoglu , M and Kuru , M . 2004 . Karyotype analyses of the tench (Tinca tinca L., 1758) living in Lake Mogan (Ankara) . Turk J Vet Anim Sci , 28 : 143 – 147 .
- Howell , WM and Black , DA . 1980 . Controlled silverstaining of nucleolus organizer regions with a protective colloidal developer: a 1 step method . Exp (Basel) , 36 : 1014 – 1015 .
- Karahan , A and Ergene , S . 2009 . Cytogenetic variation of geographically isolated four populations of Garra rufa [(Heckel, 1843) (Pisces, Cyprinidae)] in Turkey . Caryologia , 62 : 276 – 287 .
- Kuru , M . 2004 . Recent systematic status of inland water fishes of Turkey . J Gazi Educ Fac , 3 : 1 – 21 .
- Levan , A , Fredga , K and Sandberg , AA . 1964 . Nomenclature for centromer position on chromosomes . Hereditas , 52 : 201 – 220 .
- Padilla , JA , Fernández-García , JL , Rabasco , A , Martínez-Trancón , M , Rodriguez de Ledesma , I and Pérez-Regadera , JJ . 1993 . Characterization of the karyotype of the tench (Tinca tinca L.) and analysis of its chromosomal heterochromatic regions by C-banding, Ag-staining, and restriction endonuclease banding . Cytogenet Cell Genet , 62 : 220 – 223 .
- Sola , L , Cataudella , S , Gentili , G and Monaco , G . 1983 . An experimental carp x tench hybrid: karyological analysis and SEM morphological observations . Boll Zool , 50 : 159 – 171 .
- Sumner , AT . 1972 . A simple technique for demonstrating centromeric heterochromatin . Exp Cell Res , 75 : 304 – 306 .