Abstract
Iran has more than 63 species of genus Onobrychis. This genus has small chromosomes with different ploidy levels. Counting ploidy levels in somatic cells in the metaphase is difficult; therefore, this study aims to evaluate a method using counts of stomata guard cell chloroplasts, as well as using flow cytometry as methods for determining ploidy levels in annual species of Onobrychis genus. Three middle leaves of greenhouse-grown plants were randomly selected for chloroplast counts in 10 pairs of stomata guard cells of the lower surface of leaf cells. For determining ploidy levels, the usual method was counting chromosomes which determined in the metaphase cells of root tip meristems with having minimum of 10 metaphase plate mitosis for each species. All of the samples were also studied, to determine ploidy levels using flow cytometry, and to find the mode and ratio for each of them. The results indicated that numbers of chloroplast stomata guard cells and flow cytometry in the tetraploid samples were approximately twice the numbers in the diploid samples. Ploidy levels were highly correlated (r = 0.97) with mean numbers of chloroplasts in stomata guard cells, and the ploidy levels were highly correlated (r = 0.93) with modes of the flow cytometry. The mean numbers of chloroplasts in stomata guard cells were highly correlated (r = 0.95) with modes of flow cytometry in all the studied populations. Therefore, these observations determine that counting stomata guard cells in chloroplasts and flow cytometry can be recommended as a fast and simple method for determining ploidy levels in annual species of Onobrychis genus.
Keywords:
Introduction
Onobrychis genus has 63 genetically diverse species in Iran, distributed throughout different climatic regions. The species provides a valuable genetic resource for breeding crops (Ghanavati et al. Citation2010). Annual species of Onobrychis genus belong to Lophobrychis, Afghanica and Heliobrychis sections; they are widespread throughout different climatic regions of the country (Rechinger 1984). These species is valuable as forage and is used to control soil erosion and to attract bees (Lock and Simpson Citation1991; Yakovlev et al. Citation1996; Mabberley Citation1997).
As a genetic resource, annual species have the different ploidy levels of diploid and tetraploid, with different numbers of chromosomal bases 7 and 8 (Hatami and Nasirzadeh Citation2006; Hesamzadeh Hejazi and Ziaei Nasab Citation2009, Citation2010; Ranjbar et al. Citation2010; Ghanavati et al. Citation2010, 2011). These factors make breeding projects working with these species as genetic resources difficult. Sometimes different ploidy levels can be observed within a single species. Therefore, the determination of a ploidy level is not only important in the study of relative relationships of species, but also is very important in producing interspecies hybrids, and in genetics studies and breeding programs. Chromosome size in the species of this genus is very small; therefore, determination of ploidy levels by means of the usual method of counting chromosomes in root meristem cells in extensive breeding programs is very difficult and requires a large number of intact seeds. Therefore, it is necessary to develop a simpler technique for use in breeding programs and for identification of species. Recent studies have shown that the division and number of chloroplasts in leaf cells is influenced by the nuclear genome (Leech Citation1981) and that in some species there is a direct relationship between numbers of chloroplasts in stomata guard cells and ploidy levels. Counting chloroplasts in stomata guard cells, in comparison with the more usual method of counting chromosomes in meristem cells of a root tip, is easy and cheap; therefore it was used in this study to determinate ploidy levels. The results indicated that numbers of chloroplasts of stomata guard cells in tetraploid species was almost double those of the diploid species. Correlation of number of chloroplasts in stomata guard cells and ploidy level was observed in tomato (Koornneef et al. Citation1989), potato (Schreiter et al. Citation1989; Cardi et al. Citation1992), wheat (Hawke and Leech Citation1990), green pea (Murray and Standing Citation1992) and interspecies hybrids of Arachis hypoyaea L. interspecies hybrids of Arachis hypoyaea L. and A. stenosperm L. (Singsit and Oaias-Akians 1992), and alfalfa (Ghanavati et al. Citation2004).
Furthermore, nuclear DNA content of chromosomes in plant cell nuclei may also be used as an index to estimate ploidy levels. Flow cytometry and 4′,6-diamidino-2-phenylindole (DAPI) with fresh leaves of the plant were used to determinate nuclear DNA content in plant cells, and the result of the analysis is shown in the histogram figure that is related to nuclear DNA content (Properi et al. Citation1991). Flow cytometry is commonly used to determine aneuploidy and polyploidy (Kawara et al. Citation1999) and to observe disorder in cell division, because many populations of cells can be analyzed by this method in a short time. Some plant species contain phenol in their leaves, therefore 11 species of Rosaceae family with high propedium staining inhibitors were surveyed for the purpose of using the seed instead of the leaf (Jedrzejczyk and Sliwinska Citation2010). The results showed that in spite of the optimization of phenol concentration in the nuclear isolated buffer for every species, the effect of cytosol composition in the leaves did not disappear completely, whereas none of the seeds of the studied species had inhibitors.
In this survey, ploidy levels of annual species of the Onobrychis genus were determined by counting chloroplasts of stomata guard cells. The efficiency of this method was compared with that of flow cytometry by counting chromosomes in the mitosis metaphase cells.
Materials and methods
Plant material
A total of 13 seeds of five species of annual species Onobrychis, including O. crista-galli, O. caput-galli, O. micrantha, O. pulchella, O. aucheri ssp. tehranica and O. aucheri ssp. psammophila, were gathered from the main habitats of different regions (Table ). They were planted in the greenhouse of the National Plant Gene Bank of Iran. After they reached a stage of having about 10 leaves, they were used for counting chloroplasts in stomata guard cells and for flow cytometry.
Table 1. Studied species of Onobrychis and their habitats.
Counting chloroplasts of stomata guard cells
To count the chloroplasts of stomata guard cells, three samples from the middle leaves of seedlings (5–6 internodes) were selected and a small piece from the epidermis of the lower surface of the leaf was removed with a blade and put on a plate. The samples were stained with Logul solution, and the numbers of chloroplasts in the 20 pairs of stomata guard cells were counted using a light microscope with a magnification of 400. Mean and standard deviation for each species was determined.
Flow cytometry
Flow cytometry was employed to determine ploidy levels. This was done in two ways: first, two young leaves of each plant were cut at 4 cm and placed into a Petri dish, then 1600 μl DAPI buffer was poured onto the leaves and they were chopped with a blade to free the nuclei. The obtained suspension was then passed through a special filter device, a Ploidy Analyzer, to remove the cellular components and the large pieces. The filtered materials were then poured into a cylindrical tube, which was installed in the appropriate place place of the Ploidy Analyzer and the DNA content of the tested samples was determined. In the second method the same procedure was followed, but using the radicle instead of the leaf. A standard plant in experiments was used to determine accuracy of ploidy levels if the nuclear DNA properties of the standard samples were similar to the DNA properties of a tested sample, then the error of the device was taken as minimum. The standard peaks should not overlap with the targeted peaks. Expected ploidy levels for other samples were obtained based on the peak and mode ratio of the standard sample.
Counting of chromosomes
Radicles of the surveyed plants were pretreated in 8-hydroxyl-quinalin (2 mM), fixed in a solution of formalin 10% and chromic acid 1% (1:1), and stained with aceto-iron-hematoxilin solution based on Ghanavati et al. (Citation2010). Then at least 10 mitosis metaphase plate of root tip meristems were studied for each species.
Results and discussion
The results showed that two basic chromosome numbers (x = 7 and x = 8) and three ploidy levels (2n = 2x = 14, 2n = 2x = 16 and 2n = 4x = 32) were evident in the genus Onobrychis. The tests revealed evidence of one ploidy level for O. caput-galli (2n = 2x = 14) and O. pulchella (2n = 2x = 16) and two ploidy levels for O. aucheri (2n = 2x = 16, 2n = 4x = 32) and O. crista-galli (2n = 2x = 16, 2n = 4x = 32). Other studies on this genus confirm these results (Zohary Citation1972; Goldblatt Citation1981; Ansari et al. Citation2000; Hesamzadeh Hejazi and Ziaie Nasab Citation2009, 2010). The present study confirms the findings of another report (Hatami and Nasirzadeh Citation2006) that the two subspecies O. aucheri subsp. tehranica and O. aucheri subsp. psammophila are different in terms of morphological and chromosomal characteristics and therefore should be considered as two separate species.
This study also reports the existence of 2n = 2x = 16 and 2n = 4x = 32 for different populations of O. crista-galli in Iran, i.e. O. crista-galli 5 and O. crista-galli 6 with 16 chromosomes were diploid and O. crista-galli 1, O. crista-galli 2, O. crista-galli 3 and O. crista-galli 4 with 32 chromosomes were tetraploid. The first chromosomal study of Onobrychis was performed on O. crista-galli from the southeastern Mediterranean in 1931 and the number of chromosomes of this species was determined as 14. Other research on this species was carried out in 1955 and the number of chromosomes was reported as 16 (Darlington and Wylie Citation1955). Some other surveys were performed on this species from 1977 to 2006 and they obtained the result of 2n = 2x = 16 (Goldblatt Citation1981; Tamas Citation2006) but a study in 1994 reported 2n = 2x = 32 (Goldblatt and Johnson 1996). The different populations of O. crista-galli 2n = 2x = 16 in Iran have been reported by Hesamzadeh Hejazi and Ziaei Nasab (2009). Chromosome numbers and measurements were recorded in six populations of six species of Onobrychis, including the Egyptian species and most representatives of the section Lophobrychis. One of these surveyed populations was O. crista-galli and levels of x = 8, tetraploid and 2n = 4x = 32 were identified. It was declared that the evolution of O. crista-galli was associated with chromosome duplication; intra-chromosomal variability and reduction in mean chromosome length could have originated from O. ptolemaica (Abou-El-Enain Citation2002). This study on O. crista-galli showed that this species in Iran possess two ploidy levels: diploid and tetraploid, evident from a survey of two populations of O. crista-galli 5 and O. crista-galli 6 that were gathered from the same region. On completion of morphological studies, it is possible that there are two varieties for this species (Ghanavati et al. 2012). In addition, this study indicated that despite the different species and populations of Onobrychis possessing different numbers of basic chromosomes, all of them possess small chromosomes; in other words chromosome mean length of this plant is smaller than many other species and genera of other plants.
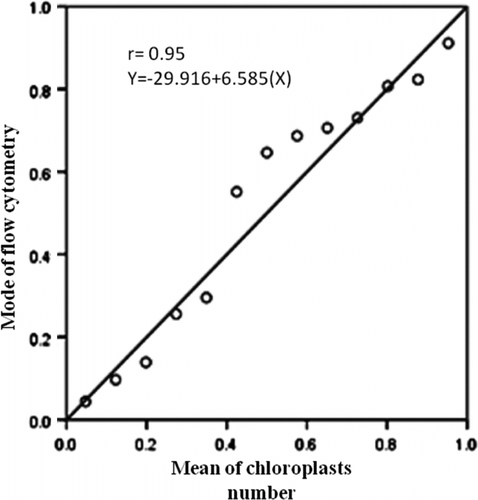
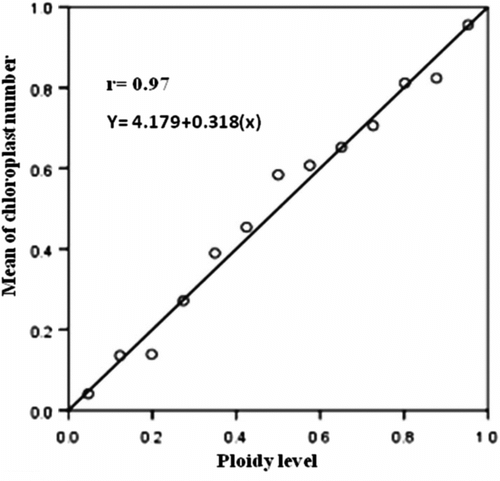
Minimum, maximum and mean numbers of chloroplasts of stomata guard cells in the mentioned species were determined (Table ). The results of the table show that numbers of chloroplasts in stomata guard cells of diploid populations was between 7 and 11 but in tetraploid populations the number ranged from 13 to 17. The mean number of chloroplasts in diploid populations was 9.10 and in tetraploid populations was 14.38. Results for O. crista-galli 1, O. crista-galli 5, O. caput-galli 1 and O. aucheri ssp. psammophila are shown in Figure . The number of chloroplasts of stomatal guard cells in tetraploid populations of O. crista-galli and subspecies of O. aucheri ssp. psammophila was nearly double that of the number of chloroplasts of stomatal guard cells in diploid populations of O. crista-galli and diploid species of O. pulchella, O. micrantha, O. caput-galli and O. aucheri ssp. tehranica. The results of this survey indicate that the ploidy level was highly correlated (r = 0.97) with the mean number of chloroplasts in stomatal guard cells (Figure ). Previous studies in melon (Fassuliotis and Newlson Citation1992), potato (Mozafari et al. Citation1997) and alfalfa (Ghanavati et al. Citation2004) have also reported that numbers of chloroplasts in the tetraploid variety was nearly double that of the diploid variety. Chloroplasts possess independent and circular DNA. The genes of each species are very stable (Martin and Hermann Citation1998). Although this organelle has special genes and there are nearly100 genes in its DNA, for the purpose of division, growth and biochemical action they connect to express genes of the nucleus (Leech Citation1981) and polyploidy increases the number of gene transcripts that relate to form chloroplasts. In this survey, the relationship between numbers of chloroplasts in stomata guard cells with ploidy levels is confirmed in annual species of Onobrychis. Chromosome counting methods are difficult, require intact seeds and take least one week to prepare the samples, but by counting the chloroplasts of stomata guard cells, ploidy levels can be determined from only a few fresh leaves in a much shorter time (about 10 minutes). Therefore based on the results of this survey, counting of chloroplasts of stomata guard cells is recommended as an economical, easy and fast method to determine ploidy levels of annual species of Onobrychis. However, the number of chromosomes (14 or 16) cannot be determined; only ploidy level (diploid or tetraploid) can be determined.
Table 2. Number of chromosomes, number of chloroplasts and ploidy level in annual species of Onobrychis.
Figure 1. Mitotic metaphase (A) and the chloroplast number of stomata guard cells (B) of (1) Onobrychis crista-galli 1, (2) O. crista-galli 5, (3) O. aucheri ssp. psammophila, and (4) O. caput-galli 1.
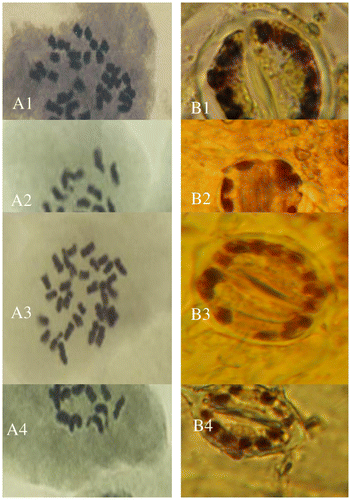
Figure 2. Linear regression between mean of chloroplast number and ploidy level in annual species of Onobrychis.
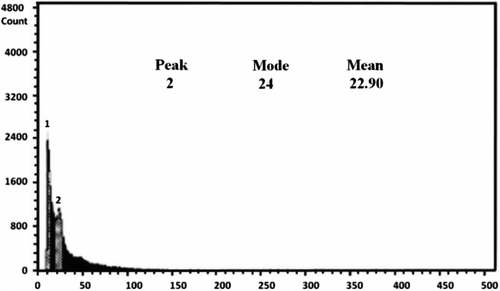
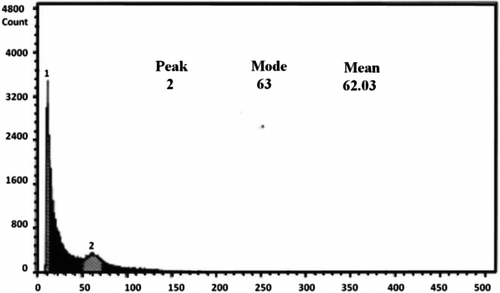
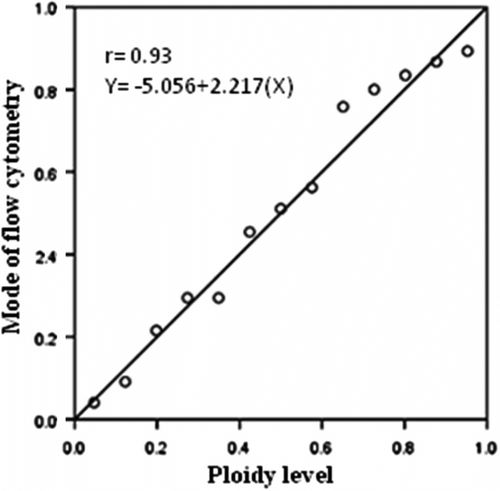
For studying the DNA content of the studied species, O. caput-galli 1, which is diploid, was used as a control (Figure 3). This sample was tested several times and eventually it became clear that the device showed the index of DNA content (mode) with an average of 24. The index of expected DNA content for a diploid Onobrychis was estimated in the range of 24–35. All tested species were mixed with a control sample, and the index of DNA content (mode) was in the same range and the peaks were consistent with each other in the diploid samples. Meanwhile, the index of DNA content (mode) was between 61 and 70 in the tetraploid populations and was nearly double that of the diploid samples; the peak demonstrated that it was separated from the control sample (Table , Figures , Figure ). The results showed that ploidy levels were highly correlated (r = 0.93) with mode of flow cytometry, and as ploidy level increased, the mode of the samples increased. This method is similar to the technique of counting chloroplasts of stomata guard cells, which is simple and requires only fresh leaves. The method can be used to replace chromosome counting, especially when the number of samples is high. However, in this method, high levels of phenol in the leaves of some species prevented rapid testing. In this survey, fresh leaves of O. aucheri subsp. tehranica and O. aucheri subsp. psammophila did not respond to flow cytometry at different times and during many repeats, despite being annual. This is because they are woody and have high levels of phenol. Therefore, a part of the plant that had a lower amount of phenol was used, i.e. the radicle. The radicle had previously had been used to determine ploidy levels in species of Rosaceae (Jedrzejczyk and Sliwinska Citation2010). The results of this present survey support the application of the radicle instead of fresh leaf in this species. The mean number of chloroplasts in stomata guard cells was highly correlated (r = 0.95) with mode of flow cytometry in all the studied populations, indicating that each of these methods can be used effectively to determine ploidy levels.
Table 3. Ratio of mode of studied annual species of Onobrychis with control based on flow cytometer peak.
Figure 3. Linear regression between ploidy level and mode of flow cytometry in annual species of Onobrychis.
References
- Abou-El-Enain , MM . 2002 . Choromosomal criteria and their phylogenetic implication in the genus Onobrychis Mill sect. Lophobrychis (Leguminosae), with special reference to Egyptian species . Bot J Linn Soc , 139 : 409 – 414 .
- Ansari Asl F, Ahmadian P, Nasirzadeh A. 2000. Cytological study of Onobrychis germplasm in Tehran province. Iran J Rangeland Forest Plant Breed Genet Res. 5:36–56. (In Persian).
- Cardi , T , Carpoto , D and Frusciante , L . 1992 . In vitro shoot regeneration and chromosome doubling in 2X and 3X potato cloned . Am Potato J , 69 : 1 – 12 .
- Darlington , CD and Wylie , AP . 1955 . In chromosome atlas of flowering plants , London : George Allen and Unwin . 519 p
- Fassuliotis , G and Newlson , BV . 1992 . Regeneration of tetraploid Muskmelon from cotyledonsand their morphological differences from two diploid Muskmelon genotypes . J Am Soc Hortic Sci , 117 : 863 – 866 .
- Ghanavati , F and Eskandari , H . 2011 . Relationship between the chloroplast number in stomatal guard cells, flow cytometry and ploidy level in Onobrychis spp . Seed Plant Improve J , 27 : 427 – 439 .
- Ghanavati , F , Eskandari , H , Bakhshi Khaniki , G , Sorkhi , B and Amirabadizadeh , H . 2010 . Karyotypic study of sect. Hymenobrychis of Onobrychis in Iran . Seed Plant Improve J , 26 : 545 – 560 . (In Persian)
- Ghanavati , F , Mozafari , J and Masumi , AA . 2004 . Determination of ploidy level with counting the chloroplast number in stomatal guard cells in Medicago sp . Seed and Plant Journal , 20 : 117 – 127 . (In Persian)
- Ghanavati , F , Nematpajooh , N , Khosrow Chahli , M and Safaei Chaeikar , S. 2012 . Cytological evaluation of annual species of the Onobrychis genus in Iran . Crop Breeding Journal , 2 ( 1 ) : 17 – 24 .
- Goldblatt P. 1981. Index to plant chromosome numbers 1975–1978. Monog Syst Botan. 5. Saint Louis Missouri. 553p
- Goldblatt P, Johnson DE. 1996. Index to plant chromosome numbers 1992–1993. Monog Syst Botan. 5. Saint Louis Missouri. 276p.
- Hatami , A and Nasirzadeh , AR . 2006 . Change in rank position of two Onobrychis subspecies according to morphological and karyotypic studies in Fars province . Pajouhesh Sazandegi , 75 : 186 – 191 . (In Persian)
- Hawke , JC and Leech , RM . 1990 . Acetyl coenzyme A carboxylase in species of Triticum of different ploidy . Plants , 81 : 543 – 546 .
- Hesamzadeh Hejazi , SM and Ziaei , Nasab M . 2009 . Cytogenetic study on several populations of diploid species of Onobrychis in natural gene bank of Iran . Iran J Rangeland Forest Plant Breed Genet Res , 16 ( 2 ) : 158 – 179 .
- Hesamzadeh Hejazi , SM and Ziaei , Nasab M . 2010 . Cytotaxonomy of some Onobrychis (Fabaceae) species and populations in Iran . Caryologia , 63 ( 1 ) : 18 – 31 .
- Jedrzejczyk I, Sliwinska E. 2010. Leaves and seeds as materials for flow cytometric estimation of the genome size of 11 Rosaceae woody species containing DNA-staining inhibitors. J Bot. Article ID 930895, 9 pages. doi:10.1155/2010/930895. Hindawi Publishing Corporation.
- Kawara , S , Takata , M and Takehara , K . 1999 . High frequency of DNA aneuploidy detected by DNA flow cytometry in Bowen, s disease . J Dermatol Sci , 21 : 23 – 26 .
- Koornneef , J , Van Diepen , AM , Hanhart , CJ , Kieboom-de Wast , AC , Martinell , C , Schoenmakers , HCH and Wijbrandi , J . 1989 . Chromosome instability in cell and tissue cultures of tomato haploid and diploid . Euphytica , 43 : 179 – 186 .
- Leech , RM . 1981 . Observation of the mechanism of chloroplast division in higher plants . New Phytol , 87 : 1 – 9 .
- Lock , JM and Simpson , K . 1991 . Legumes of West Asia: A check-list , Kew , UK : Royal Botanical Gardens . 452 p
- Mabberley DJ. 1997. The Plant Book. A portable dictionary of the vascular plants, 2nd ed. Cambridge, UK: Cambridge University Press. 342 p.
- Martin , W and Herman , RG . 1998 . Gene transfer from organelles to the nucleus: how much, what happenes and why . Plant Physiol , 118 : 9 – 17 .
- Mozafari , J , Wolyn , DJ and Ali-Khan , ST . 1997 . Chromosome doubling via tuber disc culture in dihaploid potato as determined by confocal microscopy . Plant Cell Reprod , 16 : 329 – 333 .
- Murray , H and Standing , L . 1992 . Genomic constancy during the development of Lathyrus odoratus cultivars . Heredity , 68 : 321 – 327 .
- Properi , E , Giangare , MC and Bottiroli , G . 1991 . Nuclease induced DNA structural changes assessed by flow cytometry with the intercalating dye propidium iodide . Cytometry , 12 : 323 – 329 .
- Ranjbar , M , Karamian , R and Hadadi , A . 2010 . Cytosystematics of three Onobrychis species (Fabaceae) in Iran . Caryologia , 63 ( 3 ) : 237 – 249 .
- Rechinger , KH . 1984 . Onobrychis in flora Iranica . Akademische druck and verlagsanstalt. Graz-Austria , 157 : 387 – 484 .
- Schreiter , J , Munzert , B and Moll , A . 1989 . Bestimmung des ploidiegrades durch chloroplast enzahlungen in stomata bei in-vitro kia lusregeneraten von kartoffeln . Arch Zuchtungs Forsch Berlin , 19 : 69 – 73 .
- Singsit , C and Oaias-Akians , P . 1992 . Rapid estimation of ploidy levels in-vitro regenerated interspecific Arachis hybrids . Euphytica , 64 : 183 – 188 .
- Tamas E. 2006. Cytological aspects of the Onobrychis genus. Bull USAMV. 62:154–158.
- Yakovlev , G.P. , Sytin , A.K. and Roskov , J.R. 1996 . Legumes of Northern Eurasia, a check list , Kew , UK : Royal Botanical Garden . 542p
- Zohary , M . 1972 . Flora Palestina Part Two , Jerusalem : The Israel Academy of Sciences and Humanities . 300 p