Abstract
The genus Hypostomus is distributed throughout South America and is one of the most widely studied genera among Loricariidae, with approximately 130 species described. However, correct taxonomic identification is difficult due to subtle morphological differences between species. Thus, cytogenetic data on this genus are fundamental to clarifying the evolutional history of the group. Cytogenetic analyses were carried out on three species of Hypostomus in the state of Paraná, Brazil: one from the Tibagi river basin (Hypostomus aff. ancistroides) and two from the Iguaçu river basin (Hypostomus commersonii and Hypostomus derbyi). Hypostomus aff. ancistroides had 2n = 66 chromosomes (12m; 16sm; 10st; 28a) and number of arms (fundamental number, FN) = 104, with large heterochromatic blocks identified in two chromosome pairs and nucleolar organizing regions (NORs) detected in seven chromosomes of the complement. The H. commersonii population had 2n = 68 chromosomes (12m; 12sm; 8st; 36a) and FN = 100, with low heterochromatin located pericentromerically and NORs detected in only one chromosome pair. The H. derbyi population had 2n = 66 chromosomes (6m; 10sm; 20 st; 30a), with large heterochromatic blocks dispersed throughout the karyotype and multiple sites with NORs. The karyotype markers proved efficient in separating the species studied herein and corroborate the divergent karyotype evolution in the genus Hypostomus.
Introduction
According to Vari and Malabarba (Citation1998), 30–40% of all existing diversity in Neotropical fish has yet to be recognized. The family Loricariidae has 856 described species (Froese and Pauly Citation2012) and approximately 300 species that have not yet been described (Reis et al. Citation2003). It is one of the 36 families belonging to the order Siluriformes, in which the genus Hypostomus has approximately 130 species described thus far (Ferraris Jr Citation2007). Fish belonging to this family are commonly known as armored catfish and inhabit nearly all of Central and South America, from Costa Rica to Argentina (Reis et al. 2003).
Hypostomus is one of the best cytogenetically characterized genera in the family Loricariidae (Mendes-Neto et al. Citation2011; Bueno et al. Citation2012). Its diploid number ranges from 2n = 54 chromosomes in H. plecostomus (Muramoto et al. Citation1968) to 2n = 84 chromosomes in Hypostomus sp. 2 (Cereali et al. Citation2008). The taxonomy remains relatively imprecise due to the high number of species and considerable inter-species variation (Birindelli et al. Citation2007), leading to difficulties in identification at the species level.
Cytogenetics can be used as an important taxonomic tool, especially in the case of cryptic species (Bertollo et al. Citation1986). An example of this is the distinct diploid number and karyotype formula in different species of Hypostomus not yet taxonomically described from the Xingu region of the Amazon, suggesting the presence of at least three distinct species (Milhomem et al. Citation2010).
This paper describes the karyotype of two species of Hypostomus (H. commersonii and H. derbyi) not previously analyzed in this regard. Moreover, the karyotype of H. aff. ancistroides is revised based on a population that has not previously been investigated. The results are discussed with regard to the cytotaxonomy and karyotype evolution in this genus of Neotropical fish.
Materials and methods
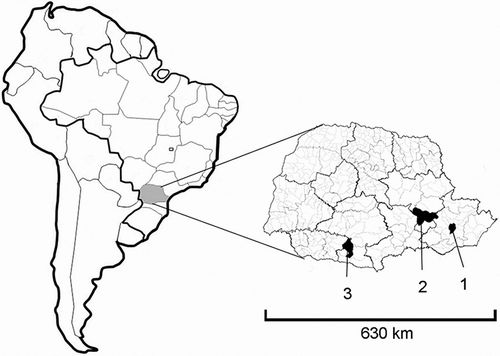
A total of 12 specimens of Hypostomus aff. ancistroides (seven males and five females) were analyzed from the upper Tibagi river basin in the municipality of Ponta Grossa, state of Paraná, Brazil (25.154103 S; 50.14703 W) (Figure ). Two female specimens of Hypostomus commersonii were analyzed from the lake formed by the Ney Braga hydroelectric plant in the mid Iguaçu river basin in the municipality of Mangueirinha, state of Paraná, Brazil (25.793709 S; 52.113562 W) (Figure ). Five specimens of Hypostomus derbyi (three males and two females) were analyzed from the upper Iguaçu river basin in the city of Curitiba, Paraná, Brazil (25.60645 S; 49.281621 W) (Figure ). The specimens were taxonomically identified by Dr. Claudio Zawadzki of the NUPELIA Ichthyology Museum of the Universidade Estadual de Maringá (Brazil). The fish were collected with the authorization of the Brazilian environmental agency (Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis – IBAMA; license number 15115-1). This study received approval from the ethics committee of the aforementioned university (process number 02-2008).
Figure 1 Map of South America, highlighting the state of Paraná, Brazil, indicating collection sites (municipalities) for species of Hypostomus studied herein: (1) Curitiba; (2) Ponta Grossa; and (3) Mangueirinha.
The fish were acclimatized in aerated aquaria and anesthetized through immersion in a 1% benzocaine solution prior to sacrifice. Mitotic preparations were obtained from cells from the anterior kidney, following in vivo treatment with colchicine (Bertollo et al. Citation1978). The nucleolar organizing regions (NORs) were visualized using silver nitrate impregnation (Ag-NORs) (Howell and Black Citation1980) and heterochromatin was evidenced using C-banding (Sumner Citation1972). The detection of chromatin regions rich in GC and AT was performed using the method described by Schweizer (Citation1976), with double staining (chromomycin and 4',6-diamidino-2-phenylindole). Fluorescence in situ hybridization (FISH) was employed to locate ribosomal DNA genes, using the 18S rDNA probe obtained from Prochilodus argenteus (Hatanaka and Galetti Citation2004). The 18S probe was labeled with biotin-14-dATP by nick translation, following the manufacturer’s instructions (Bionick Labeling System, Invitrogen). The detection and amplification of the signal were performed with avidin-FITC (Sigma-Aldrich Co. USA) and biotinylated anti-avidin (Sigma-Aldrich Co. USA). The general hybridization procedure followed the protocol described by Pinkel et al. (Citation1986). The analysis was carried out using the Applied Spectral Image system coupled to an epifluorescence microscope (Carl Zeiss Axiophot Germany). Chromosome analyses were performed in about 30 metaphases of each specimen using the Case Data Manager Expo 4.0 (Applied Spectral Imaging, Migdal HaEmek, Israel) software program.
The chromosomes were classified based on the arm ratio proposed by Levan et al. (Citation1964), with metacentric (m), submetacentric (sm) and subtelocentric (st) chromosomes exhibiting two arms and acrocentric (a) chromosomes exhibiting one arm, and arranged in decreasing size order in the karyotype.
Results
The standard karyotype of Hypostomus aff. ancistroides had 2n = 66 chromosomes (12m, 16sm, 10st and 28a) and fundamental number (FN) = 104 (Figure A). C-banding revealed two large heterochromatic blocks corresponding to the short arm of one pair of submetacentric chromosomes and slight labeling in one pair of acrocentric chromosomes (Figure A). Silver impregnation revealed four to six Ag-NORs, all in terminal positions of the chromosome arms with intraindividual variation. These same regions were revealed by double staining with CMA3/DAPI as rich in GC bases and confirmed by FISH with the 18S probe (Figure A box).
Figure 2 Karyotype of (A) H. aff. ancistroides; (B) H. commersonii; and (C) H. derbyi. Conventional Giemsa staining.
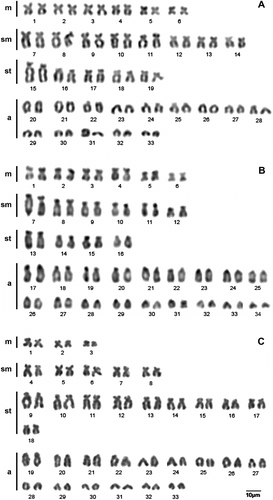
Figure 3 Mitotic metaphase showing heterochromatin distribution pattern in (A) H. aff. ancistroides; (B) H. commersonii; and (C) H. derbyi. Highlighted chromosomes with NORs labeled with silver nitrate, CMA3/DAPI and FISH with 18S probe (box).
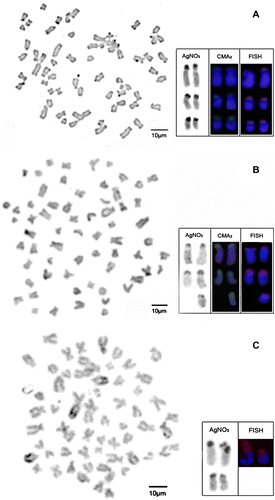
The standard karyotype of Hypostomus commersonii had 2n = 68 chromosomes (12m, 12sm, 8st and 36a) and FN = 100 (Figure B). C-banding revealed few heterochromatic tags only in the centromeric and telomeric regions of some chromosomes (Figure B). Ag-NORs were detected in up to five chromosomes with intraindividual variation. Double staining with CMA3/DAPI revealed five sites rich in GC coinciding with the Ag-NORs and confirmed by FISH with the 18S rDNA probe (Figure B box).
The standard karyotype of Hypostomus derbyi had 2n = 66 chromosomes (6m, 10sm, 20st and 30a) and FN = 82 (Figure C). C-banding revealed large heterochromatic blocks on some chromosome arms (Figure C). Ag-NORs were detected on the short arm in up to two chromosome pairs and confirmed by FISH with the 18S rDNA probe (Figure C box).
Discussion
The genus Hypostomus exhibits considerable diversity with regard to the diploid number, ranging from 2n = 54 chromosomes in H. plecostomus (Muramoto et al. Citation1968) to 2n = 84 in Hypostomus sp. 2 (Kavalco et al. Citation2005). Hypostomus emarginatus (2n = 52) has been taxonomically reallocated outside the genus Hypostomus as Squaliforma emarginata (Montoya-Burgos et al. Citation2002). Thus, the diploid number 2n = 52 chromosomes (Artoni and Bertollo Citation2001) is not valid for describing the basal condition in Hypostomus. Thus, 2n = 54 chromosomos proposed by Artoni and Bertollo (2001) as an ancestral condition for the family Loricariidae so also be considered for Hypostomus.
The present study reveals a new karyotype formula for H. aff. ancistroides, with a diploid number of 66 chromosomes (12m, 16sm, 10st and 28a), unlike the 2n = 68 chromosomes reported in other populations of this species (Artoni and Bertollo Citation1996; Alves et al. Citation2006; Endo et al. Citation2012). As Robertsonian rearrangements and pericentric inversions accompany karyotype diversification in this group of fish (Artoni and Bertollo Citation2001; Martinez et al. Citation2011), the H. aff. ancistroides population studied herein represents a more conserved condition among the karyotype geographic variants found in this species. However it still diverges from the basal condition suggested for Loricariidae with 2n = 54 chromosomes bi-armed (Artoni and Bertollo Citation2001), especially as found among Hypoptopomatinae (Ferreira et al. Citation2005).
The H. commersonii and H. derbyi karyotypes were described for the first time in the present study. In H. commersonii, the diploid number was 68 chromosomes (12m, 12sm, 8st and 36a) and the FN was 100. In H. derbyi, the diploid number was 2n = 66 chromosomes (6m, 10sm, 20st and 30a) and the FN was 82. A personal communication at scientific meetings suggests the existence of other karyotype formulas for these species in other locations (Bueno et al. Citation2012). One may hypothesize that the effects of allopatry lead to the accumulation of karyotype differences between populations of the same species, producing geographic karyotype variations, as may be occurring in the populations of H. commersonii and H. derbyi analyzed herein.
C-banding revealed a heterochromatin distribution pattern for H. commersonii in agreement with the trend proposed for Siluriformes, with low heterochromatin found mainly in the pericentromeric and telomeric regions (Gold et al. Citation1990). However, the H. aff. ancistroides and H. derbyi exhibited more evident heterochromatic blocks, although none were revealed with double fluorescent staining, demonstrating a heterogeneous nature in the composition of heterochromatin in these species.
Multiples NORs are often found in this genus (Artoni and Bertollo Citation1996), although NORs in a single pair of chromosomes have been detected in some species of Hypostomus (Artoni and Bertollo Citation2001). In the present study, all the populations had more than one pair of chromosomes with NORs (multiple NORs). Regarding the position, all NORs found here were located in terminal regions of the chromosomes, as observed commonly in the Loricariidae as well as the majority of Neotropical fish. The NOR sites were also all labeled by CMA3, indicating an arrangement in these regions rich in GC bases coincident with the 18S rDNA located by FISH. This correlation has been reported in different species of fish (Da Silva Cortinhas et al. Citation2003; Noleto et al. Citation2007, among others). However, one must consider that not all heterochromatin rich in CG is necessarily associated with NORs, as demonstrated by Artoni et al. (Citation1999) for Liposarcus anisitsi (the genus of which is related to Hypostomus), which exhibits large CG-positive heterochromatic blocks that do not correspond to NORs.
The detection of sites with ribosomal genes through FISH coincided with the Ag-NORs in both H. commersonii and H. aff. ancistroides. However, despite having found 2–4 NORs, only two fluorescent sites were identified by FISH using the 18S probe in H. derbyi (Table ). This suggests a polymorphic condition in this species. Another hypothesis is that two marks could be associated to heterochromatins (Molina and Galetti Citation2007).
Table 1. Comparative panel of karyotype and chromosome markers among H. commersonii, H. aff. ancistroides and H. derbyi.
The karyotype data presented here are useful to cytotaxonomy for differentiating the species from each other as well as differentiating these species from other allopatric populations with described karyotypes (Table ).
In conclusion, the interest in studying the genus Hypostomus is not attributed merely to its considerable morphological diversity or karyotype variability. Even within a large hydrographic basin, such as the system of the upper Paraná River to which the majority of species and populations of Hypostomus with described karyotypes belong, the increase in karyotype descriptions reveals more frequent cases of a species with different karyotype formulas in allopatry (geographic variations) or sympatry (cryptic species) as well as other cases of distinct species that resemble one another in the karyotype macrostructure. Thus, considering Hypostomus a valid genus (Armbruster Citation2004), the large complex of species that makes up this genus attracts increasing attention.
Acknowledgments
This study was funded by the Brazilian fostering agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
References
- Alves , AL , Oliveira , C , Nirchio , M , Granado , A. and Foresti , F . 2006 . Karyotypic relationships among the tribes of Hypostominae (Siluriformes: Loricariidae) with description of XO sex chromosome system in a Neotropical fish species . Genetica , 128 ( 1-3 ) : 1 – 9 .
- Armbruster , JW . 2004 . Phylogenetic relationships of the suckermouth armoured catfishes (Loricariidae) with emphasis on the Hypostominae and the Ancistrinae . Zool J Linn Soc , 141 : 1 – 80 .
- Artoni , RF and Bertollo , LAC . 1996 . Cytogenetic studies on Hypostominae (Pisces, Siluriformes, Loricariidae). Considerations on karyotype evolution in genus Hypostomus . Caryologia , 49 ( 3 ) : 81 – 90 .
- Artoni , RF and Bertollo , LAC . 2001 . Trends in the karyotype evolution of Loricariidae fish (Siluriformes) . Hereditas , 134 ( 3 ) : 201 – 210 .
- Artoni , RF , Molina , WB , Bertollo , LAC and Galetti , PM Jr . 1999 . Heterochromatin analysis in fish species Liposarcus anisitsi (Siluriformes) and Leporinus elongatus (Characiformes) . Genet Mol Biol , 22 ( 1 ) : 39 – 44 .
- Bertollo , LAC , Moreira-Filho , O and Galetti , PM Jr . 1986 . Cytogenetics and taxonomy: considerations based on chromosome studies of freshwater fish . J Fish Biol , 28 : 153 – 159 .
- Bertollo , LAC , Takahashi , CS and Moreira-Filho , O . 1978 . Cytotaxonomic considerations on Hoplias lacerdae (Pisces, Erythrinidae) . Brazil J Genet , 1 ( 2 ) : 103 – 120 .
- Birindelli , JLO , Zanata , AM and Lima , FCT . 2007 . Hypostomus chrysostiktos, a new species of armored catfish (Siluriformes: Loricariidae) from rio Paraguaçu, Bahia State . Brazil. Neotrop Ichthyol , 5 ( 3 ) : 271 – 278 .
- Bueno , V , Zawadzki , CH and Margarido , VP . 2012 . Trends in chromosome evolution in the genus Hypostomus Lacépède, 1803 (Osteichthyes, Loricariidae): a new perspective about the correlation between diploid number and chromosomes types . Rev Fish Biol Fish , 22 ( 1 ) : 241 – 250 .
- Cereali , SS , Pomini , E , Rosa , R , Zawadzki , CH , Froehlich , O and Giuliano-Caetano , L . 2008 . Karyotype description of two species of Hypostomus (Siluriformes, Loricariidae) of the Planalto da Bodoquena . Brazil. Genet Mol Res , 7 ( 3 ) : 583 – 591 .
- Da Silva Cortinhas , MC , Cestari , MM , Swarça , AC and Fenocchio , AS . 2003 . First chromosome data about the silverside Atherinella brasiliensis (Atheriniformes, Pisces) from the south coast of Brazil. Conventional, C-NOR and CMA3 bandings and FISH studies . Caryologia , 56 ( 2 ) : 187 – 191 .
- Endo , S , Martinez , ERM , Zawadzki , CH , Paiva , LRS and Julio , HF Jr . 2012 . Karyotype description of possible new species of the Hypostomus ancistroides complex (Teleostei: Loricariidae) and other Hypostominae . Acta Sci , 34 ( 2 ) : 181 – 189 .
- Ferraris , CJ Jr . 2007 . Check list of catfishes, recent and fossil (Osteichthyes: Siluriformes) and catalogue of siluriform primary types . Zootaxa , 1418 : 1 – 628 .
- Ferreira , DC , Chiachio , MC , Takako , AK , Andreata , AA , Foresti , F and Oliveira , C . 2005 . Comparative cytogenetics of nine species of Hypoptopomatinae (Teleostei: Siluriformes: Loricariidae): the importance of structural rearrangements in chromosome evolution . Caryologia , 58 ( 4 ) : 387 – 395 .
- Froese R, Pauly D. 2012. FishBase [Internet]; [cited 2012 Oct]. Available from: www.fishbase.org
- Gold , JR , Li , YC , Shipley , NS and Powers , PK . 1990 . Improved methods for working with fish chromosomes with a review of metaphase chromosome banding . J Fish Biol , 37 ( 4 ) : 563 – 575 .
- Hatanaka , T and Galetti , PM Jr . 2004 . Mapping of the 18S and 5S ribosomal RNA genes in the fish Prochilodus argenteus Agassiz, 1829 (Characiformes, Prochilodontidae) . Genetica , 122 ( 3 ) : 239 – 244 .
- Howell , WM and Black , DA . 1980 . Controlled silver staining of nucleolus organizer regions with a protective colloidal developer: a 1-step method . Experientia , 36 ( 8 ) : 1014 – 1015 .
- Kavalco , KF , Pazza , R , Bertollo , LAC and Moreira-Filho , O . 2005 . Karyotypic diversity and evolution of Loricariidae (Pisces, Siluriformes) . Hereditas , 94 ( 2 ) : 180 – 186 .
- Levan , A , Fredga , K and Sandberg , AA . 1964 . Nomenclature for centromeric position on chromosomes . Hereditas , 52 ( 2 ) : 201 – 220 .
- Martinez , ERM , Zawadzki , CH , Foresti , F and Oliveira , C . 2011 . Cytogenetic analysis of five Hypostomus species (Siluriformes, Loricariidae) . Genet Mol Biol , 34 ( 4 ) : 562 – 568 .
- Mendes-Neto , EO , Vicari , MV , Artoni , RF and Moreira-Filho , O . 2011 . Description of karyotype in Hypostomus regani (Ihering, 1905) (Teleostei, Loricariidae) from de Piumhi river in Brazil with comments on karyotype variation found in Hypostomus . Comp Cytogenet , 5 ( 2 ) : 133 – 142 .
- Milhomem , SSR , Castro , RR , Nagamachi , CY , Souza , ACP , Feldberg , E and Pieczarka , JC . 2010 . Different cytotypes in fishes of the genus Hypostomus Lacépèdde, 1803, (Siluriformes: Loricariidae) from Xingu river (Amazon region, Brazil) . Comp Cytogenet , 4 ( 1 ) : 45 – 54 .
- Molina , WF and Galetti Jr , PM . 2007 . Early replication banding in Leporinus species (Osteichthyes, Characiformes) bearing differentiated sex chromosomes (ZW) . Genetica , 130 ( 3 ) : 153 – 160 .
- Montoya-Burgos , JI , Weber , C and Le Bail , PY . 2002 . Phylogenetic relationships within Hypostomus (Siluriformes: Loricariidae) and related genera based on mitochondrial D- loop sequences . Rev Suisse Zool , 109 ( 2 ) : 369 – 382 .
- Muramoto , J , Ohno , S and Atkin , NB . 1968 . On the diploid state of the fish order Ostariophysi . Chromosoma , 24 ( 1 ) : 59 – 66 .
- Noleto , RB , Vicari , MR , Cipriano , RR , Artoni , RF and Cestari , MM . 2007 . Physical mapping of 5S e 45S rDNA loci in pufferfishes (Tetraodontiformes) . Genetica , 130 ( 3 ) : 133 – 138 .
- Pinkel , D , Straume , T and Gray , JW . 1986 . Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization . Proc Natl Acad Sci , 83 ( 9 ) : 2934 – 2938 .
- Reis RE, Kullander SO, Ferraris Jr CJ. 2003. Check list of the freshwater fishes of South America. EdiPUCRS, Porto Alegre. 599–602.
- Schweizer , D . 1976 . Reverse fluorescent chromosome banding with chromomycin and DAPI . Chromosoma , 58 : 307 – 324 .
- Sumner , AT . 1972 . A simple technique for demonstrating centromeric heterochromatin . Exp Cell Res , 75 ( 1 ) : 304 – 306 .
- Vari , RP and Malabarba , LR . 1998 . “ Neotropical ichthyology: an overview ” . In Phylogeny and classification of Neotropical Fishes , Edited by: Malabarba , LR , Reis , RE , Vari , RP , Lucena , ZMS and Lucena , CAS . 1 – 11 . EdiPUCRS : Porto Alegre .