Abstract
Cytology of a promising oilseed woody crop, Plukenetia volubilis, was analyzed for the first time here. There are in total 11 different chromosome numbers, ranging from 2n = 50 to 2n = 86 in P. volubilis. Different numbers were found not only in different root tips but also in different cells of the same tip. Among the cells, 2.78% cells had the lowest chromosome number of 2n = 50; and 1.39% cells had the highest chromosome number of 2n = 86. The most common chromosome number is 2n = 58 and about 27.79% cells had this quantity of chromosomes. The chromosomes were extremely small in size, at less than 2 μm. Thus, analysis of chromosome morphology was impaired. Polyploidy, confirmed as the significant evolutionary trend in chromosome number within this species, is briefly discussed. This species is a problematic and polymorphic taxon and our results indicate that its polyploidy can be regarded as the reason.
Introduction
Plukenetia, a poorly defined classification genus, belongs to the tribe Plukenetieae of subfamily Acalyphoideae in the family Euphorbiaceae. Plukenetia is a pantropical genus of over 20 species of twining vines and lianas, which were distributed throughout Africa and Latin America, and as far southeast as Malesia (Airy-Shaw Citation1982; Gillespie Citation1994, Citation2007; Bussmann et al. Citation2009). It is notable for its four-carpellate ovary and the associated character of four pistillate sepals, styles that are partially to entirely fused and often massive, and scandent habit (Gillespie Citation1994, Citation2007). However, on the basis of an evolutionary study of the floral characteristics of some groups in Euphorbiaceae, Plukenetia is a paraphyletic genus lacking any uniquely derived defining characters (Webster Citation1994; Gillespie Citation1994, Citation2007; Suarez-Cervera et al. Citation2001; Bussmann et al. Citation2009). The most well known and commercially valuable Plukenetia species is P. volubilis L., containing high levels of omega fatty acids in its seed (Hamaker et al. 1992; Cai et al. Citation2011). P. volubilis, a promising oilseed crop commonly known as Sacha Inchi, was native to the Amazon rainforest and was restricted to South America and the Lesser Antilles, where it has been cultivated by indigenous people for centuries (Semino et al. Citation2008). It flowers five months after being planted, and bears seeds around the eighth month (Cai Citation2011). The male flowers are small, white, and arranged in clusters. Two female flowers are located at the base of the inflorescence and it often produces seeds nearly year-round in the tropical region.
Due to their worldwide distribution and the fact that they include many economically important plants, many genera and species of the family Euphorbiaceae have been the subject of cytological studies (Perry Citation1943; Miller and Webster Citation1966; Hans Citation1973; Vosa and Bassi 1991; Vanzela et al. Citation1997; Soontornchainaksaeng and Chaiyasut Citation1999; Krahenbuhl et al. Citation2002; Aarestrup et al. Citation2008). Karyotypic changes are frequent in the evolution of higher plants and are often used as the basis for deductions about phylogenetics, thus the results of chromosomal studies may be useful in plant taxonomy and phylogeny analysis (Levin Citation2002). In addition, information about number and structure of chromosomes of both wild resources and cultivars is an important prerequisite for effective crop genetic and breeding studies (Levin Citation2002). Although some cytological data on Euphorbiaceae have been generated (Perry Citation1943; Vosa and Bassi 1991; Vanzela et al. Citation1997; Soontornchainaksaeng and Chaiyasut Citation1999; Krahenbuhl et al. Citation2002; Xu et al. Citation2007), there exists to date no literature on the karyotype analysis of the genus Plukenetia.
This is the first cytological study of P. volubilis. The aim of this study is (1) to identify the chromosome number and morphology of this oilseed crop species for further selection and breeding of new improved cultivars; and (2) to supply cytological proof for the taxonomy and phylogeny of Plukenetia, an economically important genus.
Materials and methods
Plukenetia volubilis seeds, collected from Peru, South America, were germinated on wet filter papers in Petri dishes in suitable lab conditions. Root tips approximately 1 cm long were cut and pre-treated in a saturated dichlorobenzene solution at room temperature in darkness for 2 h, then fixed with a carnoy solution, comprised of absolute alcohol:glacial acetic acid (3:1), at approximately 4°C for 1 h, then rinsed in distilled water twice for approximately 20 minutes. After hydrolysing in 1 mol l−1 HCl at 60°C for 7 minutes and staining with 1% aceto-orcein overnight, the meristematic zones of the tips was squashed for cytological observation.
Chromosome spreads were observed and photographed using a Nikon E800 microscope (Nikon, Japan). Cells with well-scattered chromosomes from five root tips were observed in detail and chromosome numbers were carefully counted.
Results
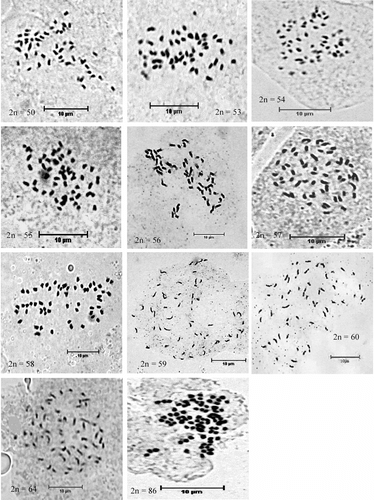
Among the 72 cells observed in P. volubilis, there are in total 11 different chromosome numbers, ranging from 2n = 50 to 2n = 86 (Figure ). Different chromosome numbers were found not only in different root tips but also in different cells of the same tip (Table ). The lowest chromosome number of 2n = 50 was found in 2.78% of the cells, and 1.39% cells had the highest chromosome number of 2n = 86. The most common chromosome number is 2n = 58 and about 27.79% cells had this quantity of chromosomes.
Table 1. Chromosome numbers found in root tip cells of P. volubilis.
The absolute length of the chromosomes of P. volubilis is less than 2 μm (Figure ), thus the chromosomes were small-sized ones. Because of that, analysis of chromosome morphology was impaired by the extremely small size of the chromosomes, restricting the comparison of the species in terms of chromosome type.
Discussion
Karyotype analysis has been useful for clarifying species by measuring number, size, arm ratio, and unique shape of chromosomes at the somatic metaphase (Levin Citation2002). Chromosomal information is an important key to the classification, phylogenetic analysis and evolutionary biology of plant species, because chromosomal information in plants, unlike other characteristics, retains its consistency (Levin Citation2002; Adams and Wendel Citation2005). The chromosome number of different cells in the metaphase of P. volubilis was so non-uniform that the single basic chromosome number of this promising oilseed crop could not been easily and correctly postulated. Perry (Citation1943) analyzed a large number of species in the Euphorbiaceae and observed that the basic chromosome numbers of unrelated species formed a primary series of x = 7, 8 and 9 and a secondary series of x = 6, 10 and 11. Vanzela et al. (Citation1997) thought the chromosome numbers detected in the subfamily Acalyphoideae were quite fragmented and did not suggest a single basic number for the subfamily and an appropriate cytotaxonomic interpretation. In addition, chromosome counts performed in the subfamily Acalyphoideae have revealed that the tribe Plukenetieae consists exclusively of polyploid species (Vanzela et al. Citation1997). Thus, P. volubilis is a polyploid plant, like 41.38% of other species in Acalyphoideae (Vanzela et al. Citation1997) and 50% of other species in the Euphorbiaceae family (Perry Citation1943; Aarestrup et al. Citation2008).
Currently available chromosome data show that polyploidy is the most significant evolutionary trend in chromosome number within Euphorbiaceae (Perry Citation1943; Hans Citation1973; Vosa and Bassi 1991; Vanzela et al. Citation1997; Krahenbuhl et al. Citation2002; Aarestrup et al. Citation2008). Polyploid plants usually have larger cells and plants are often larger, with thicker shoots, larger leaves and fruits, and a higher nutritional content, etc. (Levin Citation2002). Polyploidy is also widely acknowledged as a major mechanism of adaptation and speciation in plants (Levin Citation2002; Adams and Wendel Citation2005; Jian et al. Citation2012). Polyploids may have superior levels of adaptability and higher probabilities of survival than their diploid relatives (Soltis and Soltis Citation2000). Moreover, polyploidy is thought to imbue plants with novel features that allow them to occupy new environments, expand their geographic range, or achieve new species interactions (Levin Citation2002). Its strong and broad ecological valence would permit rapid spreading out and coexistence of different chromosome forms, which in some cases have been noted to show diverse behavior of P. volubilis plants growing under a wide range of environmental gradients in the tropical regions (Semino et al. Citation2008; Cai Citation2011; Cai et al. Citation2012), This adaptive significance of polyploidy was tested recently in wild yarrow (Achillea borealis) (Ramsey Citation2011).
Cytogenetic variation in plants has long attracted biologists’ interest (Stebbins Citation1950). What is particularly interesting about the P. volubilis described here is its surprisingly high and diverse cytological variation. The presence of a total of 11 different chromosome numbers in P. volubilis is clearly an example of a chromosomally highly variable species. It seems that polyploidy is confirmed as the most significant evolutionary trend in chromosome number within this species. The same phenomenon (numerous chromosome alterations with dysploidy resulted in high and diverse chromosome numbers) was also found in Dalechampia species in the same subfamily, Acalyphoideae (Vanzela et al. Citation1997). On the other hand, there are many reports of the widespread occurrence of chromosome races or variants within species (Murray and Young Citation2001; Severns and Liston Citation2008; Abdolkarim et al. Citation2011), especially for rare plants (Severns and Liston Citation2008). In some cases there is a clear geographic separation of the races; in others the different chromosome numbers are due to the variable presence of chromosomes, or supernumerary chromosomes, and in a third group differences in chromosome number may indicate the need for taxonomic revision (Severns and Liston Citation2008). The reason for this variation and instability is the variation in chromosome number, resulting in taxonomical problems in defining its species and genus (Vanzela et al. Citation1997; Gillespie Citation2007). Moreover, the diverse chromosome number of P. volubilis might imply that this crop has rich genetic diversity. There could be many wild resources with different cytological characters in the natural habitat, which could be further used to improve the resistance, productivity or the quality of seed oil of the widely cultivated varieties. Biparental inbreeding in normally outbreeding species may be responsible for the elevated level of chromosome variation (Parker and Wilby Citation1989). Following enforced inbreeding in a variety of plants, increased chromosome instability was observed, resulting in high levels of spontaneous breakage and reunion of chromosomes and a reduction in chiasma frequency, with an increase in univalent formation (Murray and Young Citation2001; Levin Citation2002; Severns and Liston Citation2008). There is no evidence for significant levels of inbreeding in the P. volubilis plants we examined. In addition, in many other groups in the Euphorbiaceae family, such as Euphorbia L., structural rearrangements play a marked evolutionary role in karyotype differentiation (Vosa and Bassi 1991). However, because of the extremely small size of the chromosomes of P. volubilis, the rearrangements responsible for karyotype differentiation cannot be clearly pointed out.
Acknowledgments
This work was financially supported by grants (KSCX2EWQ17, KSCX2EWZ15) from the Chinese Academy of Sciences, and by a grant (31170641) from the National Natural Science Foundation of China.
References
- Aarestrup , JR , Karam , D and Fernandes , GW . 2008 . Chromosome number and cytogenetics of Euphorbia heterophylla L . Genet Mole Res , 7 ( 1 ) : 217 – 222 .
- Abdolkarim , C , Atri , M , Sarmadi , J and Asgari , M . 2011 . Chromosome number variation in Tanacetum polycephalum Schultz Bip. (L.) (Asteraceae) in west of Iran . Caryologia , 64 ( 3 ) : 302 – 308 .
- Adams , KL and Wendel , JF . 2005 . Polyploidy and genome evolution in plants . Curr Opin Plant Biol , 8 ( 2 ) : 135 – 141 .
- Airy-Shaw , HK . 1982 . The Euphorbiaceae of central Malesia (Celebes, Moluccas, Lesser Sunda Is.) . Kew Bulletin , 26 ( 1 ) : 191 – 363 .
- Bussmann , RW , Tellez , C and Glenn , A . 2009 . Plukenetia huayllabambana sp. nov. (Euphorbiaceae) from the upper Amazon of Peru . Nord J Bot , 27 ( 4 ) : 313 – 315 .
- Cai , ZQ . 2011 . Advance in research on a special woody oilseed crop. Plukenetia volubilis . China Oils Fats , 33 ( 10 ) : 1 – 6 .
- Cai , ZQ , Jiao , DY , Tang , SX , Dao , XS , Lei , YB and Cai , CT . 2012 . Leaf photosynthesis, growth and seed chemicals of Sacha Inchi (Plukenetia volubilis) plants cultivated along an altitude gradient . Crop Sci , 52 ( 4 ) : 1859 – 1867 .
- Cai , ZQ , Yang , Q , Tang , SX and Dao , XS . 2011 . Nutritional evaluation in seeds of a woody oil crop . Plukenetia volubilis Linneo. Acta Nutrim Sin , 33 ( 2 ) : 193 – 195 .
- Gillespie , LJ . 1994 . Pollen morphology and phylogeny of the tribe Plukenetieae (Euphorbiaceae) . Ann MO Bot Gard , 81 ( 2 ) : 317 – 348 .
- Gillespie , LJ . 2007 . A revision of Paleotropical Plukenetia (Euphorbiaceae) including two new species from Madagascar . Syst Bot , 32 ( 4 ) : 780 – 802 .
- Hamaker , BR , Valles , C , Gilman , R , Hardmeier , RM , Clark , D , Garcia , HH , Gonzales , AE , Kohlstad , I and Castro , M . 1992 . Amino acid and fatty acid profiles of the Inca peanut (Plukenetia volubilis L.) . Cereal Chem , 69 ( 4 ) : 461 – 463 .
- Hans , AS . 1973 . Chromosomal conspectus of the Euphorbiaceae . Taxon , 22 ( 5 ) : 591 – 636 .
- Jian , HY , Zhang , T , Wang , QG , Li , SB , Zhang , H and Tang , KX . 2012 . Karyological diversity of wild Rosa in Yunnan . Southwestern China. Genet Resour Crop Evol , 59 ( 1 ) : 1 – 13 .
- Krahenbuhl , M , Yuan , YM and Kupfer , P . 2002 . Chromosome and breeding system evolution of the genus Mercurialis (Euphorbiaceae), implications of ITS molecular phylogeny . Plant Syst Evol , 234 ( 1 ) : 155 – 169 .
- Levin , DA . 2002 . The role of chromosomal change in plant evolution , Oxford : Oxford University Press .
- Miller , KI and Webster , GL . 1966 . Chromosome numbers in the Euphorbiaceae . Brittonia , 18 ( 4 ) : 372 – 379 .
- Murray , BG and Young , AG . 2001 . Widespread chromosome variation in the endangered grassland forb Rutidosis leptorrhynchoides F. Muell. (Asteraceae, Gnaphalieae) . Ann Bot , 87 ( 1 ) : 83 – 90 .
- Parker , JS and Wilby , AS . 1989 . Extreme chromosome heterogeneity in a small-island population of Rumex acetosa . Heredity , 62 ( Pt 1 ) : 133 – 140 .
- Perry , BA . 1943 . Chromosome number and phylogenetic relationships in the Euphorbiaceae . Am J Bot , 30 ( 7 ) : 527 – 543 .
- Ramsey , J . 2011 . Polyploidy and ecological adaptation in wild yarrow . Proc Natl Acad Sci USA , 108 ( 17 ) : 7096 – 7101 .
- Semino CA, Rojas FC, Zapata ES. 2008. Protocolo del cultivo de Sacha inchi (Plukenetia volubilis L.). La Merced, Peru. PhD thesis, pp. 1–87.
- Severns , PM and Liston , A . 2008 . Intraspecific chromosome number variation, a neglected threat to the conservation of rare plants . Conserv Biol , 22 ( 6 ) : 1641 – 1647 .
- Soltis , PS and Soltis , DE . 2000 . The role of genetic and genomic attributes in the success of polyploids . Proc Natl Acad Sci USA , 97 ( 13 ) : 7051 – 7057 .
- Soontornchainaksaeng , P and Chaiyasut , K . 1999 . Cytogenetic investigation of some Euphorbiaceae in Thailand . Cytologia , 64 ( 3 ) : 229 – 234 .
- Stebbins , GL . 1950 . Variation and Evolution in Plants , New York : Columbia University Press .
- Suarez-Cervera , M , Gillespie , L , Arcalis , E , Le Thomas , A , Lobreau-Callen , D and Seoane-Camba , JA . 2001 . Taxonomic significance of sporoderm structure in pollen of Euphorbiaceae, tribes Plukenetieae and Euphorbieae . Grana , 40 ( 1 ) : 78 – 104 .
- Vanzela , A , Ruas , PM and Marin-Morales , MA . 1997 . Karyotype studies of some species of Dalechampia Plum. (Euphorbiaceae) . Bot J Linn Soc , 125 ( 1 ) : 25 – 33 .
- Vosa , CG and Bassi , P . 1991 . Chromosome studies in the Southern African flora. 95–102: the basic karyotype of eight species of succulent Euphorbia L . Caryologia , 44 ( 1 ) : 27 – 33 .
- Webster , GL . 1994 . Classification of the Euphorbiaceae . Ann MO Bot Gard , 81 ( 1 ) : 3 – 32 .
- Xu , HG , Zhou , SD , He , XJ and Yu , Y . 2007 . Karyotype in fifteen populations belonging to thirteen species of Euphorbia (Euphorbiaceae) in China . Acta Phytotaxon Sin , 45 ( 5 ) : 619 – 626 .