Abstract
During microsporogenesis sometimes the chromatin materials migrate from one cell to another by intermeiocyte connections. In the present study, during induction of tetraploidy in sunflower, changes in the normal behaviour of chromosomes was detected. Besides chromosomal aberrations, cytomictic connections were frequently recorded. Cytomixis has been reported by many researchers in many diploid or tetraploid plants. In sunflower, cytomixis in colchiploids has not been recorded. In the current study high numbers of cytoplasmic connections were observed in the plants failing to reach higher ploidy level. Formation of cytoplasmic connections was very high, leading to many hyperploids, hypoploids and even empty pollen mother cells (PMCs). Cytomixis occurred frequently, with two to many meiocytes engaged in exchange of chromatin through one or multiple cytoplasmic connections. Few rare polyploid PMCs with cytoplasmic connections were observed, which favours formation of aneuploids and polyploids. Besides various chromosomal anomalies, fertility was also significantly affected. This work envisages the possibility that although polyploidy was not achieved in some plants there is tendency to form higher ploidy levels in this species. This illustrates the potential for formation of various genetic combinations and thus novel traits. Cytomixis is also an additional source of male sterility in sunflower and thus requires intense research.
Introduction
Induction of polyploidy is an exciting phenomenon in agricultural systems for enhancing yield. Colchicine leads to doubling of the chromosome complement and also changes in the normal behavior of the chromosome. The sunflower is an economically and commercially important plant because of its heart-healthy profile. This attracts breeders to induce polyploidy in the sunflower. Polyploidy has been successfully achieved by several studies (e.g. Singh Citation1992; Srivastava and Srivastava Citation2002). The present work was undertaken to induce polyploidy in Helianthus annuus and to study morphogenetic changes during induction.
In the present study cytomixis was recorded in the plants which reverted back during induction of colchiploidy. Chromosomal transfer by cytoplasmic connections is referred to as cytomixis. Cytomixis is an important phenomenon as it affects the chromosome count and also the fertility of plants. According to many authors this is a genetically controlled process which is affected by external factors (Zheng et al. Citation1987; Bellucci et al. Citation2003; Boldrinia and Pagliarini Citation2006; Lattoo et al. Citation2006). However, others (Ghanima and Talaat Citation2003; Boldrinia and Pagliarini 2006) believe that it has evolutionary importance. There has been controversy about the occurrence of cytomixis in pollen mother cells (PMCs). Cytomixis is a phenomenon found in normal species, hybrids and apomicts (Kamra Citation1960). However, some researchers (Semyarkhina and Kuptsou Citation1974; Singhal et al. 2007) reported it most frequently in meiocytes of autotetraploids rather than diploids. Cytomixis in colchicine-treated but undoubled plants has also been reported (Tai and Dewey Citation1966; Bauchan et al. Citation1987). The present study deals with the occurrence of cytoplasmic connections in the plants treated with colchicine. This work also demonstrates the frequency of cytomixis at different meiotic phases and its effect on pollen and ovule fertility of the plant.
Materials and methods
Dried and healthy seeds of Helianthus annuus were pre-soaked in water for 6 h. Seeds were sown in pots in replicates. Control plants were raised simultaneously. When the seeds germinated and cotyledonary leaves opened, cotton balls soaked in aqueous solutions of 0.2%, 0.4% and 0.6% colchicine were applied overnight in two sets for each concentration. The process was repeated for three consecutive days. After each treatment a recovery period of 12 h was allowed. After treatment, the plants were raised to maturity. Young floral buds were fixed in Carnoy’s fixative for cytological assessment. Pollen and ovule sterility was also recorded. Sterility of pollen was studied by staining anthers in 1% acetocarmine-glycerine stain. Stained pollen grains were considered as fertile and unstained as sterile. Ovule/seed sterility was estimated by percentage of seeds set per capitulum.
Results
In the colchicine-treated sets, out of 540 plants only 22 were tetraploids, while the remaining either reverted back or showed mixoploidy. The plants failing to achieve higher ploidy showed variable counts of cytomictic cells in each treated set. Chromatin migration through cytoplasmic channels occurred frequently during meiosis. Frequency of cytomixis was lowest at the 0.2% treatment dose and highest at the 0.6% dose. During migration the content of the cell accumulated at one end, creating an extended cytoplasm from which chromatin materials extruded. The chromosomes accumulated in groups and formed varying numbers of chromatin balls at the junction point of meiocytes. Cytoplasmic connections were formed with the nearby cells and the chromatin material passed from one cell to another through chromatin bridges. One or more cytoplasmic channels were formed to facilitate transfer of chromatin materials. Usually a connection was created between two (Figure A, B, C) or more than two cells at a time in chain (Figure G) or in a cluster. In some cases the chromatin material moved in more than one direction (Figure D, H). In a few cases the cytoplasmic connections between PMCs were so intense that many cells were seen interconnected at a time (Figure H).
Figure 1 (A) Chromatin transfer with one PMC at diakinesis; (B) migration of chromatin through cytoplasmic bridge; (C) empty cell with a remnant after migration of chromosomes; (D) complete migration of chromatin with one PMC showing bidirectional movement of chromatin; (E) cytoplasmic connection between cells with a single chromatin ball at junction, one PMC showing secondary association of chromosomes; (F) cytomixis between adjacent cells with one PMC at metaphase-II; (G) migration of chromatin material between cells in chain with one cell showing affinity to form connection; (H) simultaneous and multiple cytoplasmic connections in group of PMCs; (I) chromatin bridge between hyperploid PMCs; (J) tetrad and triad; (K) smaller and larger pollen grains (unreduced pollen); (L) fertile (stained), semi sterile (half stained) and sterile pollen grains (lightly stained).
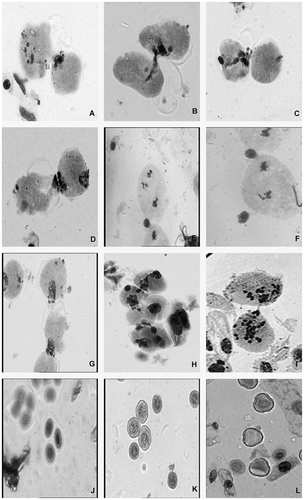
The cells engaged in chromatin migration were either at the same meiotic stage or at different stages (Figure E, F). Cytoplasmic connections were observed between cells at various stages of meiosis. Cytomixis at different meiotic stages is depicted in Table . The percentage of cytomixis increased with increasing dose of colchicine. Table shows that intermeiocyte connections were more frequent at the pachytene stage (76.12%) of the 0.6% colchicine treatment, followed by at other prophase-I stages. Formation of intermeiocyte connections was less frequent in late meiotic stages.
Table 1. Frequency (in %) of cytomixis at different stages of meiosis in control and cytomictic plants.
Cytomixis resulted in varied ploidy levels. The chromosome counts in the cytomictic cells were either more or less than in normal PMCs. However some meiocytes were found to be completely empty after chromatin migration (Figure A, C). Intermeiocyte connections were also encountered in a few PMCs with chromosomes number more than the diploid complement (Figure I).
As well as during cytomixis various chromosomal abnormalities were also reported. The control plants showed normal chromosome behaviour. Stickiness was registered in the highest frequency. Other anomalies such as scattering, precocious movement, unorientation, secondary association, bridges, laggards and micronuclei were also significant. A striking deviation in bivalent formation at diakinesis was recorded. Univalents and multivalents in the form of chains or rings were very frequent. Varying degrees of aberrations at metaphase and anaphase were observed. Tetrad abnormalities such as diads, triads and pentads were also prevalent. Triads (Figure J) were more frequent among pollen abnormalities.
Fertility was much lower in the treated plants; the sterility percentage is shown in Table . The presence of light-stained pollen grains (Figure L) showed the frequency of pollen sterility. At 0.2% and 0.4% colchicine treatments only partial sterility was found. However, the sterility increased greatly at the 0.6% dose. At the 0.6% dose the frequency of semi-sterile pollen was recorded as 14.63% and completely sterile pollen was about 13.05%. However, ovule sterility also increased to 38.42 + 1.21(%) at the highest dose treatment as compared to control plants. Some pollen grains were also found to be bigger than the normal pollen grains (Figure K).
Table 2. Pollen and ovule sterility percentage in control and cytomictic plants.
Discussion
Cytomixis is the migration of chromosomes from one cell to another through cytoplasmic channels. It has been detected by different workers in many plants. Some workers observed it in diploids (Sapre Citation1978), others showed it in genetically unbalanced plants (Salesses Citation1970; Mantu and Sharma Citation1983) and some observed it more frequently in polyploids (Semyarkhina and Kuptsou Citation1974). Cytomixis has been described in meiocytes; however, Papini et al. (Citation2010) reported intercellular channels in somatic cells of plants for the first time. There are various views regarding the occurrence of cytomixis. It has now been proved by various workers that cytomixis is a genetically controlled process. However, other environmental and physiological processes also alter cytomixis (Bellucci et al. Citation2003; Boldrinia and Pagliarini 2006; Lattoo et al. Citation2006). Fatemah et al. (Citation2010) predicted the probable cause of cytomixis to be persistence of plasmodesmata during meiosis. Cell wall dissolution between the adjacent cells may also lead to cytoplasmic connections (Falistocco et al. Citation1995). According to Dwivedi et al. (Citation1988) the probable cause of cytomixis may be the chemical action of colchicine. Colchicine is known to alter the functioning of microtubules, and hence inhibits the formation of cell wall (Hardman Citation1982). A few other workers believe that alterations in biochemical processes influence the functioning of anthers and lead to cytomixis (Risueño et al. Citation1969). Many others believe it to be due to the malfunctioning of genes (Bedi Citation1990). Nirmala and Kaul (Citation1994) predicted it to be due to the male sterile mutant gene.
The cytomixis observed here might be due to a misbalance in chromosomal activity during reversal. The chemical action of colchicine may be responsible for this. Various chromosomal abnormalities were also encountered which suggests that cytomixis is correlated with these genetic aberrations. Among the various chromosomal aberrations, stickiness was most common. Stickiness was mostly observed because of an accumulation of chromosomes during cytomixis. Due to prevalent stickiness and condensation of chromosomes during cytomixis the individuality of chromosomes was lost in most of the cases. Similar observations were also reported by Singhal and Kumar (2008). Cytoplasmic connections were recorded in most of the meiotic phases. Cytomixis was most prevalent in prophase-I and its occurrence in this phase also indicates its genetic basis. Similar observations were recorded in various plants (Guochang et al. Citation1987; Yen et al. Citation1993; Bellucci et al. Citation2003). This showed that certain changes in chromosomal properties lead to migration of chromatin materials in the surrounding cells through cytoplasmic connections. In most of the cases the migration was in the form of condensed chromatin balls. Chromatin balls have also been reported by Zhen-Qiao and Xing-Feng (2009). This migration of chromosomes resulted in unbalanced gametes, and due to this mixoploids have formed. The present analysis is in accordance with De Nettancourt and Grant (Citation1964), who suggested that cytomixis is a speciality of plants lacking balanced gametes such as aneuploids, haploids or hybrids. Ranjbar et al. (Citation2011, Citation2012) showed the occurrence of similar meiotic irregularities along with coenocytes due to cytomixis. In some cases the cells were left completely empty, showing migration of all chromatin materials. High frequency of intermeiocyte connections leads to a reduction in fertility. The reduced fertility could be a consequence of unbalanced gametes formed during cytomixis. In the cytomictic plants pollen sterility was prevalent. Abnormal tetrads, i.e. diad, triad and pentad, were recorded in considerable frequency in the plants where intermeiocyte connections were observed. This is probably due to various chromosomal abnormalities along with cytomixis leading to aberrant pollen. Similar results were shown by Bellucci et al. (Citation2003). Some pollen grains were found to be larger than the normal pollens, which may be attributed to increased chromosome number in some of the meiocytes during cytomixis. This is in accordance with results reported by Sheidai et al. (Citation2010), but contrary to the smaller size of pollen grains of colchicine-treated plants documented by Bauchan et al. (Citation1987). These unreduced pollen grains show the possibility of formation of polyploids or aneuploids.
This indicates that cytomixis directly or indirectly interferes with fertility of the plant and normal functioning of chromosomes and results in many deformities such as chromosomal anomalies, pollen sterility, tetrad abnormalities, etc. All these associated abnormalities and the high intensity of cytomixis predicts that cytomixis is due to genetic consequences and may be affected by environmental factors. Another important aspect is that cytomixis with or without chromatin degeneration may act as a cause for male sterility (Nirmala and Rao Citation1996). Pagliarini (Citation2000) and Vala et al. (Citation2011) have also predicted cytomixis to be the cause of male sterility. Although cytomixis is of little importance because of its negative effects on fertility, it provides a route for production of aneuploids and individuals with higher ploidy level. Cytomixis needs to be the focus of future study, as it has the potential to induce male sterility.
Acknowledgements
The authors are grateful to the Plant Genetics Laboratory, Department of Botany, University of Allahabad for providing invaluable technical assistance and facilities.
References
- Bauchan , GR , Linkous , L-CW and Tai , W . 1987 . Cytomixis in Agropyron cristatum . Genome , 29 : 765 – 769 .
- Bedi , YS . 1990 . Cytomixis in woody species . P Indian AS-Plant Sc , 100 : 233 – 238 .
- Bellucci , M , Roscini , C and Mariani , A . 2003 . Cytomixis in Pollen Mother Cells of Medicago sativa L . J Hered , 94 ( 6 ) : 512 – 516 .
- Boldrini , KR and Pagliarini , MS . 2006 . Cell fusion and cytomixis during microsporogenesis in Brachiaria humidicola (Poaceae) . S Afr J Bot , 72 ( 3 ) : 478 – 481 .
- De Nettancourt , D and Grant , WF . 1964 . La cytogénétique de Lotus (Leguminosae) III. Un cas de cytomixie dans un hybride interspécifique . Cytologia , 29 : 191 – 195 .
- Dwivedi , NK , Sikdar , AK , Jolly , MS , Susheelamma , BN and Suryanarayana , N . 1988 . Induction of tetraploidy in colchicine-induced mutant of mulberry. I. Morphological and cytological studies in cultivar Kanva–2 . Indian J. Genet , 48 : 305 – 311 .
- Falistocco , E , Tosti , T and Falcinelli , M . 1995 . Cytomixis in pollen mother cells of diploid Dactylis, one of the origins of 2n gametes . Heredity , 86 : 448 – 453 .
- Fatemah , F , Sheidai , M and Asadi , M . 2010 . Cytological study the genus Arenaria L. (Caryophyllaceae) . Caryologia , 63 ( 2 ) : 149 – 156 .
- Ghanima , AM and Talaat , AA . 2003 . Cytomixis and its possible evolutionary role in a Kuwaiti population of Diplotaxis harra (Brassicaceae) . Bot J Linn Soc , 143 : 169 – 175 .
- Guochang , Z , Qinglan , Y and Yongren , Z . 1987 . The relationship between cytomixis, chromosome mutation and karyotype evolution in Lily . Caryologia , 40 : 243 – 259 .
- Hardman , AR . 1982 . “ Regulation of polarity in tissues and organs ” . In The cytoskeleton in plant growth and development , Edited by: Lloyd , CW . New York , NY : Academic Press .
- Kamra , OP . 1960 . Occurrence of binucleate and multinucleate pollen mother cells in Hordeum . Hereditas , 46 : 536 – 542 .
- Lattoo , SK , Khan , S , Bamotra , S and Dhar , AK . 2006 . Cytomixis impairs meiosis and influences reproductive success in Chlorophytum comosum (Thunb) Jacq. — an additional strategy and possible implications . J Biosci , 31 : 629 – 637 .
- Mantu , DE and Sharma , AK . 1983 . Cytomixis in pollen mother cells of an apomictic ornamental Ervatamia divaricata (Linn.) Alston . Cytologia , 48 : 201 – 207 .
- Nirmala , C and Kaul , MLH . 1994 . Male sterility in pea . VI. Gene action duplicity. Cytologia , 59 : 195 – 201 .
- Nirmala , A and Rao , PN . 1996 . Genesis of chromosomal numerical mosaicism in higher plants . Nucleus , 39 : 151 – 175 .
- Pagliarini , MS . 2000 . Meiotic behavior of economically important plant species: the relationship between fertility and male sterility . Genet Mol Biol , 23 ( 4 ) : 997 – 1002 .
- Papini , A , Tani , G , Di Falco , P and Brighigna , L . 2010 . The ultrastructure of the development of Tillandsia (Bromeliaceae) trichome . Flora , 205 ( 2 ) : 94 – 100 .
- Ranjbar , M , Karamian , R and Nouri , S . 2011 . Impact of cytomixis on meiosis in Astragalus cyclophyllos Beck (Fabaceae) from Iran . Caryologia , 64 ( 3 ) : 256 – 264 .
- Ranjbar , M , Hajmoradi , F and Karamian , R . 2012 . An overview on cytogenetics of the genus Onobrychis (Fabaceae) with special reference to O. sect. Hymenobrychis from Iran . Caryologia , 65 ( 3 ) : 187 – 198 .
- Risueño , MC , Giménez-Martin , G , López-Sáez , JF and R-Garcia , MI . 1969 . Connexions between meiocytes in plants . Cytologia , 34 : 262 – 272 .
- Salesses , G . 1970 . Sur le phénomène de cytomixie chez des hybrides triploïdes de prunier . Conséquences génétiques possibles. Ann Amélior Plant , 20 : 383 – 388 .
- Sapre , AB . 1978 . Cytomixis in Trilobachne cookei (Stapf) Schenck (Poaceae) . Indian J Bot , 1 ( 1/2 ) : 29 – 33 .
- Semyarkhina , SYA and Kuptsou , MS . 1974 . Cytomixis in various forms of sugarbeet . Vests I ANBSSE Ser Biyal , 4 : 43 – 47 .
- Sheidai , M , Jaffari , F , Noormohammadi , Z and Shahid , B . 2010 . Cytomixis and unreduced pollen grain formation in six Hordeum species . Gene Conserve , 9 ( 37 ) : 136 – 151 .
- Singh , RN . 1992 . Chromosomal abnormalities and fertility in induced autotetraploid Helianthus annuus in C1 and C2 generation . Cytologia , 57 : 277 – 281 .
- Singhal , VK and Kumar , P . 2008 . Impact of cytomixis on meiosis, pollen viability and pollen size in wild populations of Himalayan poppy (Meconopsis aculeata Royle) . J Biosci , 33 ( 3 ) : 371 – 380 .
- Singhal , VK , Gill , BS and Dhaliwal , RS . 2007 . Status of chromosomal diversity in the hardwood tree species of Punjab state . J. Cytol. Genet. , 8 : 67 – 83 .
- Srivastava , R and Srivastava , GK . 2002 . Colchicine induced autotetraploidy in Helianthus annuus L . J Cytol Genet , 3 : 15 – 21 .
- Tai , W and Dewey , DR . 1966 . Morphology, cytology, and fertility of diploid and colchicine induced tetraploid crested wheatgrass . Crop Sci , 6 : 223 – 226 .
- Vala , AG , Fougat , RS , Roshni , S and Vinay , K . 2011 . Cytological causes of blond psyllium for male sterility . Electron J Plant Breed , 2 ( 1 ) : 100 – 106 .
- Yen , C , Yang , J-L and Sun , G-L . 1993 . Intermeiocyte connections and cytomixis in intergeneric hybrids of Roegneria ciliaris (Trin.) Nevski with Psathyrostachys huashanica Keng . Cytologia , 58 : 187 – 193 .
- Zheng , GC , Yang , Q and Zheng , Y . 1987 . The relationship between cytomixis, chromosome mutation and karyotype evolution in Lily . Caryologia , 40 : 243 – 259 .
- Zhen-Qiao , S and Li , Xing-Feng . 2009 . Cytomixis in pollen mother cells of Salvia miltiorrhiza . Caryologia , 62 ( 3 ) : 213 – 219 .