Abstract
We analyzed the karyological criteria of the Japanese water shrew, Chimarrogale platycephala in central Honshu. Only a few studies on the karyotype of this species for samples from Aomori Prefecture, northernmost Honshu, the Japanese Islands have been published. Our current results indicate that the chromosomal constitutions are fundamentally identical to those of previous studies. In addition, heteromorphism in the largest autosomal pair (no. 1 homologue) was also observed, in agreement with previous data. This heteromorphism of the no. 1 homologue is considered a usual phenomenon in C. platycephala based on previous and current results. In addition, another autosomal heteromorphism on medium-sized subtelocentrics was observed as the first example. The heteromorphic regions were positively stained by C-banding. Moreover, a cline seemed to appear in the degree of the heteromorphic status along south to north in comparison with previous studies. In C. platycephala, there are several kinds of heterochromatinizations; such heteromorphisms would be neutral differentiations karyologically for natural selections from an evolutionary standpoint.
Introduction
The Japanese water shrew, Chimarrogale platycephala (Soricomorpha, Mammalia), is endemic to the Japanese Islands Honshu and Kyushu (Koyasu Citation1998; Ohdachi et al. Citation2009). This species has a specific habitat along clear streams (Abe Citation2003) and a few genetically related groups, based on mitochondrial DNA analysis, have been identified on other parts of the islands (Iwasa and Abe Citation2006). According to Iwasa and Abe (Citation2006), this species has relatively high genetic diversity.
The chromosomal configuration of C. platycephala was studied using conventional and differential staining methods by Obara and Tada (Citation1985) and Obara et al. (Citation1996). These studies indicated that C. platycephala heteromorphisms are usual for the segment length neighboring the secondary constriction at the short arm of the pair of no. 1 chromosomes (no. 1 homologue). Interestingly, the segments were shown to be constantly heterochromatic by C-banding (Obara and Tada Citation1985). The results of Obara and Tada (Citation1985) and Obara et al. (Citation1996) were obtained from shrew samples collected exclusively from Aomori Prefecture, northernmost Honshu, Japan. Thus, whether there are heteromorphisms in C. platycephala from other locations is unknown. To understand the chromosomal diversity and differentiation status in this species, it will be necessary to confirm whether the presence of such heteromorphisms is a local phenomenon in Aomori Prefecture, northernmost Honshu.
In this study, we studied karyotypes of C. platycephala from Kanagawa Prefecture, central Honshu, Japan, and investigated their heteromorphic status. We then improved the findings with those from previous studies of samples from Aomori Prefecture. In particular, the heteromorphic conditions between our and previous samples were compared statistically and evaluated from the standpoint of chromosomal evolution.
Materials and methods
Shrew samples
The shrew samples were collected from four localities (A–D in Table ) using mesh live traps and baited with small fish (sardines or mackerel) for one night at Tanzawa Mountains in Kanagawa Prefecture, central Honshu, Japan. All shrew samples are preserved in the MAI private collection (Table ).
Table 1. Cytogenetic data of Chimarrogale platycephala samples examined in this study.
Chromosomal analysis
Chromosome preparations were performed from fibroblast cells and bone marrow cells. For fibroblast cell samples, cells were cultured from tail vertebrae tissue in Minimum Essential Medium (MEM) (Nissui Pharmaceutical Co., Ltd., Tokyo) including 15% bovine calf serum for 2–3 weeks. Colchicine (final concentration 0.025 μg ml–1) was added 45 min before harvest. For bone marrow cell samples, the cells were cultured in the same MEM containing colchicine (final concentration 0.025 μg ml–1) at 37°C for 50 min. Subsequently, both types of cell were treated in 0.075 M KCl at 37°C for 20 min as a hypotonic treatment, followed by fixation with Carnoy’s fixative (methanol:acetic acid = 3:1). Air-dried cells were stained conventionally and differentially stained by G- and C-banding techniques (Sumner Citation1972; Iwasa and Tsuchiya Citation2000). For G-banding, the urea method (Kato and Yosida Citation1972) with slight modifications was used. Under microscopic observations, there was fundamentally no difference between fibroblast cells and bone marrow cells regarding morphological and other characteristics on the chromosomal constitutions. Therefore, we treated both cells as the same somatic data. Karyotyping was done according to the numbering system of Obara and Tada (Citation1985).
The relative length of each terminal segment neighboring secondary constriction on the short arm to the length of the long arm in the no. 1 homologue was calculated. The difference in the relative lengths in the longer segment of the short arm (LSA) and in the shorter segment of the short arm (SSA) were statistically analyzed by Student’s t-test to determine the significance of the heteromorphism. In addition, we compared statistically the heteromorphic status between the data of Obara et al. (Citation1996) and the present study.
Results
All 10 individuals carried the same chromosomal constitutions: 2n = 52, FNa (fundamental arm number of autosomes) = 100. Of them, nine individuals had 17 metacentrics (M), four submetacentrics (SM), and four subtelocentrics (ST) as well as a large-sized submetacentric X chromosome and a medium-sized acrocentric Y chromosome (Figure ). In the no. 1 homologue showing the largest size, there were apparent secondary constrictions (Figure ), as observed in previous studies (Obara and Tada Citation1985). These secondary constrictions varied between the homologous chromosomes. In addition, the residual individual of the 10 individuals examined had the same chromosomal constitutions but showed an additional heteromorphism in the ST autosomes (Figure ).
Figure 1 Conventionally stained (a), G-banded (b) and C-banded (c) karyotypes of C. platycephala (specimen nos.: MAI-461 for conventionally stained and G-banded karyotypes and MAI-598 for C-banded ones).
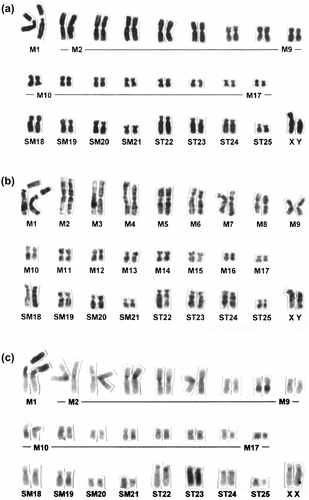
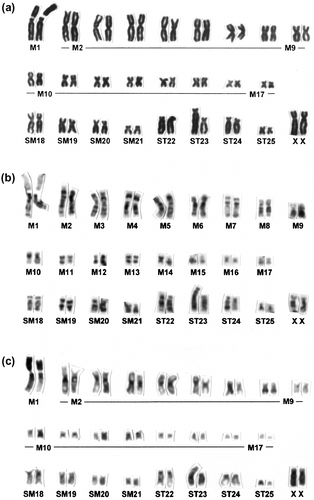
Figure 2 Conventionally stained (a), G-banded (b) and C-banded (c) karyotypes of C. platycephala showing a heteromorphic pair at the no. 23 homologue (specimen no.: MAI-462).
The G-band patterns of our samples seemed to be identical to those reported previously (Obara and Tada Citation1985). In particular, the large chromosomes were identified easily by G-band patterns, but it was difficult to identify the small ones (). Moreover, current G-band patterns also seemed to be identical to those of C. himalayica in Taiwan, which is closely related to the Japanese water shrew (Motokawa et al. Citation2006).
The C-banding revealed that there were fundamentally large C-band-positive segments at the short arms of the no. 1 homologue and centromeric heterochromatins (). In addition, the proximal–interstitial regions of the long arms of the no. 8 homologue and the entire Y chromosome were positively stained by the C-banding (), although, in previous studies, it has been reported that these chromosomes were positively stained at terminal regions (Obara and Tada Citation1985). Moreover, three individuals showed that entire regions except for the terminal regions in ST homologues were positively stained by C-banding (). Such ST homologues (including homomorphic and heteromorphic pairs) in two of the three individuals above were identified as the no. 23 autosomal pair (). Namely, in our C-banding analysis, the short arm segments of the no. 1 homologue and the long arm segments of the no. 8 homologue were positively stained as the heterochromatinization differentiation. In addition, the entire regions except for the terminal of the no. 23 homomorphic or heteromorphic pair were heterochromatinized ().
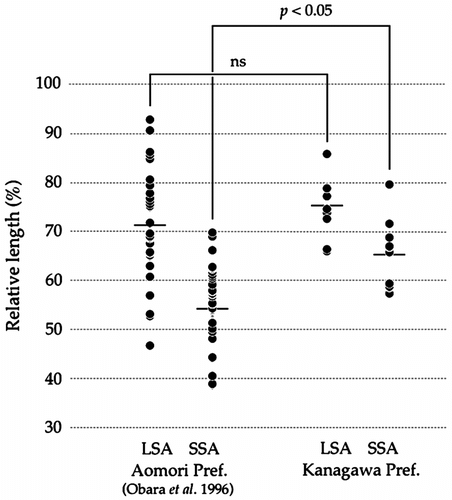
According to the conventionally stained chromosomes, we calculated LSA and SSA and show the results in Table and Figure . LSA and SSA ranged from 57.3 to 79.0% with standard deviation (SD = 4.79–7.75). All the individuals showed the heteromorphism in the no. 1 homologue (p < 0.05) statistically . In comparison with the data of Obara et al. (Citation1996) by t-test, the averages of LSA of each our Kanagawa Prefecture individuals did not differ from those of Aomori Prefecture at p = 0.05. However, SSA averages of each Kanagawa Prefecture individual were significantly longer than those of Aomori Prefecture (p < 0.05) and the SSA of current samples seemed to be longer than that of Aomori Prefecture (Figure ).
Figure 3 Relationships between the relative lengths of the heteromorphic constitutive heterochromatin blocks (C-block) in the no. 1 homologue among all the individuals of C. platycephala examined in this study. Left and right in each individual indicate LSA and SSA, respectively. Vertical lines and horizontal lines indicate ranges and averages, respectively. Solid rectangles indicate standard deviation. Locality codes (A–D) are explained in Table .
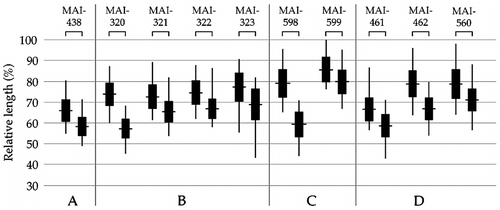
Figure 4 A comparison of heteromorphisms of C. platycephala samples from Aomori and Kanagawa Prefectures, Japan, according to Obara et al. (Citation1996) and the present data. In SSA, the averages of each individual from Aomori Prefecture and those of each individual from Kanagawa Prefecture are significantly different (asterisk, p < 0.05), but in LSA the averages of each individual from both prefectures do not differ significantly (ns). Horizontal bars indicate averages.
Discussion
The present and previous results (Obara et al. Citation1996) suggest that the heteromorphic status of the no. 1 homologue is a specific karyological criterion in C. platycephala (). With regard to the cause of the heteromorphism, Obara et al. (Citation1996) reported that there was “unequal crossing-over” during the meiotic process at the heterochromatic segment of the no. 1 homologue. Unequal crossing-over is assumed to occur in the long repetitive satellite DNA region, which shows heterochromatinization (Philip and Greenbaum Citation1990; Sumner Citation2003). In this study, we did not observe the meiotic stages, but the variation of lengths of heterochromatic segments was confirmed in all the individuals. Therefore, the occurrence of unequal crossing-over is a satisfactory reason for the variable heterochromatic lengths. Moreover, intercellular variation of lengths of the no. 1 heterochromatic segments was observed (Figure ). Such variation also may be caused by unequal somatic crossing-over (Therman and Kuhn Citation1981).
On the basis of the present analysis, the heteromorphic ST pair was observed in three individuals (Figure ), but such heteromorphism was not observed in the individuals from Aomori Prefecture (Obara et al. Citation1996). The entire regions without terminal region of the homomorphic/heteromorphic ST pairs and probably the no. 23 homologue were positively stained by C-banding (). Such heterochromatinization was not observed in the individuals from Aomori Prefecture (Obara et al. Citation1996). Thus, the heteromorphism and heterochromatinization in the ST pair currently are considered to be a characteristic of the individuals from Kanagawa Prefecture. In addition, some C-band-positive regions were recognized at the proximal–interstitial region of the long arms of the no. 8 homologue, the entire Y chromosome (). These C-band-positive regions were not observed in the individuals from Aomori Prefecture (Obara et al. Citation1996). Motokawa et al. (Citation2006) also revealed the other type of C-band distribution in the individuals of C. platycephala from Wakayama Prefecture in western Honshu. Accordingly, we conclude that C. platycephala usually has heterochromatic polymorphisms causing heteromorphisms as an intraspecific variation. Such quantitative variations of the heterochromatin and its polymorphisms are observed in many animal species and seem to have less effect on fertility related to post-mating isolation (King Citation1980; Baverstock et al. Citation1982; Hatanaka et al. Citation1998). Namely, carrying the heterochromatic polymorphism in the autosomes of C. platycephala is not uncommon, but the property and function of heterochromatin are currently unclear.
As a closely related taxon, the genus Neomys carried a similar karyotype to C. platycephala, but the heteromorphism in the chromosomal size, such as the no. 1 homologue, was not observed (Fredga and Levan Citation1969; Zima et al. Citation1998; Chassovnikarova et al. Citation2009). In addition, Motokawa et al. (Citation2006) reported the karyotype of C. himalayica in Taiwan, which seemed to have the homomorphic no. 1 homologue carrying heterochromatic blocks. Accordingly, the heteromorphism of the no. 1 homologue is confirmed only in C. platycephala. Moreover, in comparison with the results of Obara et al. (Citation1996) regarding specimens from Kanagawa Prefecture, C. platycephala individuals from Aomori Prefecture tend to have strongly heteromorphic status in the no. 1 homologue (Figure ). If these tendencies in the genus Chimarrogale are valid, the heteromorphism of the no. 1 homologue seems to be strong along latitudes from south to north. To explain this gradient, an analysis of the karyotypes of C. platycephala individuals from western Japan is necessary. Considering the variable degree of the heteromorphisms and the heterochomatinizations, we estimate that these phenomena have been differentiated during the evolutionary process of C. platycephala because these chromosomal polymorphisms are considered as neutral for viability (King Citation1993).
Acknowledgments
We are grateful to Mayuh Tabata, Asumi Tamada, Yuki Haga and Yuri Kawaharada for their kind cooperation in collecting shrew samples. This study was supported in part by Grants-in-Aid for Scientific Research (no. 17770074) from the Ministry of Education, Science, Sports and Culture, Japan and also partially supported by a grant from Research Fund for 2007 of College of Bioresource Sciences, Nihon University.
References
- Abe H. 2003. Trapping, habitat, and activity of the Japanese water shrew, Chimarrogale platycephala. Mammal Sci. 43:51–65. [in Japanese with English abstract].
- Baverstock , PR , Gelder , M and Jahnke , A . 1982 . Cytogenetic studies of the Australian rodent, Uromys caudimaculatus, a species showing extensive heterochromatin variation . Chromosoma , 84 : 517 – 533 .
- Chassovnikarova , TG , Atanassov , NI and Dimitrov , HA . 2009 . Cytogenetic characteristic of the southern water shrew, Neomys anomalus (Insectivora; Soricidae), in the Strandzha Mountains South East Bulgaria) . Folia Zool , 58 : 416 – 419 .
- Fredga , K and Levan , A . 1969 . The chromosomes of the European water shrew (Neomys fodiens) . Hereditas , 62 : 348 – 356 .
- Hatanaka , T , Tambasco , AJ and Galetti , PM Jr . 1998 . Heterochromatin heterogeneity and chromosome heteromorphism in Cerdocyon thous (Mammalia, Canidae) . Genet Mol Biol , 21 : 227 – 231 .
- Iwasa , MA and Abe , H . 2006 . Colonization history of the Japanese water shrew Chimarrogale platycephala, in the Japanese Islands . Acta Theriol , 51 : 29 – 38 .
- Iwasa , MA and Tsuchiya , K . 2000 . Karyological analysis of the Eothenomys sp. from Nagano City, central Honshu . Japan. Chromosome Sci , 4 : 31 – 38 .
- Kato , H and Yosida , TH . 1972 . Banding patterns of Chinese hamster chromosomes revealed by new techniques . Chromosoma , 36 : 272 – 280 .
- King , M . 1980 . C-banding studies on Australian hylid frogs: secondary constriction structure and the concept of euchromatin transformation . Chromosoma , 80 : 191 – 217 .
- King , M . 1993 . Species evolution: The role of chromosome change , Cambridge : Cambridge University Press .
- Koyasu K. 1998. Natural history of Japanese Soricidae. In Yokohata Y, Abe H, editors. The Natural History of Insectivora (Mammalia) in Japan. Shobara: Hiba Society of Natural History; p. 201–267. [in Japanese].
- Motokawa , M , Harada , M , Apin , L , Yasuma , S , Yuan , SL and Lin , LK . 2006 . Taxonomic study of the water shrews Chimarrogale himalayica and C. platycephala . Acta Theriol , 51 : 215 – 223 .
- Obara , Y , Izumi , J , Tanaka , T and Koseki , J . 1996 . Heteromorphism of the No. 1 homologue in the Japanese water shrew . Chimarrogale himalayica platycephala. Chromosome Inf Serv , 61 : 20 – 22 .
- Obara , Y and Tada , T . 1985 . Karyotypes and chromosome banding patterns of the Japanese water shrew, Chimarrogale platycephala platycephala . Proc Japan Acad B , 61 : 20 – 23 .
- Ohdachi , SD , Ishibashi , Y , Iwasa , MA and Saitoh , T . 2009 . The wild mammals of Japan , Kyoto : Shoukadoh .
- Phillip , DS and Greenbaum , TF . 1990 . Unequal crossing over and heterochromatin exchange in the X-Y bivalents of the deer mouse, Peromyscus beatae . Chromosoma , 99 : 183 – 189 .
- Sumner , AT . 1972 . A simple technique for demonstrating centromeric heterochromatin . Exp Cell Res , 75 : 304 – 306 .
- Sumner , AT . 2003 . Chromosome: organization and function , Malden : Blackwell .
- Therman , E and Kuhn , EM . 1981 . Mitotic crossing-over and segregation in man . Human Genet , 59 : 93 – 100 .
- Zima , J , Lukacova , L and Macholan , M . 1998 . “ Chromosomal evolution of shrews ” . In Evolution of shrews , Edited by: Wolsan , M and Wojcik , JM . 175 – 218 . Bialowieza : Mammal Research Institute, Polish Academy of Sciences .