Abstract
Two varieties of Saxifraga diversifolia, diversifolia and parnassifolia, collected from the moist alpine slopes around Gauri Kund, 3930 m (Manimahesh Hills, Himachal Pradesh, India), are studied for detailed male meiosis, chiasma frequency and pollen fertility. Both varieties exist at diploid level (based on x = 8), and show a meiotic chromosome count of n = 8 at diakinesis and metaphase-I, and regular chromosome distributions at anaphases-I/II. The meiotic chromosome count of n = 8 ascertained here represents a new aneuploid cytotype, supplementing the earlier report of a diploid cytotype with 2n = 20 from the north-west Himalayas in India and the Nepal Himalayas. Of the two varieties, var. diversifolia showed the presence of multiple chromosomal associations and univalent chromosomes at diakinesis and metaphase-I of meiosis-I. On the other hand, var. parnassifolia does not have multivalent formation but showed only 2–4 univalent chromosomes at diakinesis and metaphase-I. Occurrence of univalents in pollen mother cells of var. diversifolia and parnassifolia reduced the chiasma frequency significantly and also caused some pollen sterility (7–8%). The paper herein discusses for the first time the occurrence of structural heterozygosity and univalent chromosomes and their apparent affect on chiasma frequency and pollen fertility in S. diversifolia.
Introduction
Associations of more than two chromosomes in a diploid taxon might indicate that at least partial homology of chromosomes extends to some non-homologous pairs, which is possible due to either the hybrid nature of the taxon or heterozygosity for reciprocal translocations (Singhal Citation1982). Reciprocal translocations, also known as chromosomal interchanges, are a type of chromosomal aberration arising from the exchange of broken segments of two nonhomologous chromosomes (Mahama and Palmer Citation2003). Such chromosomal aberrations occur in nature at a low rate, probably in all higher organisms (Grant Citation1981). Translocation heterozygosity occurs independently of hybridization in some species, where it typically results in reduced fertility (Levin Citation2002). Reciprocal translocations were reported for the first time in Stizolobium deeringianum by Belling in Citation1914. Since then, their occurrence and consequences have been reported in a number of flowering plants (Gohil and Koul Citation1978; Singhal and Gill Citation1981; Sharma and Gohil Citation2003, Citation2008, Citation2011; Talukdar and Biswas Citation2006; Kim et al. Citation2008; Talukdar Citation2008, Citation2010; Ghaffari et al. Citation2009; Gupta et al. Citation2010; Golczyk Citation2011; Kohli and Gohil Citation2011) and groups of organisms including human beings (Mather Citation1936; Hultén Citation1974). These may arise spontaneously or be caused by a variety of factors including chemical or irradiation treatments. Reciprocal translocations are sources of intraspecific chromosomal structural polymorphism and have been considered as an important genetic aberration utilized by geneticists as well as plant breeders (Müntzing & Prakken Citation1941; Hrishi et al. Citation1969; Candela et al. Citation1979; Mahama et al. Citation1999). Generally few chromosomes are involved in reciprocal translocations; however, the entire chromosome complement has also been reported to be involved in the process in Oenothera (Cleland Citation1972), Rhoeo spathacea (Sax Citation1931; Verma and Ohri Citation1979; Koul et al. Citation2011), Chelidonium majus (Pilquin Citation1981), Pennisetum americanum (Vari and Bhowal Citation1991) and Allium roylei (Sharma and Gohil Citation2003, Citation2008; Kohli and Gohil Citation2011).
Among the vital steps of chromosomal behaviour during meiosis, pairing of homologous chromosomes is very essential for completion of this important event in sexual reproduction. Chromosomes may fail to pair either due to asynapsis or desynapsis, resulting in the presence of univalents. These univalents not only interfere with the completion of meiosis, but the very survival of the individual through sexual propagation is greatly impaired (Soost Citation1951). The presence of such univalent chromosomes resulted in a decrease in chiasma frequency. Chiasma formation is the most sensitive and delicate stage in the meiotic process and in most cases reduced pairing of chromosomes and consequently the presence of univalents during the meiotic process influence chiasma frequency (Sjödin Citation1970). Reduced chiasma frequency in turn considerably lowers gametic fertility.
During the course of cytomorphological explorations in the alpine regions of the north-west Himalayas we found multiple associations of four chromosomes in Saxifraga diversifolia var. diversifolia Wall. ex Ser (Family: Saxifragaceae) and univalent chromosomes in var. parnassifolia (D. Don) Engl. Saxifraga L. is the largest genus in the family Saxifragaceae (Soltis et al. Citation2001) and consists of about 400 species (Webb Citation1993; Soltis et al. Citation1993, Citation1996; Healy and Gillespie Citation2004). Engler (Citation1930) reported that Saxifragaceae are morphologically diverse assemblage of annual, biennial and perennial herbs, shrubs, trees and vines. It comprises 17 subfamilies (Schulze-Menz Citation1964). In recent attempts to clarify the circumscription and relationships of the morphologically diverse members of Saxifragaceae using molecular systematic tools (18S rDNA and rbcL), Soltis and Soltis (Citation1997) and Soltis et al. (Citation1993, Citation2001) concluded that Saxifragaceae is a polyphyletic family of the Saxifragales clade, that encompasses the subfamilies of Rosidae, Dilleniidae and Hamamelidae. Soltis and Soltis (Citation1997) suggested that many of the families (Cunoniaceae, Droseraceae, Cephalotaceae, Gunneraceae, Rosaceae and Greyiaceae) traditionally considered close relatives of Saxifragaceae are only distantly related to this narrowly defined family. The MatK and rbcL gene sequence data have also indicated that the genus Saxifraga is polyphyletic (Soltis et al. Citation1996).
S. diversifolia grows as a perennial herb, having stem, leaves and inflorescence covered with crimson glandular hairs. Bright yellow flowers appear during the months of July–September. The species, which is mainly distributed in Western China, Bhutan, north-west Indian Himalayas, Myanmar, Nepal and Sikkim, grows on open alpine moist slopes and in rock crevices between altitudes of 2700 and 4300 m.
The aim of the present work was to assess the chromosome number in the two varieties and to study the effect of chromosomal associations and univalent chromosomes on chiasma frequency, course of meiosis and pollen fertility.
Material and methods
For the male meiotic studies, material of the two varieties of S. diversifolia were collected from the moist alpine slopes of Gauri Kund (Manimahesh Hills, Chamba, Himachal Pradesh, 32°24.11′ N; 76°38.25′ E, altitude 3930 m) in July 2008. The two varieties, diversifolia and parnassifolia, differ in some leaf characters, such as presence or absence of glandular hairs on leaf margins and cauline type leaves. (i) Var. diversifolia is characterized by having glabrous stem and leaves, and upper leaves petiolate. (ii) Var. parnassifolia, also known under the name of S. parnassifolia D. Don, is recognized by its Parnassia-like upper sessile leaves with glandular hairs on the margins. Sepals in this variety are characteristic having crimson glandular hairs on the margins.
The young developing floral buds from healthy plants were fixed in freshly prepared Carnoy’s fixative (1 glacial acetic acid: 3 chloroform: 6 ethanol, v:v:v) for 24 hours and subsequently stored in 70% ethanol in a refrigerator. Developing anthers from floral buds were squashed in 1% acetocarmine and preparations were studied for chromosome counts, and detailed meiotic behaviour in pollen mother cells (PMCs) at prophase-I, metaphase-I (M-I), anaphases-I/II (A-I/II), telophases-I/II (T-I/II) and sporad stage. A total of 10–30 PMCs were examined for determining the chromosome counts while 20–50 slides were prepared from different anthers/flowers for analysis of chromosomal associations.
Pollen fertility was estimated through stainability tests for which anthers of mature flowers were squashed in glyceroacetocarmine mixture (1:1) and 1% aniline blue dye. A total of 100–300 pollen grains were analysed in each case for pollen fertility. Well-filled pollen grains with uniformly stained cytoplasm were scored as fertile/viable while shrivelled pollen with unstained or poorly stained cytoplasm were counted as sterile/unviable. Photomicrographs from the temporary mounts were taken using a Nikon Eclipse 80i microscope (Melville, USA).
Results
A Meiotic course
(i) Var. diversifolia
The chromosome number in var. diversifolia was confirmed from the presence of eight unequal sized ring and rod type bivalents (two bivalents are large sized) at diakinesis (Figure ) and M-I (Figure ), and 8:8:8:8 daughter chromosomes distribution at A-II (Figure ). The presence of multivalents and univalents were observed in 43.48% of the PMCs (Table ). Analysis of 115 PMCs at diakinesis and M-I revealed that 6.52% of the chromosomes were involved in multivalent formation of which 3.26% were ring type and 2.17% and 1.09% were chain and alternate (zigzag ring type), respectively. Meanwhile, 4.13% of the chromosomes remained as univalents (Table ). Chain and ring type quadrivalents were recorded in equal number of PMCs (8.70%) (Figure , 5). The presence of two alternate (zigzag ring) type quadrivalents were recorded in 4.35% of PMCs (Figure ). Univalents (up to two) were observed in 21.73% of cases (Figure ). In some of the PMCs large sized bivalents were observed to show late disjunction during anaphase-I (Figure ).
Figures 1–13 Meiotic chromosome numbers and their behaviour during meiosis in the two varieties. (1–8) Var. diversifolia: (1, 2) PMCs showing n = 8 with two large bivalents (arrowed) at diakinesis and metaphase-I; (3) a PMC showing 8:8:8:8 daughter chromosomes distributions at four poles of anaphase-II; (4) a PMC with 6II+1IV (chain) at diakinesis (arrowed); (5) a PMC with 6II+1IV (ring) at metaphase-I (arrowed); (6) a PMC with 4II+2IV (two zigzag rings, arrowed); (7) PMC with 7II+2I (arrowed); (8) late disjunction of one bivalent at anaphase-I (arrowed). (9–13) Var. parnassifolia: (9, 10) PMCs showing n = 8 at diakinesis and metaphase-I; (11) a PMC with 7II+2I (arrowed); (12) a PMC with 6II+4I (arrowed); (13) a PMC with laggards at anaphase-I (arrowed). Scale bar = 10 μm.
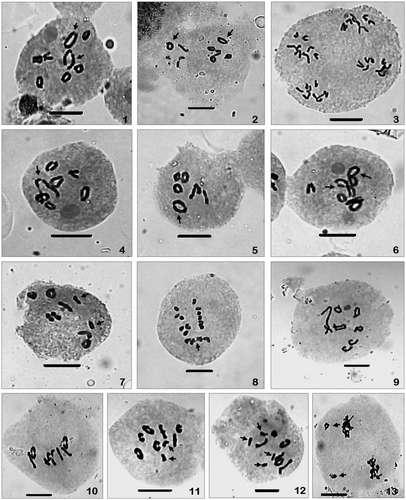
Table 1. Analysis of chromosomal associations at diakinesis/M-I and chiasma frequency in Saxifraga diversifolia.
(ii) Var. parnassifolia
Meiotic studies in this variety also showed the same diploid chromosome number, n = 8 as confirmed by the presence of eight bivalents in PMCs at diakinesis (Figure ) and M-I (Figure ). None of the PMCs in this variety showed any multivalent formation, but some of the PMCs depicted the presence of univalent chromosomes. Analysis of 98 meiocytes revealed that 57.14% of the observed PMCs showed the presence of eight bivalents. The number of ring type bivalents in such PMCs ranges from 2 to 7 and the rest of the bivalents are rod shaped. In 42.86% of the PMCs at M-I, 2–4 chromosomes remained as univalents (Figures , 12, Table ) which lagged during the movements of chromosomes to opposite poles at anaphases (Figure ).
B Chiasma frequency
(i) Var. diversifolia
On the basis of chromosomal associations, PMCs are put into four categories: (a) PMCs with eight bivalents; (b) PMCs with bivalents and univalents; (c) PMCs with multivalents and univalents; and (d) PMCs with bivalents and multivalents. The mean chiasma frequency per PMC in the PMCs with eight bivalents is 12.91. However, the chiasma frequency per PMC is reduced significantly to 11.16 in the PMCs having 2–4 univalents. In the PMCs depicting structural heterozygosity, the mean chiasma frequency was recorded to be the highest (13.68). The presence of multivalents in the PMCs with univalents also showed increased mean chiasma frequency per PMC (12.50) compared to the PMCs with 2–4 univalents and no multivalents. Data on chiasma frequency in the PMCs having different chromosomal associations and pollen fertility are given in Table .
(ii) Var. parnassifolia
As none of the PMCs in this variety showed any multivalent formation there are only two categories of PMCs: (a) PMCs with eight bivalents; and (b) PMCs with bivalents and univalents. A decrease in the mean chiasma frequency per PMC was recorded in PMCs having 2–4 univalents (11.20) when compared to PMCs with eight bivalents (12.33). Details on the number of ring and rod type bivalents and univalents per PMC and pollen fertility are provided in Table .
C Pollen sterility
Due to the presence of univalent chromosomes, lagging chromosomes, structural heterozygosity and late disjunction of some bivalents, nearly 7–8% of the pollen grains were recorded to be sterile/unstained.
Discussion
Heterozygosity for reciprocal translocations, which is generally identified by reduced reproductive capacity and the presence of multivalents during reduction division, has the potential to create and conserve specific gene combinations (Sharma and Gohil Citation2011). The presence of 1–2 quadrivalents (rings or chains) along with 4–6 bivalents in the PMCs of var. diversifolia (2n = 2x = 16) indicates heterozygosity for reciprocal translocations. Depending upon the number of chiasmata, the quadrivalents in the PMCs can be depicted either as a ring (four chiasmata) or a chain (three chiasmata) for the configuration at diakinesis and M-I. Both chain and ring configurations have been detected. Due to the presence of multiple chromosomal associations, some pollen sterility (7%) resulted in this structural heterozygote. The orientation of multivalents at M-I decides whether or not a translocation heterozygote causes sterility (Gohil and Koul Citation1978; Sharma and Gohil Citation2003). Adjacent disjunction is supposed to be responsible for the formation of sterile pollen grains containing duplications and deficiencies. Burnham (Citation1956) and Ghaffari et al. (Citation2009) considered that in many plant species individuals with reciprocal translocations are usually semi-sterile (50% pollen and ovule abortion) and 50% of the meiocytes display the alternate type configuration of the interchange complex at M-I. Totally sterile plants due to reciprocal translocations have also been reported in Allium consanguineum by Gohil and Koul (Citation1978) and in Allium roylei by Sharma and Gohil (Citation2003). In the present investigation, adjacent orientation of multivalents at M-I coupled with the presence of univalent chromosomes seems to be the probable cause of some pollen sterility in the species.
Chromosome pairing at meiosis-I is well determined in diploids, where each chromosome consistently finds its homologous counterpart to give rise to disomic segregation (Jannoo et al. Citation2004). The normal meiotic behaviour in 57.14% of the observed PMCs in var. parnassifolia (2n = 2x = 16) depicted the complete homology between chromosomes and is indicative of the normal constitution of a diploid taxon as has been observed by Singhal (Citation1982) in many woody species. However, 2–4 chromosomes remained as univalents in 42.86% of the observed PMCs. These univalent chromosomes failed to get included at the poles and were left as laggards during A-I. Consequently some pollen sterility (8%) is resulted.
In a number of studies (Soost Citation1951; Thomas and Rajhathy Citation1966; Sjödin Citation1970; Kitada and Omura Citation1983; Jongedijk and Ramanna Citation1989; Higgins et al. Citation2004) it has been demonstrated that occurrence of univalents has a negative effect on the chiasma frequency of the rest of the bivalents in the PMCs. In the present investigation, the mean chiasma frequency per PMC and per bivalent reduced significantly in the PMCs with univalents in both the varieties (Table ), compared to PMCs with only bivalents and PMCs with multivalents. However, the difference in the mean chiasma frequency per PMC and per bivalent in the PMCs with univalents is not significant in either variety. In var. diversifolia the values of chiasma frequency in the PMCs with multivalents is higher than in PMCs without multivalents, which indicated a positive effect of the presence of reciprocal translocations on chiasma frequency. Thus there is a negative effect of the occurrence of univalents on the pairing of chromosomes during meiosis and consequently on pollen fertility. Deleterious effects of univalents on meiotic course and pollen fertility have also been documented by several workers (Singhal Citation1982; Fiona et al. Citation1988; Leggett Citation1998; Maity and Datta Citation2009a, Citation2009b; Datta et al. Citation2010; Goyal and Khan Citation2010). On the other hand, Dnyansagar and Sudhakaran (Citation1970) in Vinca rosea, Merker (Citation1971) in Triticale, Gatt et al. (Citation2000) in some hybrids of Dahlia, and Palma-Silva et al. (Citation2004) in Vriesea and Aechmea showed that frequency of univalents is not correlated directly with reduced pollen viability. Among the other reasons put forth to explain the reduction in chiasma frequency, Hultén (Citation1974) argued that terminalization of chiasmata occurs when there is contraction of the bivalents during diakinesis which may reduce chiasma frequency.
The occurrence of univalent chromosomes in diploid plants has been attributed to the hybrid constitution of the genome (Stebbins and Pun Citation1953; Singhal Citation1982; Leggett Citation1998; Kumar et al. Citation2012). However, in the present case, the fact that the univalent chromosomes in both varieties of S. diversifolia are not associated with any multiple association seems to be the result of asynapsis/desynapsis, as has been suggested by Singhal (Citation1982), Maity and Datta (Citation2009b) and Datta et al. (Citation2010). Asynapsis/desynapsis may originate spontaneously (Kaul and Murthy Citation1985; Singh Citation2002; Sharma et al. Citation2010; Ranjbar et al. Citation2012) or be induced by factors such as temperature, humidity, nutrients, radiations, mutagens and chemicals influencing chromosome pairing during meiosis (Prakken Citation1943; Ahloowalia Citation1969; Sjödin Citation1970; Singh et al. Citation1977; Koduru and Rao Citation1981; Vishnuvardhan and Lakshmi Citation1987; Rao and Kumar Citation2003; Kumar and Rai Citation2006, Citation2007; Gulfishan et al. Citation2010; Avijeet et al. Citation2011). The presently studied individuals of the species grow in a high altitude alpine area of the Manimahesh Hills, where the temperature at the time when these plants enter the flowering/bud stage is very low (2–15°C). The low temperature seems to be the probable cause of these synaptic irregularities in chromosomes of this species during meiosis.
We here report for the first time the existence of structural heterozygosity due to reciprocal translocations in the species. The presently counted meiotic chromosome number of n = 8 for var. diversifolia and var. parnassifolia adds a new cytotype to the already recorded chromosome counts of 2n = 20 from other regions of the north-west Himalayas in India (Mehra and Dhawan Citation1971) and Nepal Himalayas (Malla et al. Citation1984).
Acknowledgements
The authors wish to thank the University Grants Commission (UGC), New Delhi for providing financial assistance under DRS SAP I and II, ASIST programme, and Dr D.S. Kothari Post-Doctoral Fellowship {Award Letter No. F.4-2/2006(BSR)/13-427/2011(BSR)} to Dr Puneet Kumar. Further support was provided by the Council of Scientific and Industrial Research (CSIR) under the Senior Research Fellowship to the senior author. Thanks are also due to the Head, Department of Botany, Punjabi University, Patiala for necessary laboratory and internet facilities.
References
- Ahloowalia , BS . 1969 . Effect of temperature and barbiturates on a desynaptic mutant of rye grass . Mutat Res , 7 ( 2 ) : 205 – 213 .
- Avijeet , C , Shukla , S , Rastogi , A , Mishra , BK , Ohri , D and Singh , SP . 2011 . Impact of mutagenesis on cytological behaviour in relation to specific alkaloids in Opium Poppy (Papaver somniferum L.) . Caryologia , 64 ( 1 ) : 14 – 24 .
- Belling , J . 1914 . The mode of inheritance of semi-sterility in the offspring of certain hybrid plants . Mol Genet & Genomics , 12 ( 1 ) : 303 – 342 .
- Burnham , CR . 1956 . Chromosomal interchanges in plants . Bot Rev , 22 ( 7 ) : 419 – 552 .
- Candela , M , Figueiras , AM and Lacadena , JR . 1979 . Maintenance of interchange heterozygosity in cultivated rye, Secale cereale L . Heredity , 42 ( 3 ) : 283 – 289 .
- Cleland , RE . 1972 . Oenothera: Cytogenetics and Evolution: Experimental Botany , New York : Academic Press .
- Datta , AK , Mukherjee , S , Saha , A and Das , A . 2010 . Seasonal influence on the chromosome behaviour of diploid (Solanum nigrum L.) and hexaploid (S. americanum Mill.) species of Solanum . Asian J Exp Biol Sci , 1 ( 1 ) : 193 – 196 .
- Dnyansagar , VR and Sudhakaran , IV . 1970 . Induced tetraploidy in Vinca rosea L . Cytologia , 35 : 227 – 241 .
- Engler A. 1930. Saxifragaceae. In Engler A, Prantl K, editors. Die natuflichen pflanzenfamilien, 2nd ed. Vol. 18a. Leipzig: Engelmann. p. 74–226.
- Fiona , DW , Grigg , PR , Smith , PR , Stenersen , MA and Murray , BG . 1988 . Variable pollen fertility and abnormal chromosome behaviour in the pepino (Solanum muricatum Ait., Solanaceae) . Sci. Hortic , 35 ( 3–4 ) : 259 – 268 .
- Gatt , M , Hammett , K and Murray , B . 2000 . Interspecific hybridization and the analysis of meiotic chromosome pairing in Dahlia (Asteraceae-Heliantheae) species with x = 16 . Pl Syst Evol , 221 ( 1–2 ) : 25 – 33 .
- Ghaffari , SM , Karimzadeh , G and Najafi , AA . 2009 . Occurrence of reciprocal translocations in Lathyrus boissieri Sirj (Fabaceae) from Iran . Cytologia , 74 ( 2 ) : 195 – 199 .
- Gohil , RN and Koul , AK . 1978 . Structural hybridity in Allium consanguineum . Cytologia , 43 ( 2 ) : 243 – 247 .
- Golczyk , H . 2011 . The arrangement of pericentromeres during meiotic prophase I in the permanent translocation heterozygote Rhoeo spathacea . Caryologia , 64 ( 2 ) : 197 – 202 .
- Goyal , S and Khan , S . 2010 . Cytology of induced morphological mutants in Vigna mungo (L.) Hepper . Egypt J Biol , 12 ( 2 ) : 81 – 85 .
- Grant , V . 1981 . Plant speciation , New York : Columbia University Press .
- Gulfishan , M , Khan , AH and Bhat , TA . 2010 . Studies on cytotoxicity induced by DES and SA in Vicia faba L. var. major . Turk J Bot , 34 ( 1 ) : 31 – 37 .
- Gupta , RC , Himshikha , Rana PK , Kumar , P and Singhal , VK . 2010 . First report of structural heterozygosity in Artemisia parviflora (Asteraceae) from Parvati Valley in Kullu District (Himachal Pradesh, India) . Bot Serb , 34 ( 1 ) : 63 – 66 .
- Healy , C and Gillespie , L . 2004 . A systematic analysis of the alpine Saxifrage complex (Saxifragaceae) in the Canadian Arctic Islands using morphology and Chloroplast DNA data . Can Field Nat , 118 ( 3 ) : 326 – 340 .
- Higgins , JD , Armstrong , SJ , Franklin , FCH and Jones , GH . 2004 . The Arabidopsis MutS homolog AtMSH4 functions at an early step in recombination: evidence for two classes of recombination in Arabidopsis . Genes Dev , 18 : 2557 – 2570 .
- Hrishi , N , Müntzing , A and Ramulu , KS . 1969 . Further data on structural heterozygosity in a strain of Secale kuprijanovii . Hereditas , 61 ( 20 ) : 339 – 347 .
- Hultén , M . 1974 . Chiasma distribution at diakinesis in the normal human male . Hereditas , 76 ( 3 ) : 55 – 78 .
- Jannoo , NL , Grivet , J , D’Hont , DA and Glaszmann , J . 2004 . Differential chromosome pairing affinities at meiosis in polyploid sugarcane revealed by molecular markers . Heredity , 93 ( 5 ) : 460 – 467 .
- Jongedijk , E and Ramanna , MS . 1989 . Synaptic mutants in potato, Solanum tuberosum L. II. Concurrent reduction of chiasma frequencies in male and female meiosis of ds-1 (desynapsis) mutants . Genome , 32 ( 6 ) : 1054 – 1062 .
- Kaul , MLH and Murthy , TG . 1985 . Mutant genes affecting higher plant meiosis . Theor Appl Genet , 70 ( 5 ) : 449 – 466 .
- Kim , JS , Oginuma , K and Tobe , H . 2008 . Analysis of meiotic chromosome behaviour in diploid individuals of Chrysanthemum zawadskii and related species (Asteraceae): evidence for chromosome rearrangements . Cytologia , 73 ( 4 ) : 425 – 435 .
- Kitada , K and Omura , T . 1983 . Genetic control of meiosis in rice Oryza sativa L.II. Cytogenetical analysis of desynaptic mutants . Jpn J Genet , 58 ( 6 ) : 567 – 577 .
- Koduru , PRK and Rao , KM . 1981 . Cytogenetics of synaptic mutants in higher plants . Theor Appl Genet , 59 ( 4 ) : 197 – 214 .
- Kohli , B and Gohil , RN . 2011 . Is Allium roylei Stearn still evolving through multiple interchanges? . Nucleus , 54 ( 1 ) : 19 – 23 .
- Koul , KK , Nagpal , R and Alok , A . 2011 . Spectrum of chromosomal configurations in pollen mother cells of Rhoeo spathacea (Swartz) Stearn . Caryologia , 64 ( 2 ) : 135 – 145 .
- Kumar , G and Rai , P . 2006 . Induced desynaptic male sterile lines in soybean . Cytologia , 71 ( 4 ) : 337 – 343 .
- Kumar , G and Rai , P . 2007 . Comparative genotoxic potential of mercury and cadmium in soybean . Turk J Biol , 31 ( 1 ) : 13 – 18 .
- Kumar , P , Singhal , VK and Kaur , D . 2012 . Impaired male meiosis due to irregular synapsis coupled with cytomixis in a new diploid cytotype of Dianthus angulatus (Caryophyllaceae) from Indian cold deserts . Folia Geobot , 47 ( 1 ) : 59 – 68 .
- Leggett , JM . 1998 . Chromsosmes and genomic relationship between the diploid species of Avena strigosa and A. eriantha and the tetraploid A. macroccana . Heredity , 80 ( 3 ) : 316 – 363 .
- Levin , DA . 2002 . The role of chromosomal change in plant evolution , London : Oxford University Press .
- Mahama , AA , Deaderick , LM , Sadanaga , K , Newhouse , KE and Palmer , RG . 1999 . Cytogenetic analysis of translocations in soybean . J Hered , 90 ( 6 ) : 648 – 653 .
- Mahama , AA and Palmer , RG . 2003 . Translocation breakpoints in soybean classical genetic linkage groups 6 and 8 . Crop Sci , 43 ( 5 ) : 1602 – 1609 .
- Maity , S and Datta , AK . 2009a . Meiosis in nine species of Jute (Corchorus) . Indian J Sci Technol , 2 ( 2 ) : 27 – 29 .
- Maity , S and Datta , AK . 2009b . Spontaneous desynapsis in Corchorus fascicularis Lamk. (Family: Tiliaceae) . Indian J Sci Technol , 2 ( 3 ) : 34 – 36 .
- Malla , SB , Bhattarai , S , Saiju , HK , Kayastha , M and Pandey , I . 1984 . In IOPB chromosome number reports . LXXXII. Taxon , 33 ( 1 ) : 126 – 134 .
- Mather , K . 1936 . The determination of position in crossing-over. 1 . Drosophila melanogaster. J Genet , 33 ( 2 ) : 207 – 235 .
- Mehra , PN and Dhawan , H . 1971 . In IOPB chromosome number reports . XXXIV Taxon , 20 ( 6 ) : 785 – 797 .
- Merker , A . 1971 . Cytogenetic investigations in hexaploid Triticale . Hereditas , 68 ( 2 ) : 281 – 290 .
- Müntzing , A and Prakken , R . 1941 . Chromosomal aberrations in rye populations . Hereditas , 27 ( 3–4 ) : 273 – 308 .
- Palma-Silva , C , Dos Santos , DG , Kaltchuk-Santos , E and Bodanese-Zanettini , MH . 2004 . Chromosome numbers, meiotic behaviour, and pollen viability of species of Vriesea and Aechmea genera (Bromeliaceae) native to Rio Grande do Sul . Brazil. Am J Bot , 91 ( 6 ) : 804 – 807 .
- Pilquin , IP . 1981 . An unusual case of a complex heterozygote presenting no taxonomical problem in Chelidonium majus L. (Papaveraceae) . Experientia , 37 ( 4 ) : 341 – 342 .
- Prakken , R . 1943 . Studies of asynapsis in rye . Hereditas , 29 ( 3–4 ) : 475 – 495 .
- Ranjbar , M , Hajmoradi , F and Karamian , R . 2012 . An overview on cytogenetics of the genus Onobrychis (Fabaceae) with special reference to O. sect . Hymenobrychis from Iran. Caryologia , 65 ( 3 ) : 187 – 198 .
- Rao , SR and Kumar , A . 2003 . Cytological investigations in a synaptic variant of Anogeissus sericea var. sericea Brandis (Combretaceae), an important hardwood tree of Rajasthan . Bot J Linn Soc , 142 ( 1 ) : 103 – 109 .
- Sax , K . 1931 . Chromosome ring formation in Rhoeo discolor . Cytologia , 3 ( 1 ) : 36 – 53 .
- Schulze-Menz GK. 1964. Saxifragaceae. In: Melchior H, editor. A. Engler’s Syllabus der Pflanzenfamilien. Berlin: Gebruider Born- traeger. p. 201–206.
- Sharma , G and Gohil , RN . 2003 . Cytology of Allium roylei Stearn. I. Meiosis in a population with complex interchanges . Cytologia , 68 ( 2 ) : 115 – 119 .
- Sharma , G and Gohil , RN . 2008 . Intrapopulation karyotypic variability in Allium roylei Stearn – a threatened species . Bot J Linn Soc , 158 ( 2 ) : 242 – 248 .
- Sharma , G and Gohil , RN . 2011 . Occurrence of differential meiotic associations and additional chromosomes in the embryo-sac mother cells of Allium roylei Stearn . J Genet , 90 ( 1 ) : 45 – 49 .
- Sharma , SK , Bisht , MS and Pandit , MK . 2010 . Synaptic mutation-driven male sterility in Panax sikkimensis Ban. (Araliaceae) from Eastern Himalaya . India. Pl Syst Evol , 287 ( 1 ) : 29 – 36 .
- Singh , RB , Singh , BD , Vijayalakshmi , T and Singh , RM . 1977 . Meiotic behaviour of spontaneous and mutagen induced partial desynaptic plants in pearl millet . Cytologia , 42 ( 1 ) : 41 – 47 .
- Singh , RJ . 2002 . Plant cytogenetics , 2nd ed , Boca Raton , (FL) : CRC Press .
- Singhal VK. 1982. Cytomorphological studies on some members of Polypetalae from Northern and Central India [PhD thesis]. [Patiala]: Department of Botany, Punjabi University.
- Singhal , VK and Gill , BS . 1981 . Structural heterozygosity in Citrus jambhiri Lush. (Rutaceae) . Cell Chromosome Newslett , 4 ( 3 ) : 58 – 60 .
- Sjödin , J . 1970 . Induced asynaptic mutants in Vicia faba L . Hereditas , 66 ( 2 ) : 215 – 232 .
- Soltis , DE and Soltis , PS . 1997 . Phylogenetic relationships in Saxifragaceae sensu lato: A comparison of topologies based on 18S rDNA and rbcL sequences . Am J Bot , 84 ( 4 ) : 504 – 522 .
- Soltis , DE , Morgan , DR , Grable , A , Soltis , PS and Kuzoff , R . 1993 . Molecular systematics of Saxifragaceae sensu stricto . Am J Bot , 80 ( 9 ) : 1056 – 1081 .
- Soltis , DE , Kuzoff , RK , Conti , E , Gornall , R and Ferguson , K . 1996 . MatK and rbcL gene sequence data indicate that Saxifraga (Saxifragaceae) is polyphyletic . Am J Bot , 83 ( 3 ) : 371 – 382 .
- Soltis , DE , Kuzoff , RK , Mort , ME , Zanis , M , Fishbein , M , Hufford , L , Koontz , J and Arroyo , MK . 2001 . Elucidating deep-level phylogenetic relationships in Saxifragaceae using sequences for six chloroplastic and nuclear DNA regions . Ann Missouri Bot Gard , 88 ( 4 ) : 669 – 693 .
- Soost , RK . 1951 . Comparative cytology and genetics of asynaptic mutants in Lycopersicon eseulentum Mill . Genetics , 36 ( 4 ) : 410 – 434 .
- Stebbins , GL and Pun , FT . 1953 . Artificial and natural hybrids in the Gramineae, tribe Hordeae. V. Diploid hybrids of Agropyron . Am J Bot , 40 ( 6 ) : 444 – 449 .
- Talukdar , D . 2008 . Cytogenetic characterization of seven different primary tetrasomics in grass pea (Lathyrus sativus L.) . Caryologia , 61 ( 4 ) : 402 – 410 .
- Talukdar , D . 2010 . Reciprocal translocations in grass pea (Lathyrus sativus L.): pattern of transmission, detection of multiple interchanges and their independence . J Hered , 101 ( 2 ) : 169 – 176 .
- Talukdar , D and Biswas , AK . 2006 . An induced mutant with different flower colour and stipule morphology in grass pea (Lathyrus sativus L.) . Indian J Genet , 66 ( 4 ) : 365 – 367 .
- Thomas , H and Rajhathy , T . 1966 . A gene for desynapsis and aneuploidy in tetraploid Avena . Can J Genet Cytol , 8 ( 3 ) : 506 – 515 .
- Vari , AK and Bhowal , JG . 1991 . Origin and meiotic behaviour of two multiple interchange trisomics in pearl millet (Pennisetum americanum (L.) Leeke) . Cytologia , 56 ( 3 ) : 315 – 318 .
- Verma , SC and Ohri , D . 1979 . Breakdown of the classical meiotic system in Rhoeo spathacea (Commelinaceae) . Cytologia , 44 ( 1 ) : 91 – 102 .
- Vishnuvardhan , Z and Lakshmi , N . 1987 . Environmental influence on a desynaptic double trisomic pearl millet Pennisetum americanum (L.) Leeke . Cytologia , 52 ( 3 ) : 571 – 575 .
- Webb DA. 1993. Saxifraga. In: Tutin TG, Burges NA, Chater AO, Edmondson JR, Heywood VH, Moore DM, Valentine DH, Walters SM, Webb DA, editors. Flora Europaea. 2nd ed. Vol. 1. Cambridge, (UK): Cambridge University Press. p. 437–458.