Abstract
Silver nanoparticles are one of the most commonly used nanomaterials. However, there remains insufficient information on their genotoxic effects. The goal of this study was to investigate cytotoxic and genotoxic effects of chitosan-coated AgNPs using the well-established Allium test. Root tip cells were treated with solutions at different concentrations (1, 2.5, 5 and 50 mg l−1) of chitosan-coated AgNP (size: 10–30 nm; organic coat: 2–5 nm). On the same slide mitotic abnormalities, chromosome aberrations and micronuclei were detected. Also mitotic and phase indexes were analyzed. No cytotoxic or genotoxic effects were found at concentrations below 5 mg l−1. The absence of induction of chromosomal and mitotic abnormalities by chitosan-capped AgNPs at low concentrations is possibly due to the capping, which may partly protect the cells from direct interaction with the AgNPs. Mitotic and chromosomal abnormalities and micronuclei were detected at a concentration of 50 mg l−1. Significant increase in mitotic index was found at 5 and 50 mg l−1 concentrations of AgNP. The data demonstrated that chitosan-coated AgNP exhibit both clastogenic and aneugenic activity.
Introduction
The nanotechnology industry is developing rapidly and many engineered nanomaterials have been used for their benefits without recognition of their harmful effects on human health and the environment (Singh et al. Citation2009).
Silver is one of the most frequently used nanomaterials due to its antibacterial properties. The antimicrobial activity of silver nanoparticles (AgNP) has many applications (Kim et al. Citation2007; Choi et al. Citation2008; Singh et al. Citation2009; Wijnhoven et al. Citation2009; Quadros and Marr Citation2010; Rai et al. Citation2012). In particular, the beneficial effect of AgNP on infection prophylaxis led to the development of medical devices (Chaloupka et al. Citation2010). However AgNP can be toxic not only for prokaryotic cells. Nanoparticles as well as other medicinal agents require toxicity and cytotoxicity testing to ensure their safety (Modallal et al. Citation2008; Aye et al. Citation2013; Hu et al. Citation2013). AgNP toxicity and genotoxicity has been found in different eukaryotic cellular models, such as human lung fibroblasts, human hepatoma cells (HepG2) (AshaRani et al. Citation2009; Kawata et al. Citation2009), rat alveolar macrophages (Carlson et al. Citation2008) or plant and algae cells (Navarro et al. Citation2008; Kumari et al. Citation2009; Ruffini Castiglione et al. 2009; Panda et al. Citation2011; Klancnik et al. 2011).
However, AgNP remains a controversial research area with regard to its genotoxicity and toxicity (Ghosh et al. Citation2012). Therefore extensive studies are required to understand the effect of AgNP on human health and the environment.
An important task of today’s nanotechnology is searching for nanomaterials with safe biocompatible properties. It is known that the biological activity of nanoparticles depends on the size, shape and coat of nanoparticles, and if these properties change, the bio effects can differ dramatically. For example, coating (capping) has been shown to significantly reduce cytotoxicity (Derfus et al. Citation2004; Hoshino et al. 2004; Hardman Citation2006; Reijnders Citation2008).
Biological assessment with different markers is required in order to provide information that will allow the informed design of future nanomaterials, ensuring their biocompatibility and minimizing potential adverse health risks (Singh et al. Citation2009). Such knowledge is of great importance for nanotechnology to grow in a responsible and sustainable manner (Asare et al. Citation2012).
Plant bioassays are important tests in the detection of genotoxic and toxic agents and contamination in the environment (Adamakis et al. Citation2013; Dixit et al. Citation2013; Frescura et al. Citation2013). Allium cepa L. has been used to evaluate DNA damage, such as chromosome aberrations, micronuclei and disturbances in the mitotic cycle (Bakare et al. Citation2012; Frescura et al. Citation2012; Olorunfemi et al. Citation2012; Achary et al. Citation2013). Due to its sensitivity, the A. cepa test was the first of nine plants assay systems evaluated by the Gene-Tox Program of the US Environmental Protection Agency (Grant Citation1994). The Allium test is now frequently used for environmental monitoring (Fiskesjo Citation1993; Leme and Marin-Morels Citation2009), laboratory research (Pesnya and Romanovsky Citation2013) and has also bee proposed for assessing nanomaterials (Kumari et al. Citation2009; Klancnik et al. 2011).
Therefore, in the present study, genotoxic and cytotoxic effects of chitosan-coated AgNP on root meristematic cells of Allium cepa L. were investigated.
Materials and methods
Silver nanoparticles
The dispersion of chitosan-coated AgNP in deionized water, synthesized by an electrochemical method (Rodriguez-Sanchez et al. Citation2000) was a gift from SPA “Likom”, Yaroslavl, Russia. According to the manufacturer, the concentration of AgNP in dispersion is 5 mg l−1, density 270–300 nanoparticles/μl3, size of nanoparticles 10–35 nm, and size of coat is 2–5 nm, spherical in shape, surface area 24.6 m2 g−1, purity: 99.5%.
Measurements of AgNP performed using scanning probe microscope FemtoScan (Moscow, Russia). The atomic force microscopy (AFM) method was used. Data obtained were processed by software Femtoscan Online (Advanced Technologies Center, Moscow, Russia; http://www.nanoscopy.net). The AFM images (Figure ) obtained of AgNP revealed the particles to be spherical in shape, well distributed, with small aggregation, in the size range of 10–40 nm as specified by the manufacturer.
The stock dispersion of AgNP was serially diluted at different concentrations and vortexed (1, 2.5, 5 and 50 mg l−1).
2.2. Allium test and treatment
As a method of analysis of genotoxic activity was used Allium test (Constantin and Owens Citation1982; Fiskesjo 1985).
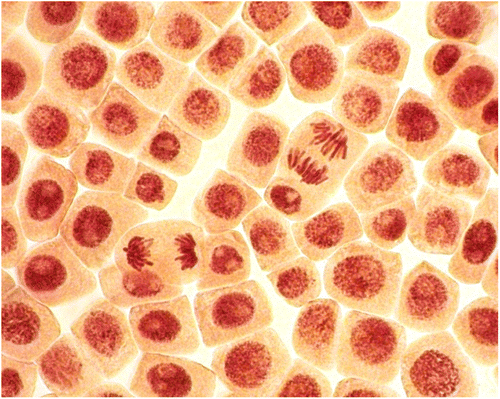
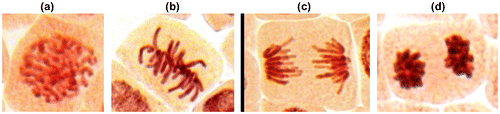
Onion bulbs (Allium cepa L., 2n = 16) of the Stuttgarten-Risen variety, average weight 25 g, were placed in small glass jars with their basal ends dipped in distilled water, and germinated at room temperature (24 ± 3°C). When the newly emerged roots were 0.50 cm in length, they were used in the test. Roots of A. cepa were treated with a series of concentrations of AgNP, i.e. 1, 2.5, 5 and 50 mg l−1 for 96 h in the absence of direct light. Control groups were treated with deionized distilled water. After treating root-tips were placed in a solution of ethanol (96%) and glacial acetic acid (3:1) for 48 h then washed with distilled water and dyed using aceto-orcein for 1 h. The squash technique was applied for the study of the mitotic index (MI) and phase indexes, mitotic and chromosomal aberrations and micronuclei. Five replicates (bulbs) were performed for each group and scoring was given from the three roots of each replicate (15 slides for each concentration) (Barbério et al. 2011). The MI was calculated for each treatment as a number of dividing cells per 700 cells and also scored the proportions of mitotic phases (Figure ).
Figure 2. (Color online) Stages of mitosis in the meristematic cells of A. cepa: (a) prophase; (b) metaphase; (c) anaphase; (d) telophase.
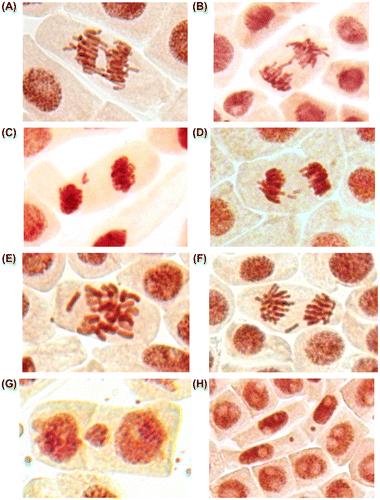
Chromosomal aberrations (chromatid (single) and chromosome (double) bridges and fragments) were scored in 100 ana-telophases per slide. Mitotic abnormalities (lagging chromosomes and polyploidy) were scored in 1000 mitotic cells per slide. Micronuclei frequency was expressed as the number of interphase cells with micronuclei per 3000 for every slide. All examinations were done under a light microscope at 400× magnification. The most frequent abnormalities (Figures , , ) and normal cells (Figures , ) are shown in photomicrographs. The results regarding MI and phase indexes and the frequency of mitotic and chromosomal abnormalities in root-tip cells of A. cepa are summarized in Tables –.
Table 1. Data on mitotic and phase indexes (mean ± SD) in the meristematic cells of A. cepa roots treated with solutions of silver nanoparticles.
2.3. Statistical analysis
Statistical calculations were done using Statistica 8.0. The differences in the mitotic index and phase indexes between treated and control groups were tested applying the non-parametric Mann–Whitney test. Frequencies of chromosome aberrations, micronuclei and mitotic abnormalities were statistically analyzed by Student’s t-test and ANOVA. The level of significance was accepted at p ≤ 0.05 (*).
3. Results and discussion
3.1. Mitotic and phase indexes
AgNP at concentrations 5 and 50 mg l−1 significantly increased the number of dividing cells in A. cepa root meristem. The most pronounced effect was registered at 50 mg l−1 concentration (Table ). It should be noted that MI significantly higher than that in the control can be harmful to cells, leading to a disordered cell proliferation and even to the formation of tumor tissues (Leme and Marin-Morels Citation2009). Additionally, phase indexes are affected only at this concentration of AgNP. The prophase index and metaphase index are increased, while that of the anaphase index and telophase index are diminished (Table ). According to Prokhorova et al. (Citation2008), increasing frequency of prophases is associated with the violation of the chromosomal supramolecular structure. The situation when the frequency of metaphases increases, while that of anaphases and telophases decreases, can be associated with the action of AgNP on the achromatic spindle. In this case chromosomal segregation cannot occur, which may result in the appearance of genomic mutations (e.g. polyploidy and aneuploidy) (Prokhorova et al. Citation2008). Several mechanisms have been proposed for the occurrence of polyploidy (Zimmet and Ravid Citation2000; Gautam and Kumar 2013; Beyaz et al. Citation2013)
AgNP at 1 and 2.5 mg l−1 concentrations caused slight, but not statistically significant, increases in MI and prophase index (Table ). Our results indicate that chitosan-capped AgNP can stimulate mitotic activity in plant tissue and disturb proportions of mitotic phases, which may result in mitotic abnormalities.
Mitotic abnormalities
In the control root tip cells were registered lagging chromosomes with frequency 0.05 ± 0.02% (spontaneous level). AgNP caused significant increasing in the frequency of lagging chromosomes at a concentration of 50 mg l−1 (Table , Figure E, F).
Table 2. Data on the number of mitotic disturbances (mean ± SD) in the meristematic cells of A. cepa roots treated by AgNP.
At concentrations of 5 and 50 mg l−1 polyploid cells were registered (Figures , ). However, the frequency of polyploid cells was considerable only at 50 mg l−1. Induction of polyploidy is considered to be the result of interference with components of the mitotic spindle during chromosome segregation or by blocking of cytokinesis (Honma et al. Citation2012). It is not clear whether chitosan coated AgNP produces polyploidy by a similar mechanism. It should be noted that observed polyploid cells did not look like classical colchicine induced polyploidy, according to Levan’s (Citation1938) micrographs. It is well known that colchicine has strong mito-depressive activity, while observed silver nanoparticles exhibit stimulating properties.
Figure 5. (Color online) Giant polyploid cells (prophases, telophases and interphases) in root meristem of A. cepa after incubation in solution of 50 mg l−1 AgNP.
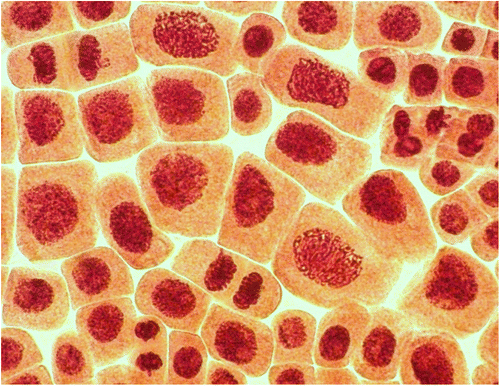
Figure 4. (Color online) Giant polyploid cells (anaphases and interphases) in root meristem of A. cepa after incubation in solution of 50 mg l−1 AgNP.
In addition, three or four nucleoli per nucleus were detected in the polyploid cells at interphase (Figure ). Normally the diploid nucleus of Allium cepa contains one or two (Figure ) nucleoli (sometime two nucleoli fuse into a single large nucleolus) (Wusheng et al. Citation1994; Panzera et al. Citation1996). It was established that the distribution of nucleoli number in an interphase nucleus can be used as an indirect practical method to distinguish diploid and polyploid cells (Dabrowska Citation1989). Thus, in the present study three or four nucleoli per nucleus may indicate polyploidy.
In the in the roots treated with 1 and 2.5 mg l−1 of silver nanoparticles polyploidy was not detected (Table ).
Chromosomal abnormalities and micronuclei
Spontaneous frequencies of chromosomal aberrations (bridges and fragments) were 0.60 ± 0.16% and for micronuclei 0.011 ± 0.004 (Table ).
Table 3 Data on the number of chromosomal aberrations and micronuclei (mean ± SD) in the meristematic cells of A. cepa roots treated with colloidal solutions of silver nanoparticles.
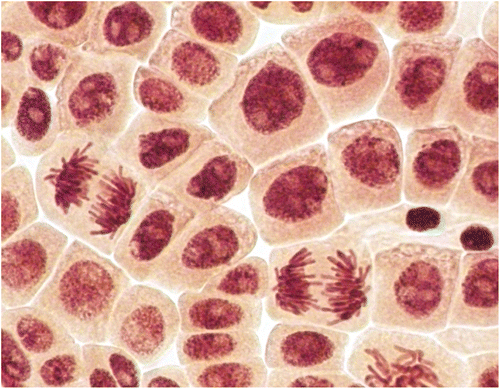
Treatments with 1, 2.5 and 5 mg l−1 concentrations did not increase the frequencies of chromosomal abnormalities or micronuclei over the control values. The absence of induction of abnormalities by chitosan-capped AgNPs at low concentrations is possibly due to the capping, which may partly protect the cells from direct interaction with the AgNP (Nymark et al. 2012; de Lima et al. Citation2012; Ju et al. Citation2013). A significant increase in the frequency of chromosomal aberrations was observed only after exposure to 50 mg l−1 of AgNP. Bridges (Figure A, B) and fragments (Figure C, D) were registered in root meristems of A. cepa at this concentration. Additionally, significant increase in frequency of micronuclei (Figure G, H) was registered (Table ). Micronuclei may arise mostly from acentric fragments or lagging chromosome (Fenech Citation2000). Fragments (Figure C, D) can be derived from chromosomal breakages caused by clastogenic effect or they may alternatively derive from chromosome aberrations, such as chromosomal bridges, which break up and originate acentric fragments (Fiskesjo Citation1993; Yi and Meng Citation2003; Leme and Marin-Morales 2008).These observations verify previous reports and show that AgNP can induce chromosomal aberrations and micronuclei in plant cells (Kumari et al. Citation2009; Panda et al. Citation2011). Additionally, new type of abnormalities (polyploidy) were registered (Table , Figures , ). These findings indicate that organic-coated AgNP can affect the whole genome.
Figure 6. (Color online) Mitotic and chromosome abnormalities and micronuclei in root meristematic cells of A. cepa: bridges (A, B), acentric fragment (C), double fragments (D), lagging chromosomes (E, F) and micronuclei (G, H).
Thus, many engineered nanoparticles, including AgNP with different chemical properties, have been shown to be cytotoxic and genotoxic both to plant and mammalian cells (Kumari et al. Citation2009; Singh et al. Citation2009; Foldbjerg et al. Citation2011; Panda et al. Citation2011; Klančnik et al. Citation2011; Teodoro et al. Citation2011). AgNP are capable of entering the nucleus, and directly or indirectly interacting with nuclear material, leading to alterations in DNA integrity (Kruszewski et al. Citation2011; Asare et al. Citation2012). Although the exact mechanism underlying genotoxicity of AgNP is yet to be elucidated, several studies have suggested that AgNP-induced DNA damage was apparently mediated through oxidative stress (Oberdorster Citation2004; Sayes et al. Citation2005; Reeves et al. Citation2008; Panda et al. Citation2011; Liu et al. Citation2012). Cellular interaction of AgNP which leads to the generation of reactive oxygen species has been shown to be related to the physicochemical characteristics of nanoparticles: size, coating, shape, surface charge (Carlson et al. Citation2008; Panda et al. Citation2011; Kim and Ryu Citation2013). Possible mechanisms for induction of oxidative stress by AgNP include direct generation of ROS from the surface of the particles, soluble compounds such as transition metals, and altered function of mitochondria or NADPH oxidase (Kim et al. Citation2011).
Conclusions
To summarize, the results of this study show that chitosan-coated AgNP did not exhibit cytotoxicity and genotoxicity at concentrations of 1 and 2.5 mg l−1. Considerable genotoxic effects of chitosan-coated AgNP at concentration 5 and 50 mg l−1 were observed. The mitotic index was significantly increased at 5 and 50 mg l−1 concentrations of AgNP. However phase indexes were modified only at 50 mg l−1 concentration of AgNP. Polyploid cells were detected at 5 mg l−1 and 50 mg l−1 concentrations of AgNP. The total frequency of mitotic abnormalities (polyploidy, chromosome lagging), chromosome aberrations (fragments, bridges) and micronuclei were significantly increased at 50 mg l−1 concentrations of AgNP.
Thus chitosan-coated AgNPs exhibit both clastogenic and aneugenic activity in plant cells.
Declaration of interest
The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgments
The author would like to express gratitude to Tatiana S. Pesnya, Alexander S. Pesnya, Anton V. Romanovsky, Sergey E. Bolotov and Inna M. Prokhorova for their helpful advice and notes.
References
- Achary VM, Parinandi NL, Panda BB. 2013. Calcium channel blockers protect against aluminium-induced DNA damage and block adaptive response to genotoxic stress in plant cells. Mutat Res. 751(2):130–138.
- Adamakis IS, Panteris E, Cherianidou A, Eleftheriou EP. 2013. Effects of bisphenol A on the microtubule arrays in root meristematic cells of Pisum sativum L. Mutat Res. 750(1–2):111–120.
- Asare N, Instanes C, Sandberg WJ, Refsnes M, Schwarze P, Kruszewski M, Brunborg G. 2012. Cytotoxic and genotoxic effects of silver nanoparticles in testicular cells. Toxicol In Vitro. 291(1–3):65–72.
- AshaRani PV, Low Kah Mun G, Hande MP, Valiyaveettil S. 2009. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 3(2):279–290.
- Aye M, Di Giorgio C, Berque-Bestel I, Aime A, Pichon BP, Jammes Y, Barthélémy P, De Méo M. 2013. Genotoxic and mutagenic effects of lipid-coated CdSe/ZnS quantum dots. Mutat Res. 750(1–2):129–138.
- Bakare AA, Adeyemi AO, Adeyemi A, Alabia OA, Osibanjo O. 2012. Cytogenotoxic effects of electronic waste leachate in Allium cepa. Caryologia. 65(2):94–100.
- Barbério A, Voltolini JC, Mello MLS. 2011. Standardization of bulb and root sample sizes for the Allium cepa test. Ecotoxicology. 20(4):927–935.
- Beyaz R, Alizadeh B, Gürel S, Özcan SF, Yildiz M. 2013. Sugar beet (Beta vulgaris L.) growth at different ploidy levels. Caryologia. 66(1):90–95.
- Carlson C, Hussain SM, Schrand AM, Braydich-Stolle LK, Hess KL, Jones RL, Schlager JJ. 2008. Unique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen species. J Phys Chem B. 112(43):13608–13619.
- Chaloupka K, Malam Y, Seifalian AM. 2010. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 28(11):580–588.
- Choi O, Deng KK, Kim N Jr, Ross L, Rao YS, Hu Z. 2008. The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res. 42(12):3066–3074.
- Constantin MJ, Owens ET. 1982. Introduction and perspectives of plant genetic and cytogenetic assay. Mutat Res. 99(1):1–12.
- de Lima R, Seabra AB, Durán N. 2012. Silver nanoparticles: a brief review of cytotoxicity and genotoxicity of chemically and biogenically synthesized nanoparticles. J Appl Toxicol. 32(11):867–879.
- Derfus AM, Chan WCW, Bhatia SN. 2004. Probing the cytotoxicity of semiconductor quantum dots. Nano Letters. 4(1):11–18.
- Dixit V, Prabha R, Chaudhary BR. 2013. Effects of EMS and SA on meiotic cells and thymoquinone content of Nigella sativa L. cultivars. Caryologia. 66(2):178–185.
- Dabrowska J. 1989. Number of nucleoli in diploids and polyploids of the genus Achillea L. Acta Soc Botanuc Polon. 58(4):541–548.
- Fenech M. 2000. The in vitro micronucleus technique. Mutat Res. 455(1–2):81–95.
- Fiskesjo G. 1985. The Allium test as a standard in environmental monitoring. Hereditas. 102(1):99–112.
- Fiskesjo G. 1993. The Allium cepa test in wastewater monitoring. Environ Toxicol Water Qual. 8(3):291–298.
- Foldbjerg R, Dang DA, Autrup H. 2011. Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Arch Toxicol. 85(7):743–750.
- Frescura VD, Kuhn AW, Laughinghouse IV HD, Nicoloso FT, Lopes SJ, Tedesco SB. 2013. Evaluation of the allelopathic, genotoxic, and antiproliferative effect of the medicinal species Psychotria brachypoda and Psychotria birotula (Rubiaceae) on the germination and cell division of Eruca sativa (Brassicaceae). Caryologia. 66(2):138–144.
- Frescura VD, Laughinghouse IV HD, Tedesco SB. 2012. Antiproliferative effect of the tree and medicinal species Luehea divaricata on the Allium cepa cell cycle. Caryologia. 65(1):27–33.
- Gautam N, Kumar G. 2013. Consequences of colchicine induced intermeiocyte connections in Helianthus annuus. Caryologia. 66(1):65–69.
- Ghosh M, Ja M, Sinha S, Chakraborty A, Mallick SK, Bandyopadhyaye M, Mukherjee A. 2012. In vitro and in vivo genotoxicity of silver nanoparticles. Mutat Res. 749(1–2):60–69.
- Grant WF. 1994. The present status of higher plant bioassays for the detection of environmental mutagens. Mutat Res. 310(2):175–185.
- Hardman RA. 2006. Toxicological review of quantum dots: Toxicity depends on physicochemical and environmental factors. Environ Health Persp. 114(2):165–172.
- Honma M, Takahashi T, Asada S, Nakagawa Y, Ikeda A, Yamakage K. 2012. In vitro clastogenicity and phototoxicity of fullerene (C60) nanomaterials in mammalian cells. Mutat Res. 749(1–2):97–100.
- Hu P, Wang T, Xu Q, Chang Y, Tu H, Zheng Y, Zhang J, Xu Y, Yang J, Yuan H, Hu F, Zhu X. 2013. Genotoxicity evaluation of stearic acid grafted chitosan oligosaccharide nanomicelles. Mutat Res. 751(2):116–126.
- Ju L, Zhang G, Zhang C, Sun L, Jiang Y, Yan C, Duerksen-Hughes PJ, Zhang X, Zhu X, Chen FF, Yang J. 2013. Quantum dot-related genotoxicity perturbation can be attenuated by PEG encapsulation. Mutat Res. 753(1):54–64.
- Kawata K, Osawa M, Okabe S. 2009. In vitro toxicity of silver nanoparticles at nontoxic doses to HepG2 human hepatoma cells. Environ Sci Technol. 43(15):6046–6051.
- Kim HR, Kim MJ, Lee SY, Oh SM, Chung KH. 2011. Genotoxic effects of silver nanoparticles stimulated by oxidative stress in human normal bronchial epithelial (BEAS-2B) cell. Mut Res. 726(2):113–122.
- Kim S, Ryu D-Y. 2013. Silver nanoparticle-induced oxidative stress, genotoxicity and apoptosis in cultured cells and animal tissues. J Appl Toxicol. 33(2):78–89.
- Kim Y-K, Lee Y-S, Jeong DH, Cho M-H. 2007. Antimicrobial effect of silver nanoparticles. Nanomedicine. 3(1):95–101.
- Klančnik K, Drobne D, Valant J, Dolenc Koce J. 2011. Use of a modified Allium test with nano TiO2. Ecotox Environ Saf. 74(1):85–92.
- Kruszewski M, Brzoska K, Brunborg G, Asare N, Dobrzynska M, Duzinska M, Fjellsbo LM, Georgantzopoulou A, Gromadzka-Ostrowska J, Gutleb AC. 2011. Toxicity of silver nanomaterials in higher eukaryotes. Adv Mol Toxicol. 5:179–259.
- Kumari M, Mukherjee A, Chandrasekaran N. 2009. Genotoxicity of silver nanoparticles in Allium cepa. Sci Total Environ. 407(19):5243–5246.
- Leme DM, Marin-Morales MA. 2008. Chromosome aberration and micronucleus frequencies in Allium cepa cells exposed to petroleum polluted water—a case study. Mut Res. 650(1):80–86.
- Leme DM, Marin-Morels MA. 2009. Allium cepa test in environmental monitoring: a review on its applications. Mutat Res. 682(1):71–81.
- Levan A. 1938. The effect of colchicine on root mitoses in Allium. Hereditas. 24(4):471–486.
- Liu Y, Guan W, Ren G, Yang Z. 2012. The possible mechanism of silver nanoparticle impact on hippocampal synaptic plasticity and spatial cognition in rats. Toxicol Letters. 209(3):227–231.
- Modallal N, Abderrahman SM, Papini A. 2008. Cytogenetic effect of Arum maculatum extract on the bone marrow cells of mice. Caryologia. 61(4):383–387.
- Navarro E, Piccapietra F, Wagner B, Marconi F, Kaegi R, Odzak N, Sigg L, Behra A. 2008. Toxicity of silver nanoparticles to Clamydomonas reinhardtii. Environ Sci Technol. 42(23):8959–8964.
- Nymark P, Catalán J, Suhonen S, Järventaus H, Birkedal R, Clausen PA, Jensen KA, Vippola M, Savolainen K, Norppa H. 2012. Genotoxicity of polyvinylpyrrolidone-coated silver nanoparticles in BEAS 2B cells. Toxicology. 313(1):38–48. http://dx.doi.org/10.1016/j.tox.2012.09.014
- Oberdorster E. 2004. Manufactured nanomaterials (fullerenes, C60) induce oxidative stress in the brain of juvenile largemouth bass. Environ Health Persp. 112(10):1058–1062.
- Olorunfemi D, Duru E, Okieimen F. 2012. Induction of chromosome aberrations in Allium cepa L. root tips on exposure to ballast water. Caryologia. 65(2):147–151.
- Panda KK, Achary VM, Krishnaveni R, Padhi BK, Sarangi SN, Sahu SN, Panda BB. 2011. In vitro biosynthesis, genotoxicity bioassay of silver nanoparticles using plants. Toxicol In Vitro. 25(5):1097–1105.
- Panzera F, Giménez-Abián MI, López-Sáez JF, Giménez-Martín G, Cuadrado A, Shaw PJ, Beven AF, Cánovas JL, De la Torre C. 1996. Nucleolar organizer expression in Allium cepa L. chromosomes. Chromosoma. 105(1):15–19.
- Pesnya DS, Romanovsky AV. 2013. Comparison of cytotoxic and genotoxic effects of plutonium-239 alpha particles and mobile phone GSM 900 radiation in the Allium cepa test. Mutat Res. 750(1–2):27–33.
- Prokhorova IM, Kovaleva MI, Fomicheva AN, Babanazarova OV. 2008. Spatial and temporal dynamics of mutagenic activity of water in lake Nero. Inland Water Biol. 1(3):1–25.
- Quadros ME, Marr LC. 2010. Environmental and human health risks of aerosolized silver nanoparticles. J Air Waste Manage. 60(7):770–781.
- Rai MK, Deshmukh SD, Ingle AP, Gade AK. 2012. Silver nanoparticles: the powerful nanoweapon against multidrug-resistant bacteria. J Appl Microbiol. 112(5):841–852.
- Reeves JF, Davies FS, Dodd NJF, Jha AN. 2008. Hydroxyl radicals (OH) are associated with titanium dioxide (TiO2) nanoparticle-induced cytotoxicity and oxidative DNA damage in fish cells. Mutat Res. 640(1–2):113–122.
- Reijnders L. 2008. Hazard reduction in nanotechnology. J Ind Ecol. 12(3):297–306.
- Rodrıguez-Sanchez L, Blanco MC, López-Quintela MA. 2000. Electrochemical synthesis of silver nanoparticles. J Phys Chem B. 104(41):9683–9688.
- Ruffini Castiglione M, Cremonini R. 2009. Nanoparticles and higher plants. Caryologia. 62(2):161–165.
- Sayes CM, Gobin AM, Ausman KD, Mendeza J, West JL, Colvin VL. 2005. Nano-C60 cytotoxicity is due to lipid peroxidation. Biomaterials. 26(36):7587–7595.
- Singh N, Manshian B, Gareth JS, Jenkins GJS, Griffiths SM, Williams PM, Maffeis TGG, Wright CJ, Doak SH. 2009. Nanogenotoxicology: the DNA damaging potential of engineered nanomaterials. Biomaterials. 30(23–24):3891–3914.
- Teodoro JS, Simões AM, Duarte FV, Rolo AP, Murdoch RC, Hussain SM, Palmeira CM. 2011. Assessment of the toxicity of silver nanoparticles in vitro: a mitochondrial perspective. Toxicol In Vitro. 25(3):664–667.
- Wijnhoven SWP, Peijnenburg WJGM, Herberts CA, Hagens WI, Oomen AG, Heugens EHW, Roszek B, Bisschops J, Gosens I, Van de Meent D, Dekkers S, De Jong WH, Zijverden MV, Sips AJAM, Geertsma RE. 2009. Nanosilver – a review of available data and knowledge gaps in human and environmental risk assessment. Nanotechnology. 3(2):109–138.
- Wusheng J, Donghua L, Maoxue L. 1994. Effects of Cd on the nucleolus in root tip cells of Allium cepa. J Environ Sci. 6(3):382–386.
- Yi H, Meng Z. 2003. Genotoxicity of hydrated sulfur dioxide on root tips of Allium sativum and Vicia faba. Mutat Res. 537(1):109–114.
- Zimmet J, Ravid K. 2000. Polyploidy: occurrence in nature, mechanisms, and significance for the megakaryocyte-platelet system. Exp Hematol. 28(1):3–16.