Abstract
The aim of this study was to evaluate possible genotoxic damage of dichlorophene stress in rats by chromosomal aberration (CA), micronucleus (MN) and mitotic index (MI) assays in bone marrow cells. The study was carried out in vivo using three sublethal concentrations, 66.9 mg, 133.8 mg and 200.7 mg kg–1 body weight of rat of dichlorophene administered intraperitoneally. The bone marrow cells were evaluated in each of the three treated groups at multiple durations. The MN and CA frequencies were increased significantly. A positive time- and dose-response relationship in all exposures was observed. However, the MI significantly decreased at each concentration compared to normal control. The results confirm the cytotoxic and genotoxic damage in Rattus norvegicus, and the suitability of the parameters for the screening of the genotoxicant is further discussed.
Introduction
The threat of some chemicals is so serious that their routine use may be mutagenic to human population (Saghir et al. Citation2001). Studies on industrial workers and farmers have shown that exposure to such hazardous chemicals cause somatic as well as hereditary mutations (Antonelli et al. Citation2003; Farah et al. Citation2003). A few studies on mammals demonstrated the mutagenic potential of chemical compounds can be assessed by using CAs as an indicator of mutagenic potential (Sharma et al. Citation2000). However, studies determining the genotoxic potential of organochlorine pesticides in mammals using CAs are rare. A survey of the literature showed no studies investigating the genotoxicity of dichlorophene in mammalian system.
This could partly be because dichlorophene is a relatively recently introduced compound. Chemically, it is represented as 2,2′-methylenebis(4-chlorophenol), a halogenated phenolic compound with wide applications. It is used as a fungicide, bactericide and antiprotozoan (Gemmel and Johnston Citation1981; Kintz et al. Citation1997). Dichlorophene spray also has therapeutic use in the disease digital dermatitis (Ghashghaei 2007). In guinea pigs, a few studies obtained mixed results in dicholorophene sensitization tests (Yamarik 2004). Some derivatives, such as chlorinated bisphenol, used as antibacterial and antifungal agents, are indicated to be potent inhibitors of glucose-6-phosphate dehydrogenase in yeast. There have been other studies of related compounds including dichlorophen (Wang and Buhler Citation1981; Kintz et al. Citation1997) but research on dichlorophene is lacking.
We propose to evaluate the in vivo mutagenic potential of dichlorophene in Rattus norvegicus by using CAs to look for breaks, gaps, rings, translocations and multiple aberrations, MN induction and MI using bone marrow cells (BMCs).
Materials and methods
Specimens
The animals were procured from Central Drug Research Institute (CDRI), Lucknow, India, and acclimatized for a week. The regular feed included commercial standard food and water ad libitum. All rats were 8–10 weeks of age, had an average weight of 100 ± 10 g, and were kept in controlled conditions (12 h dark and light period; temperature 22 ± 2°C; and humidity 70–80%). Of the five groups, two groups served as controls (positive and normal) and three groups received treatments with a specific concentration of dichlorophene for a specified time. The sacrifice of rats was in compliance with the ethical regulations formulated by the Ethical Committee of the Aligarh Muslim University, Aligarh.
Chemicals
Bearing CAS No. 97-23-4, 99.6%, dichlorophene was supplied by Sigma-Aldrich Laborchemikalein, Niedersachsen, Germany. Chemicals used in various other formulations were also of the highest purity analytical grade. These were potassium chloride, methanol, colchicine, glacial acetic acid, foetal bovine serum, Giemsa stain, May–Grunwald stain, ethanol, potassium dihydrogenphosphate and disodium hydrogen phosphate.
Treatment
A stock solution was prepared by dissolving dichlorophene in distilled water. Sublethal concentrations were prepared on the basis of LD50 values of dichlorophene 669 mg kg–1 body weight (bw) in rat. Sublethal concentrations of 66.9, 133.8 and 200.7 mg kg–1 bw were administered intraperitoneally, comprising five animals per treatment. Bone marrow flushed and the cells were screened after completion of specified durations. Concurrently positive control cyclophosphamide (0.02 mg g–1) and normal control (distilled water) runs were also made and evaluated in the same manner. A comparative analysis of the data was prepared.
Slide preparation
Two hours prior to sacrificing of rats, colchicine, 0.004 mg kg–1 bw was injected intraperitoneally. This was based on prior runs for scoring the optimal aberrations.
Chromosomal aberrations
The slide preparation and the staining followed the protocol of Preston et al. (Citation1987). In brief: both femurs of each rat were extracted and bone marrow was flushed using a syringe having 0.56% KCl solution. The KCl served as a hypotonic solution incubated for 30 m at 37°C, following centrifugation of 10 m at 1500 rpm. The contents were fixed in glacial acetic acid:methanol (1:3 v/v). These cells were then prepared for microscopic examination by pouring 3–4 drops of cell suspension on pre-cleaned, chilled, ethanol-dipped slides, flame- and air-dried and stained with 5% Giemsa for 25–30 m.
Micronucleus test
The micronucleus test was carried out according to Schmid (Citation1975) on the animals treated with dichlorophene and the control groups. The flushing of BMCs from both the femurs was collected as a fine suspension into a tube containing 1 ml foetal bovine serum (FBS). The centrifugation was carried out for 10 min at 1000 rpm. The pellet was resuspended in FBS. The suspension was smeared onto pre-cleaned and air-dried slides. Following fixation in 100% methanol for 5 min, staining was carried out in May–Grunwald and Giemsa. The clearing of slides for both CA and micronucleus test was done in xylene and they were permanently mounted in DPX Dibutyl Phathalate Xylene. An appropriate number of slides were sorted. The selection of slides was made on the basis of staining quality, and they were coded and scored randomly. A maximum of 6000 cells in each group were examined at 10× and 100× magnifications. Identification of MN followed Schmid’s recommendations (1975).
Mitotic indices
The method of Hedges et al. (1995) was followed. The MI was calculated from a total of 2000 cells scored in each concentration category. A separate lot saved from slides as prepared for CA assessment was used for MI estimation. More than 500 cells per animal in all individual groups were used to evaluate the potential toxicity of the test chemical using the formula: MI = total no. of dividing cells × 100/total no. of cells observed. In these calculations interphase, prometaphase and other sub-stages were excluded.
Statistical analysis
All the scorings were done from slides under code. The Mann–Whitney U test was applied on CA and MN data to calculate the percentage of frequencies of CAs and MN and the mean percentage, standard error and to see the difference (significant or non-significant) for required groups computed with the help of Statistical Package for Social Sciences (SPSS) version 16.0. For the comparison of proportions of mitotic indices between the control and exposed groups, the chi-square test was applied by using Med Calc version 12.0.
Results
A summary of MN and CA counts, mean frequency and standard deviation is provided in Tables and . The administration of intraperitoneal doses of dichlorophene displayed a significant induction of MN. A high value of MN induction occurs in PCEs polychromatic erythrocytes at 200.7 mg kg–1 bw concentration for 24 h duration, registering a mean frequency of 1.05 ± 0.50 with 16 micronucleated cells. The frequency of MN in PCEs is in a concentration and duration dependent manner. However, at 24 h post-administration the frequency of MN formation declined so that significantly low incidences were observed in subsequent durations. The concentration and duration dependent profiles of MN are more conspicuous in Figure .
Table 2. Incidence of in vivo chromosomal aberrations recorded in the bone marrow cells of Rattus norvegicus exposed to multiple doses of dichlorophene
Table 1. Micronuclei scoring in bone marrow cells of Rattus norvagicus treated in vivo with different doses of Dicholorophene
Figure. 1 Multiple concentration and duration-dependent profiles of (MN) by dichlorophene at different intervals in Rattus norvegicus along with their standard percent error depicted by error bars. Normal control (distilled water); positive control (cyclohosphamide); DCP1 (66.9 mg); DCP2 (133.8 mg); DCP 3 (200.7 mg); *Statistically significant values at 0.05.
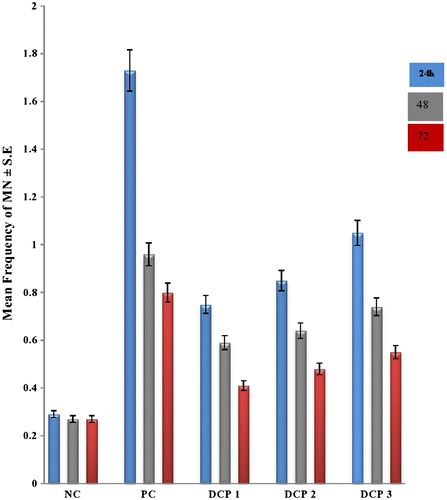
The PCE/NCE (NCE - Normochromatic erythrocytes) ratio was also studied using this compound. In each category of concentration the ratio showed a decreasing trend from 0.38 to 0.33 for respective concentrations as the time elapsed following administration of pesticide.
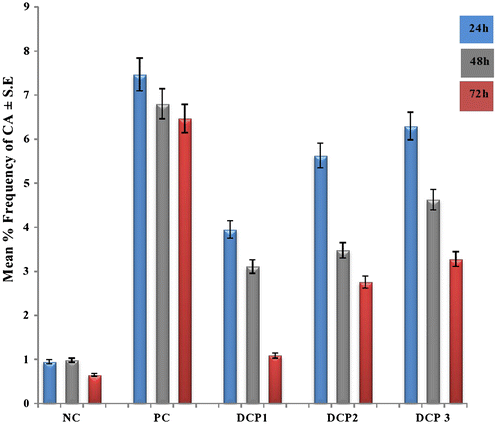
Chromosomal aberrations for this compound were also observed. With a maximum at 24 h duration, a significant decrease in mean frequency was observed as the duration increased. The results are presented in Table . Most conspicuous is the maximum effect at the high concentration: chromosomal damage was recorded as 6.30 ± 1.69 No, they dont need units, which is close to the severity of cyclophosphamide. In the remaining two concentrations the injurious effect measured by CA is less. The studies of discrimination between chromatid and chromosomal aberrations showed a marginal increase in favour of chromatid type errors. Hence, the multiple aberrations were found in significant numbers. The breaks, gaps, translocations, stickiness and pulverization were not unique for any treatment group qualitatively; however, a dose-dependent response for total aberrations was typically observed. CA revealed a concentration-dependant increase and the maximal response in test chemical was observed at 24 h treatment in all the concentrations. The profiles of CAs are illustrated in Figure .
Figure. 2 Multiple concentrations and duration-dependent profiles of (CAs) by dichlorophene at different intervals in Rattus norvegicus along with their standard percent error depicted by error bars. Normal control (distilled water); positive control (cyclohosphamide); DCP1 (66.9 mg/kg bw); DCP2 (133.8 mg/kg bw); DCP 3 (200.7 mg/kg bw); *Statistically significant values at 0.05.
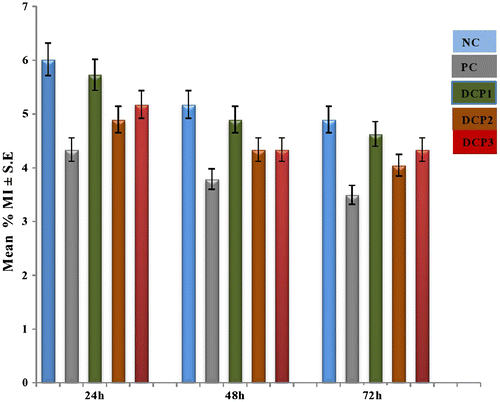
The results of the MI assay are summarized in Table . Although the trend indicated a dose-dependent inhibition, it was less convincingly time-dependent. A minimum value (4.05 ± 1.26) No, they dont need units was observed for 133.8 mg kg–1 bw; as opposed to the normal value. The maximum decrease was seen at 72 h in a concentration of 133.8 mg kg–1 bw. This pattern is shown in Figure .
Table 3. Incidence of in vivo chromosomal aberrations recorded in the bone marrow cells of Rattus norvegicus exposed to multiple doses of dichlorophene
Figure. 3 Multiple concentrations and duration-dependent profiles of (MI) by dichlorophene at different intervals in Rattus norvegicus along with their standard percent error depicted by error bars. Normal control (distilled water); positive control (cyclohosphamide); DCP1 (66.9 mg/kg bw; DCP2 (133.8 mg/kg bw); DCP 3 (200.7 mg/kg bw); *Statistically significant values at 0.05.
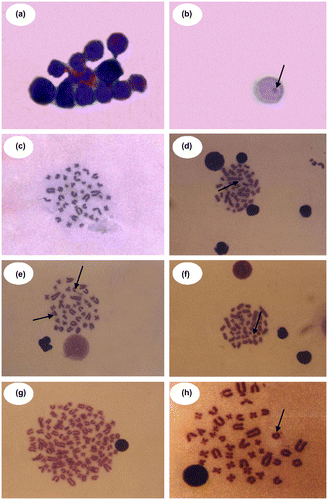
The chromatid and chromosomal aberrations included permanent features of damage such as breaks and gaps. Other aberrations such as rings, dicentrics, polyploidy, stickiness, acentric fragments and pulverization, scored as the multiple aberrations class (MA). Figure shows selected micrographs of above bone marrow cell with micronucleus and metaphase chromosome (BMCh).
Figure 4 (Color online) Photomicrograph showing polychromatic erythrocytes (a) and micronucleated cell (b) in the bone marrow cells of Rattus norvegicus treated in vivo with different doses of dicholorophene intraperitoneally and metaphase plates of bone marrow cells for different types of chromosomal aberrations with dicholorophene (c–h); (c) Normal metaphase; (d) Dicentric; (e) Acentric fragment and Gap; (f) Break; (g) Polyploidy; (h) Ring (100X oil immersion lens).
Discussion
To the best of our knowledge, only one study has been carried out on the cytotoxic and genotoxic effects of dichlorophene on plant systems (Shaikh et al. 2012), despite the fact that plant test systems are efficient material for cytogenetic studies (Dixit et al. Citation2013; Frescura et al. Citation2013).
Likewise, only a few reports are available in the mammalian system on the genotoxic effect of dichlorophene; the present investigation on Rattus norvegicus is therefore important. Our results show that this pesticide induces significant cytogenetic damage in BMCs, resulting in an increase in MN induction and CAs. The tests adopted in the study are standard one and have received much attention as parameters for genotoxic assessment (Avishai et al. Citation2002; Klobucar et al. Citation2003). Other studies also recommend using CAs for qualitative and quantitative assessment to detect clastogenic activity (Prasad et al. 2009). The MN assay is advocated for assessing clastogenic effects and damage to the mitotic apparatus with aneugenic consequences (Yoshioka et al. Citation2007; Dimitrov et al. Citation2006). A combination of these two tests is preferred (Brzovic et al. Citation2009). The time period chosen in the present study, i.e. 24, 48 and 72 h, is justified in the light of earlier studies (Nazam et al. Citation2013; Nabeel et al. Citation2008), as it allows a sufficient window period to detect clastogen and spindle poisons.
During our observations on CAs, as also in an earlier study on rat (Bird et al. Citation1982), metaphase analysis shows more chromatid breaks than chromosome breaks, pointing to DNA strand damage in the late S phase. The type of breaks indicates that dichlorophene is active in the G1 and S phase of the cell cycle; a similar conclusion was arrived at by Arzt et al. (Citation1989). The phenomenon of a decline in the frequency of aberrations in later intervals could be due to factors such as elimination of chemicals or metabolites from the body, repairing of the damaged genetic material, and elimination of chromosomes with damaged genetic material (Shyama and Rahiman Citation1993).
Induction of micronuclei in the PCE of bone marrow cells has been regarded as a sensitive bioassay for mutagenic toxicity of candidate compounds (Hammam and Foda Citation2004). The frequency of dichlorophene-induced micronucleated polychromatic erythrocytes (MNPCEs) increases, especially at 24 h post treatment, but gradually decreases. The PCE/NCE ratio and the percentage of polychromatic cells show a little variation for this chemical. Generally the ratio is regarded as an indicator of inhibition of nucleated erythropoeitic cell divisions, and therefore, important for MNT. Such changes are suggested because of unbalanced changes in number of PCEs and NCEs (Suzuki et al. Citation1989). The altered ratio points to acceleration of differentiation of erythrocytes from erythroblasts, inhibition of erythroblast division or because of recovery of erythroblast division (Suzuki et al. Citation1993).
The MI assay can help to characterize proliferating cells, another standard way of identifying compounds that inhibit or induce mitotic progression. Such an inhibition could indicate a possible cellular death or delay in the cell proliferation kinetics (Öcal and Eroglu Citation2012). This trend is not very obvious in our case, as the response to dichlorophene seems not to follow the time–response manner. Could lower concentrations of this chemical stimulate the rate of cell division (Kalcheva et al. Citation2009)? This has to be verified.
Declaration of interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgements
The research grants of University Grants Commission, New Delhi; 40-355/2011(SR), 40-3 (M/S)/2009(SA-III-MANF) and Council of Science and Technology, U.P.; CST/D-598/2011 are acknowledged. The authors are also grateful to the Chairman, Department of Zoology, Aligarh Muslim University, Aligarh for providing the necessary laboratory facilities.
References
- Antonelli A, Portesi E, Cozzoli A, Tradanico R, Balzarini P, Grigolato PG, Cosciani Cunico S. 2003. The collecting duct carcinoma of the kidney: a cytogenetical study. Europ Urol. 43:680–685.
- Arzt ES, Castelo SF, Diaz A, Finkielman S, Nahmod VE. 1989. The muscarinic agonist pilocarpine inhibits DNA and interferon-γ synthesis in peripheral blood mononuclear cells. Int J Immunopharmacol. 11:275–281.
- Avishai N, Rabinowitz C, Moiseeva E, Rinkevich B. 2002. Genotoxicity of the Kishon River, Israel: The application of an in vitro cellular assay. Mutat Res. 518:21–37.
- Bird RP, Draper HH, Bassur PK. 1982. Effects of malonaldehyde and acetaldehyde on cultured mammalian cells. Production of micronucleus and chromosomal aberrations. Mutat Res. 101:237–246.
- Brzovic V, Miletic I, Zeljezic D, Mladinic M, Kasuba V, Ramic S, Anic I. 2009. In vitro genotoxicity of root canal sealers. Int Endodont J. 42:253–263.
- Dimitrov BD, Gadeva PG, Benova DK, Bineva MV. 2006. Comparative genotoxicity of the herbicide Roundup, Stomp and Reglone in plant and mammalian test systems. Mutagenesis. 21:375–382.
- Dixit V, Prabha R, Chaudhary BR. 2013. Effects of EMS and SA on meiotic cells and thymoquinone content on Nigella sativa L. cultivars. Caryologia. 66:178–185.
- Farah MA, Ateeq B, Ali MN, Ahmad W. 2003. Evaluation of genotoxicity of PCP sand 2, 4-D by micronucleus test in freshwater fish C. punctatus. Ecotoxicol Environ Safe. 54:25–29.
- Frescura VDS, Kuhn AW, Laughinghouse HD IV, Nicoloso FT, Lopes SJ, Tedesco SB. 2013. Evaluation of the allelopathic, genotoxic and antiproliferative effect of the medicinal species Psychotria brachypoda and Psychotria birotula (Rubiaceae) on the germination and cell division of Eruca sativa (Brassicaceae). Caryologia. 66:138–144.
- Gemmel MA, Johnston PD. 1981. Cestodes. Antibiot Chemother. 30:54–114.
- Ghashghaei. . 2007. Clinical evaluation of the therapeutic effect of dichlorophen spray in treatment of digital dermatitis in dairy cows. J Vet Med. 1:23–29.
- Hammam FM, Foda IH. 2004. Mutagenic studies on the effect of Aldicarb “Temik” and vitamin C as antioxidant agent on the white rat: Chromosomal aberrations and micronucleus tests. Egypt J Hosp Med. 17:143–154.
- Hedges AR, Shieh WJ, Sikorski CT. 1995. Use of cyclodextrins for encapsulation in the use and treatment of food products. ACS Symp Ser. 590:60–71.
- Kalcheva PV, Dragoeva AP, Kalcheva KN, Enchev DD. 2009. Cytotoxic and genotoxic effects of Br-containing oxaphosphole on Allium cepa L. root tip cells and mouse bone marrow cells. Genet Mol Biol. 32:389–393.
- Kintz P, Jamey C, Doray S, Ludes B. 1997. Acute fatal poisoning with dichlorophen. Int J Legal Med. 110:95–96.
- Klobucar GIV, Pavlica M, Erben R, Papes D. 2003. Application of the micronucleus and comet assays to mussel Dreissena polymorpha haemocytes for genotoxicity monitoring of freshwater environments. Aquat Toxicol. 64:15–23.
- Nabeel M, Abderrahman S, Papini A. 2008. Cytogenetic effect of Arum maculatum extract on the bone marrow cells of mice. Caryologia. 61:383–387.
- Nazam N, Lone MI, Sheikh S, Ahmad W. 2013. Assessment of genotoxic potential of an insecticide Dichlorvos using cytogenetic assays. Interdiscipl Toxicol. 6:101–106.
- Öcal A, Eroglu HE. 2012. In vitro cytogenetic effects of Hypericum heterophyllum in human peripheral blood lymphocytes. Bangladesh J Pharmacol. 7:36–41.
- Prasad S, Srivatava S, Singh M, Shukla Y. 2009. Clastogenic effects of glyphosate in bone marrow cells of Swiss albino mice. J Toxicol. Article ID 308985, 6 pages, doi: 10.1155/2009/308985
- Preston RJ, Dean BJ, Galloway S, Holden H, McFee AF, Shelby M. 1987. Mammalian in vivo cytogenetic assay: analysis of chromosomal aberrations in bone marrow cells. Mutat Res. 189:157–165.
- Saghir SA, Fried K, Rozman KK. 2001. Kinetics of monochloroacetic acid in adult male rats after intravenous injection of a subtoxic and toxic dose. J Pharmacol Exp Therapeut. 296:612–622.
- Schmid W. 1975. The micronucleus test. Mutat Res. 31:9–15.
- Shaikh S, Nazam N, Lone MI, Ahmad W. 2012. Dichlorphen and dichlorvos mediated genotoxic and cytotoxic assessment on root meristem cells of Allium cepa. Science Dilliman. 24:13–22.
- Sharma N, Trikha P, Akthar M, Raisuddin S. 2000. Inhibitory effect of Emblica officinalis on the in-vivo clastogenicity of benzo [a] pyrene and cyclophosphamide in mice. Human Exp Toxicol. 22:643–653.
- Shyama SK, Rahiman MA. 1993. Progestin (norethisterone)-induced genetic damage in mouse bone marrow. Mutat Res. 300:215–221.
- Suzuki Y, Nagae Y, Li J, Sakaba H, Mozawa K, Takahashi A, Shimizu H. 1989. The micronucleus test and erythropoiesis: effects of erythropoietin and a mutagen on the ratio of polychromatic to normochromatic erythrocytes (P/N ratio). Mutagenesis. 4:420–424.
- Suzuki Y, Shimizu H, Nagae Y, Fukumoto M, Okonogi H, Kadokura M. 1993. Micronucleus test and erythropoiesis: Effect of cobalt on the induction of micronuclei by mutagens. Environ Mol Mutagen. 22:101–106.
- Wang JL, Buhler DR. 1981. Effect of chlorinated bisphenolson torula yeasts glucose-6-phosphate dehydrogenase. J Toxicol Environ Health. 8:639–648.
- YamarikTA. 2004. Safety assessment of dicholorophene and chlorophene. Int J Toxicol. 23:1–27.
- Yoshioka H, Shimizu H, Toyama Y, Miyakoshi Y, Suzuki Y, Takagi R. 2007. Genotoxicity study of illegal drug MDMA and its nitroso derivative N-MDMA by micronucleus and chromosomal tests using Chinese hamster lung fibroblast cell line. Environ Health Prevent Med. 12:129–137.