Abstract
Amsonia orientalis Decne. (Apocynaceae) is a medically useful and critically endangered plant which has very restricted distribution world-wide. The root tips and flower buds of A. orientalis were used as an experimental material and they were squashed in aceto-orcein. Fresh flowers and a liquid medium were used for pollen germination tests. In spite of the small size of the cells and chromosomes, all phases of mitosis and meiosis were observed. Generally the phases of mitosis were regular but there were a few abnormalities, such as laggard chromosome in metaphase and bridge formation in anaphase. There was a good relationship between the stages of pollen development and floral bud length. As a result of the cytokinesis, tetrahedral types of tetrads were occurred in microsporogenesis. The tetrad nuclei resulting from the simultaneous type of meiosis were found to be of equal size. Pollen germination had started from the first hour and pollen tube lengths and germination percentages regularly increased with increasing time. This is the first study that identifies mitotic and meiotic cycles and pollen germination in A. orientalis. Our findings about the mitosis and reproduction biology of this critically endangered plant will be useful for in vitro and in situ conservation, taxonomic and genetic studies.
Introduction
Amsonia orientalis Decne. (Apocynaceae) is a medically useful plant which has a very restricted distribution, only in the west of Turkey and east of Greece. Its preferred common name is Eastern blue star and its synonym is Rhazya orientalis. Karyological information on Amsonia species is restricted mostly to chromosome counting (Noori-Daloii et al. Citation1996; Baker et al. Citation2009). Literature on A. orientalis, which is critically endangered in nature according to IUCN categories, is very limited in number.
Nevertheless, the anatomy and morphology of A. orientalis have been investigated and it was found that extracts of this plant have strong antimicrobial activity (Akyalçın et al. Citation2006). Furthermore, it is known to have anti-tumor and anti carcinogenic effects because of its various alkaloids (Dabine Lengyel et al. Citation1986; Rahman and Zaman Citation1988; Rahman et al. Citation1989; Ekim et al. Citation2000; Akyalçın et al. Citation2006; Özen Citation2006). In addition, it has been shown that A. orientalis prefers slightly alkaline, sandy–loamy soils, saltys, mid-calcareous, with poor organic material, and very rich in iron and magnesium (Özen Citation2006). The noteworthy contributions to the embryological studies of the Apocynaceae were made by Fyre and Blodgett (Citation1905), Guignard (Citation1917), Anderson (Citation1931), Schnarf (Citation1931), Meyer (Citation1938) and Rau (Citation1940). While summarizing the embryological data on Apocynaceae, Davis (Citation1966) mentioned the controversy concerning the nature of division of microspore mother cells, and due to the occurrence of different types of embryogeny Apocynaceae was termed a heterogeneous group by Maheshwari in Citation1971.
There have been no reports on the mitosis and the reproduction biology of A. orientalis. This study provides a comprehensive description of the mitosis in root tip cells, microsporogenesis and in vitro pollen tube formation of A. orientalis during anthesis.
Materials and methods
Mitotic experiments
A. orientalis specimens were collected from Balıkesir and planted in the field at Kocaeli University. The root tips of A. orientalis were obtained form tissue culture regenerated from basal parts of shoots in Murashige and Skoog medium containing 0.5% indole acetic acid in a plant growth chamber at 23 ± 1°C and 16 h light and 8 h dark photoperiod for 30 days. The root tips were taken at 9:00–10:00 am and immediately fixed in Carnoy’s solution for 24 h and preserved in 70% ethanol. From the first experiments it was observed that the root tip cells were full of globular molecules, so they were stained with lugol in order to determine whether they were starch. After staining with lugol we found that the globular molecules that prevent us seeing the chromosomes were not starch but may be lipids and/or alkaloids. Then the root tips were treated with 75% acetone for 4 minutes to remove these molecules. Then they were hydrolyzed in 1 N HCl at 60°C for 5–10 min followed by squashing in 1% aceto-orcein.
Meiotic experiments
The flower buds in different sizes and developmental stages were taken at 09:00–10:00 am from healthy and vigorous plants in April–May. They were immediately fixed in Carnoy’s solution for 24 h and then they were transferred to 70% ethanol and stored in a refrigerator until use. Some of the flower buds were directly squashed in 1% aceto-orcein and some of them were squashed in 1% hematoxylin. For pollen viability test, a drop of aniline blue in lactophenol, a stain often used for pollen viability studies (Hauser and Morrison Citation1964), was added onto each slide. The prepared slides were kept at +4°C until examination.
In vitro pollen germination
For pollen germination tests fresh flowers during anthesis and a liquid medium containing 10% sucrose, 0.01% H3BO3, 0.01% CaCl2, 0.02% MgSO4.7H2O and 0.01% KH2PO4 were used. The anthers were hydrated by placing them in a glass vial placed in a covered tray with wet filter paper for 60 min at room temperature. Then, approximately 0.02 g of pollen with anthers was placed on a 35-mm Petri dish with 1–2 ml of liquid germination medium and they were incubated in the dark at 26 ± 1°C for 1–6 hours. Pollen grains which produced a tube equal to or longer than their own diameter (approximately 45 μm) were counted as germinated (Imani et al. Citation2011). Data were collected from four Petri dishes, counts were made at random in three fields (each of 500 pollen grains) under 100× magnification. The germination percentages of pollen grains were recorded in hours 1–6 and 12. The final percentage germination was defined as: germination % = (number of germinated pollen per field/total number of pollen per field) × 100
The in vitro elongation of pollen tubes was measured on germinated pollen grains in artificial media. The photographs of the slides were taken by Olympus BX51 light and fluorescent microscope. (Olympus Corporation, Tokyo, Japan)
Statistical analyses
The association between pollen germination and time was tested using ANOVA (p < 0.05). Duncan’s multiple range tests were used for mean separations for the effect of time during pollen germination and pollen tube length. Statistical analyses were performed with SPSS 17.0 statistical software (SPSS, Chicago, IL, USA).
Results
Mitotic observations
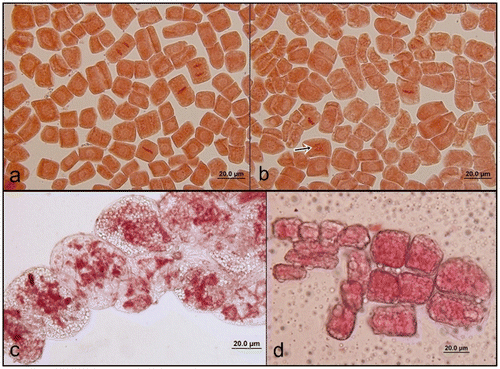
In spite of the small size of the cells and chromosomes, we observed all the phases of mitosis (Figure a–c). Generally the phases of mitosis were regular but there were a few abnormalities such as laggard chromosome in metaphase and bridge formation in anaphase. At the beginning of our study we mostly observed many molecules in meristematic root tip cells that prevented us observing mitotic divisions of A. orientalis (Figure d).
Meiotic observations
The microspore mother cells undergo two consequent meiosis divisions and form a microspore tetrad. Cell division of the microspore mother cell begins with early prophase I (Figure ) and ends with late telophase II (Figure ), passing through metaphase I (Figure ), anaphase I (Figure ), telophase I (Figure ), prophase II (Figure ), metaphase II (Figure ) and anaphase II (Figure ). During meiosis II, the spindles are oriented parallel to each other forming microspore tetrads (Figure ). Cytokinesis in microspores follows both types simultaneously; on the other hand, no cell plate was laid down after meiosis I, and the spindle fibers of this division disappear during the metaphase of meiosis II.
Figure 3. (Color online) Second meiotic division in pollen mother cells of A. orientalis stained with orcein. (a) prophase II; (b) metaphase II; (c) anaphase II; (d) telophase II; (e) tetrads; (f) microspore with one nucleus.
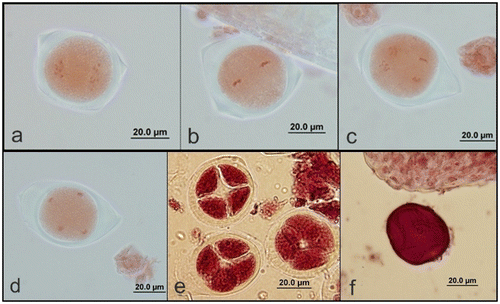
Figure 2. (Color online) First meiotic division in pollen mother cells of A. orientalis stained with orcein. (a) Prophase I; (b) metaphase I; (c) anaphase I; (d) telophase I.
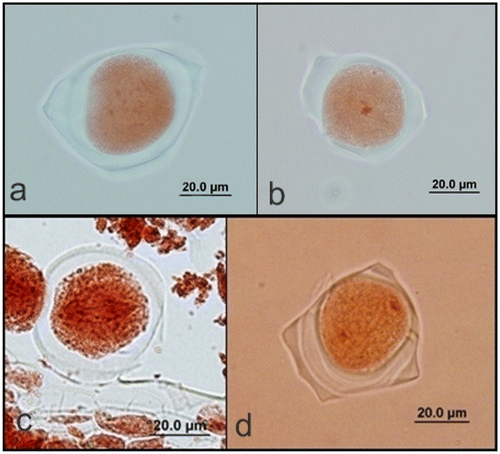
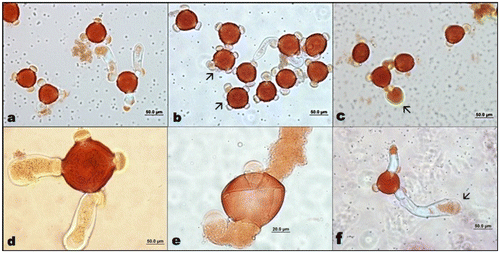
After the four daughter nuclei have become organized, they assumed a tetrahedral arrangement and a spindle was reformed between every two nuclei. It is followed by formation of constriction furrows which start at the periphery and proceed inward until they meet at the center, so that there is simultaneous division of protoplast into four cells microspores (Figure ). The microspores (Figure ) are soon separated from each other and individually developed into pollen grains (Figure ).
Figure 4. (Color online) Mature pollen grains of A. orientalis (a) stained with orcein; (b) stained with lactophenol aniline blue; (c) auto-fluorescence.
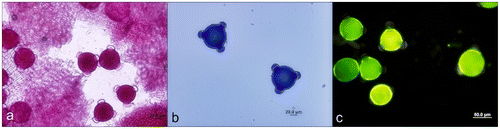
The mature pollen grains were usually triporate but some of them were tetraporate surrounded by exine and intine; they are three-celled: one large vegetative cell and a central generative cell with two nuclei during shedding.
Pollen germination
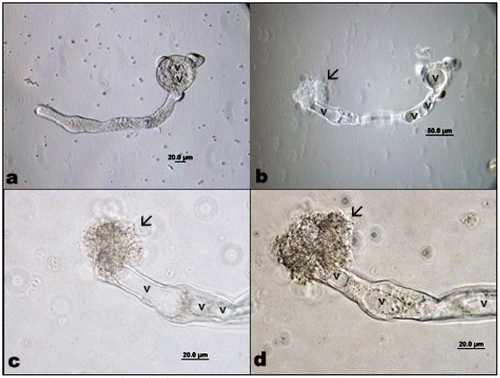
During germination, a defined area in the pollen plasma membrane – the tip growth domain to which post-Golgi vesicles are targeted and fused, promoting directional growth – is established, and the pollen tube elongation begins, often reaching astounding rates of growth. Directional growth of the pollen tube through the pistil toward the ovary is critical for egg fertilization. We observed that pollen germination in A. orientalis started in the first hour and the length and germination percentages of pollen tubes increased parallel to increasing time, as shown in Table .
Table 1. Pollen tube lengths and pollen germination percentages in A. orientalis in hours 1–6 and 12.
After the 12th hour some of the pollen tube tips got weaker and adjacent parts were swollen somewhat; growth had stopped but cyclosis continued, until after several minutes the pollen tube exploded when turgor pressure overwhelmed the weakened wall (Figure –d). Pollen germination was generally regular and monosiphonious but some polysiphonious pollen tubes were also observed (Figure ).
Discussion
Karyological information on A. orientalis is restricted only to chromosome counting. Chromosomal studies showed that the basic chromosome number was 11 (2n = 22) (Noori-Daloii et al. Citation1996). Based on our mitotic and meiotic studies, the same basic chromosome number of 11 (2n = 22) was also obtained. In spite of the small size of the cells and chromosomes, all the phases of mitosis and meiosis were observed. Generally the phases of mitosis were regular but there were a few abnormalities, such as laggard chromosome in metaphase and bridge formation in anaphase.
Many factors can affect pollen development. Kumar and Singhal (Citation2013) reported that the low temperature could cause synaptic irregularities in chromosomes of Saxifraga diversifolia during meiosis. Via do Pico and Dematteis (Citation2012) observed normal meiotic behavior for the diploid species Chrysolaena platensis (Spreng.); however, the polyploid taxa C. obovata, C. lithospermifolia (tetraploid) showed many meiotic irregularities such as multivalent formation, chromosomes outside plate, early segregation of chromosomes, laggards and bridges in anaphase and telophase. We did not observe any important irregularities in chromosomes of A. orientalis during meiosis except that we observed both metaphase and anaphase in one cell together during second meiotic division. There was a good relationship between the stages of pollen development and floral bud length. As a result of the cytokinesis isobilateral and tetrahedral types of tetrads occurred in microsporogenesis. The tetrad nuclei resulting from the simultaneous type of meiosis were found to be of equal size. Pollen germination and callose plug formations had started from the first hour, and pollen tube lengths regularly increased with increasing time. Although Akyalçın et al. (Citation2006) reported that the pollen grains of A. orientalis were triporate, we observed tetraporate pollen grains as well (Figure ). The P (polar axis) value was 47.5–52.5 μm (W) and the mean value was 50.36 μm. The E (equatorial diameter) value was 56–60 μm (W) and mean value was 59.18 μm (W). Pollen shape was suboblate and it was compressed in their polar areas (Akyalçın et al. Citation2006).
Microsporogenesis is the production of microspores from microspore mother cells in plants. The existing typological convention for microsporogenesis in angiosperms recognizes two patterns, i.e. simultaneous and successive, although intermediate patterns called modified simultaneous can also been seen (Sampson Citation1969). The successive division involves the formation of centrifugal cell plates, whereas simultaneous division involves the formation of centripetal furrows, although this may not be always the case (Furness and Rudall Citation1999). The developmental events of microsporogenesis and pollen formation are exquisitely timed and choreographed, occurring in a precise chronological order that correlates with the floral bud size. Early developmental events in microsporogenesis are known to play a role in pollen morphology and tetrad shape. Successive cytokinesis, particularly common in monocots (Rudall et al. Citation1997; Furness and Rudall Citation1999) generally leads to tetragonal, decussate, T-shaped or linear tetrads. Simultaneous cytokinesis, on the other hand, is mostly associated with tetrahedral tetrads. This type is found in monocots, but is the rule in eudicots (Rudall et al. Citation1997; Furness and Rudall Citation1999; Furness et al. Citation2002). The majority of genera in Apocynaceae follow the simultaneous pattern of microsporogenesis (Davis Citation1966; Johri et al. Citation1992). However, successive cytokinesis occurs in Apocynum androsaefolium, Holarrhena antidysentrica (Lattoo Citation1974), Plumeria diffusa and Plumeria rubra (Chauhan Citation1979). Godoy et al. (Citation2012) reported that after telophase II, meiocytes underwent simultaneous cytokinesis and formed tetrads of haploid microspores (n) during microsporogenesis of Hippocrateae volubilis. A. orientalis pollen tubes can grow about 45 μm per hour within 6 hour and extend a maximum of 1678 μm after the 12th hour. Pollen tube lengths similar to those recorded in the present study (Table ) were reported for several plant species when pollen was grown on artificial media, such as 1000–1800 mm for corn (Binelli et al. Citation1985), 450–1400 mm for peanuts (Kakani et al. Citation2002) and 20–60 mm for muskmelon (Maestro and Alvarez Citation1988).
Our findings about the mitosis and reproduction biology of this critically endangered plant will be useful for in vitro and in situ conservation studies. This study also provides support for taxonomic and evolutionary studies, for future programs on the genetic improvement of this species.
Acknowledgments
This study was supported by the research project “BAP 2009/50” and funded by Kocaeli University. We also thank Prof. Fazıl Özen and PhD student Arda Acemi for obtaining the materials from the tissue culture of A. orientalis.
References
- Akyalçın H, Özen F, Dulger B. 2006. Anatomy, morphology and palynology and antimicrobial activity of Amsonia orientalis Decne. (Apocynaceae) growing in Turkey. Int J Bot. 2(1):93–99.
- Anderson A. 1931. Studies on the embryology of the family Celastraceae. Oleaceae and Apocynaceae. Acta Universita Lund. 27:1–112.
- Baker M, Rebman J, Parfitt B, Pinkava D, Christy C, Salywon A, Puente-Martinez R. 2009. Chromosome numbers of miscellaneous angiosperm taxa. J Bot Res Inst TX. 3 (1): 279–283.
- Binelli G, De Manincor EV, Ottaviano E. 1985. Temperature effects on pollen germination and pollen tube growth in maize. Genetica Agraria. 39:269–281.
- Chauhan TS. 1979. Morphological studies in Apocynaceae. II. Embryology of Plumeria. J. Indian Bot Soc. 58:363–368.
- Dabine Lengyel E, Turiak G, Nyaradiné-Szabady J, Zambo I, Tétényi P, Hermecz I. 1986. Determination of secologanin content from shoots of Rhazya orientalis (Decne) using the HPLC method. Herba Hungarica. 25 (2): 141–150.
- Davis GL. 1966. Systematic embryology of angiosperms. New York: Wiley.
- Ekim T, Koyuncu M, Vural M, Duman H, Aytaç Z, Adıgüzel N. 2000. Red data book of Turkey (Türkiye Bitkileri Kırmızı Kitabı). 178. Ankara: Türkiye Tabiatını Koruma Derneği Yayını.
- Furness CA, Rudall PJ. 1999. Microsporogenesis in monocotyledons. Ann Bot. 84:475–499.
- Furness CA, Rudall PJ, Sampson FB. 2002. Evolution of microsporogenesis in angiosperms. Int J Plant Sci. 163:235–260.
- Fyre TC, Blodgett EB. 1905. A contribution to the life history of Apocynum androsamifolium. Bot Gaz. 40:49–53.
- Godoy SM, Alonso-Pereiraa AR, Romagnolob MB, Risso-Pascottoa C. 2012. Complex of pollen mother cell and polyad production during microsporogenesis in Hippocratea volubilis (Hippocrateaceae). Caryologia. 65 (4): 335–339.
- Guignard L. 1917. The egg in Apocynaceae and Asclepiadaceae. Mem Acad Sci Inst France. 55:1–34.
- Hauser EJP, Morrison JH. 1964. The cytochemical reduction of nitroblue tetrazolium as an index of pollen viability. American Journal of Botany. 51 : 748–752.
- Imani J, Li L, Schafer P, Kogel KH. 2011. STARTS – A stable root transformation system for rapid functional analyses of proteins of the monocot model plant barley. The Plant Journal. 67 : 726–735.
- Johri BM, Ambegaokar KB, Srıvastava PS. 1992. Comparative embryology of angiosperms. Berlin: Springer-Verlag.
- Kakani VG, Prasad PVV, Craufurd PQ, Wheeler TR. 2002. Response of in vitro pollen germination and pollen tube growth of groundnut (Arachis hypogaea L.) genotypes to temperature. Plant Cell Environ. 25:1651–1661.
- Kumar P, Singhal VK. 2013. Reduction in chiasma frequency and pollen fertility due to multiple chromosomal associations and univalents in Saxifraga diversifolia from alpine regions of northwest. Caryologia. 66 (2): 120–127.
- Lattoo CS. 1974. Morphology and embryology of Holarrhena antidysenterica Wall. Bot Gaz. 135:173–180.
- Maheswari DH. 1971. Embryology of Apocynaceae. I. Plumiereae. J. Indian Bot. Soci. 50 : 74–85.
- Maestro MC, Alvarez J. 1988. The effects of temperature on pollination and pollen tube growth in muskmelon Cucumis melo L. Scient Hortic. 36:173–181.
- Meyer S. 1938. Studies in the family Apocynaceae. J Depart. Sci Calcutta Univ. 1:131–158.
- Noori-Daloii MR, Aliyari R, Ebrahimzahed H. 1996. Study of chromosomes and soluble proteins of Rhazya stricta Decasine. and Nerium oleander L. Science. Int J. Iran. 7 (4): 209–216.
- Özen F. 2006. Türkiye’de tükenme tehlikesinde olan bir türün otekolojisi: Amsonia orientalis Decne. (Apocynaceae). BAÜ Fen Bilimleri Enstitüsü Dergisi. 8:1.
- Rahman AU, Qureshi MM, Zaman K, Malik S, Ali SS. 1989. The alkaloids of Rhazya stricta and R. orientalis. Fitoterapia. 60 (4): 291–322.
- Rahman AU, Zaman K. 1988. Studies on the alkaloids of Rhazya stricta. Phytochemistry. 27 (6): 1926–1928.
- Rau MA. 1940. Studies in Apocynaceae. J Indian Bot Soc. 19:33–44.
- Rudall PJ, Furness CA, Chase MW, Fay MF. 1997. Microsporogenesis and pollen sulcus type in Asparagales (Lilianae). Can J Bot. 75:408–430.
- Sampson FB. 1969. Cytokinesis in pollen mother cells of angiosperms with emphasis on Laurelia novae-zelandiae (Monimiaceae). Cytologia. 34:627–634.
- Schnarf K. 1931. Vergleichende embryologie der angiospermen. Berlin: Borntraeger.
- Via do Pico GM, Dematteis M. 2012. Chromosome number, meiotic behavior and pollen fertility of six species of Chrysolaena (Vernonieae, Asteraceae). Caryologia. 65 (3): 176–181.