Abstract
Polyploidy is a common phenomenon that has played a significant role in the evolutionary history of angiosperms. The allopolyploid Gossypium species (AD-genome) is the result of hybridization between two diploid species (A-genome and D-genome). In order to further explore the possible A- and D-genome donor of extant tetraploid cotton species, two molecular markers (random amplified polymorphic DNA [RPAD] and simple sequence repeat [SSR]) were employed on 13 cotton species including nine diploid (two A-genome and seven D-genome) and four tetraploid cotton species (AD-genome). Genomic in situ hybridization (GISH) was also used to further confirm the genomic origin and organization of tetraploid cotton species. The result showed that 26 of 40 RAPD primers and 49 of 120 SSR primers were polymorphic by 65% and 40.8% respectively. Clustering analyses for RAPD and SSR results indicated that the genome of G. raimondii is the nearest to the D-subgenome of tetraploid species, and it may be the ancestral D-genome donor of the tetraploid cotton. Because of the greatest genetic similarity coefficient between G. herbaceum and G. arboreum, two A-genome diploid species are phylogenetically equidistant from extant tetraploid cotton. GISH using one parental gDNA from A- or D-genome as a labeled probe together with an excess of another as blocking DNA, and tetraploid cotton as target, was found to a superior method for clearly distinguishing between two putative parental genomes. The phylogenetic and GISH results in this paper supported the hypothesis that the donors of the parental genomes are sisters of G. arboreum or G. herbaceum and G. raimondii and provided direct evidence for the origin and monophyly of the polyploidy Gossypium species.
Introduction
Polyploidy is a common and often recurrent phenomenon that has played a significant role in the evolutionary history of angiosperms (Soltis et al. Citation2009). Wood et al. (Citation2009) also reported that 15% of angiosperm and 31% of fern speciation events are accompanied by ploidy increase. Polyploidy is also a natural hybridization process. The role of hybridization in evolution has been debated for over a century and recent molecular genetic studies indicate that hybridization is surprisingly frequent in natural populations (Rieseberg Citation2008). The reunion of genomes through hybridization and allopolyploidy is conservatively estimated to account for 2–4% of speciation events in flowering plants and 7% in ferns (Otto and Whitton Citation2000).
The genus Gossypium has a long history of taxonomic and evolutionary study. It includes 51 perennial species distributed globally, of which 46 are diploid (2n = 2x = 26) and the other five are allopolyploids (AD-genome, 2n = 4x = 52) (Fryxell Citation1992). Gossypium allopolyploids are the result of hybridization between two diploid species (A-genome and D-genome), possibly in the mid-Pleistocene (Wendel Citation1989; Seelanan et al. Citation1997; Cronn et al. Citation2002). Since their parental genome species were thought to exist in diploid form and in different hemispheres, it became a hot research topic to explore its true progenitor for tetraploid cotton species. For a long time Gossypium tetraploids have been the best material for exploring the evolution of duplicated genes and duplicated genomes (Cronn et al. Citation1999; Liu et al. Citation2001). Over the decades, various kinds of tools have been used (Wendel and Cronn Citation2003), collectively demonstrating that the best extant models of the ancestral genome donors are G. arboreum or G. herbaceum (A-genome) and G. raimondii (D-genome). These two genome groups diverged from each other early in the evolution of the genus, perhaps 7 to 11 million years ago (Seelanan et al. Citation1997; Cronn et al. Citation2002).
Against this background, it is worthwhile to comprehensively examine the genus of Gossypium for its origins. In order to address the above question, we carried out a two-pronged study utilizing both molecular markers (random amplified polymorphic DNA [RPAD] and simple sequence repeat [SSR]) and genomic in situ hybridization technique (GISH) to elucidate the possible A- and D-genome donor of extant tetraploid cotton species and to further test the hypothesis of a monophyletic origin of polyploid cotton species.
Materials and methods
Plant materials
Thirteen cotton accessions including nine diploid (two A-genome and seven D-genome) and four tetraploid cotton species (AD-genome) were evaluated in the present study. The details of all accessions are presented in Table . The plants were planted in a greenhouse on the farm of Zhejiang University.
Table 1. The details of cotton accessions used in the experiment.
DNA extraction
Total genomic DNA was extracted from fresh young leaves of each accession using the Cetyltrimethyl Ammoniumbromide (CTAB) method (Paterson et al. Citation1993). DNA quality was evaluated by electrophoreses in 0.8% (w/v) agarose gel. Its concentration was estimated at 260 nm and quantified by means of comparison with DNA ladder of DL2000 (TaKaRa) ranging from 100 to 2000 bp.
PCR amplification and RAPD analysis
RAPD primers were provided by Shanghai Biotechnology (Shanghai, China). DNA amplification was carried out in a volume of 20 μl containing 2 μl template DNA (50 ng μl−1), 2 μl RAPD primer (10 μmol l−1), 0.5 μl Taq polymerase (2 U μl−1), Sangon, Shanghai, China), 0.5 μl dNTPs (10 mM), 2 μl 10 × PCR buffer (including Mg2+), and 13 μl ddH2O.
The following PCR profile was used in a DNA mastercycler (Eppendorf, Hamburg, Germany). Initial denaturation at 94°C for 2 minutes followed by denaturation at 94°C for 30 s, annealing at 38°C for 40 s and extension at 72°C for 1 min, then held at 4°C after 40 cycles.
PCR amplification and SSR analysis
The SSR primer sequences were obtained from the Cotton DB database (http://algodon.tamu.edu/htdocs 2cotton/cot2tondb.Html). They were synthesized by Shanghai Biotechnology.
DNA amplification was carried out in a volume of 20 μl containing 2 μl 10 × buffer, 1.6 μl MgCl2 (25 mM), 0.2 μl dNTPs (10 mM), 6 μl template DNA (50 ng μl−1), 2 μl primers (2.5 μM) (1 μl of forward and reverse primer each), 0.2 μl Taq polymerase (5 U μl−1, Sangon), and 8 μl ddH2O.
The PCR profile of SSR was initial denaturation at 94°C for 3 min followed by 35 cycles of denaturation at 94°C for 50 s, annealing at 58°C for 50 s, extension at 72°C for 2 min, followed by a final extension at 72°C for 10 min, and then held at 4°C.
In all cases, PCR reactions were performed at least twice in order to ensure that absence was a real one and not a failed reaction.
Electrophoresis
The PCR products were analyzed directly on 2.0% agarose gels in TBE buffer at 5 V cm−1. A DNA ladder, labeled with DL2000 (TaKaRa), ranging from 100 to 2000 bp, was used to determine the size of the RAPD and SSR fragments.
Data statistics and cluster analysis
The RAPD and SSR reproducible fragments were classified as present (1) or absent (0), and were typed into a computer file as a binary matrix, one for each molecular marker. The similarity coefficient was used to construct a dendrogram by the Unweighted Pair Group Method with Arithmetic Averages (UPGMA) according to Rohlf (Citation1993). Clustering analyses were performed using NTSYS-pc (version 2.02h) to calculate the genetic similarity matrices (Applied Biostatistical Inc, New York).
Chromosome preparations
Chromosome spreads were made using the modified enzyme digestion method of Li and Zhang (Citation1998). After observation under a phase contrast microscope, good samples were selected for GISH analysis.
Genomic DNA isolation and labeling
Total genomic DNA from the putative parental species was extracted from fresh young leaves by the CTAB method as described by Paterson et al. (Citation1993), and the DNA was further purified by RNase A. When used as a probe, genomic DNA was boiled to a length of 1–12 kb before labeling. Blocking DNA was autoclaved to 100–300 bp fragments. Total genomic DNA probes were labeled with digoxigenin-11-dUTP using a Dig-Nick Translation Mix (Roche, Mannheim, Germany) following the manufacturer’s protocol.
Genomic in situ hybridization
Pretreatment, denaturation, hybridization and detection followed the Wang et al. (Citation1999) protocol. The slides were examined under a Leica DM IRB fluorescence microscope (Leica Microsystems, Wetzlar, Germany). Chromosome images were obtained by a Leica DFC 300 FX camera and analyzed with the Leica-QFISH software package.
Results
Genetic diversity among the cotton germplasms
In total 26 out of 40 RAPD primers were polymorphic by 65% with 254 polymorphic bands ranging between 250 to 1600 bp. SSR primers (120 pairs) were used for amplification of which 49 pairs were polymorphic by 40.8%, which produced a total of 99 distinct alleles with an average of 2.0 alleles per primer pair. A total of 587 major distinct SSR bands were observed. These reproducible polymorphic DNA fragments ranged from 50 to 500 bp, which reflects a large difference in the number of repeats between the different alleles.
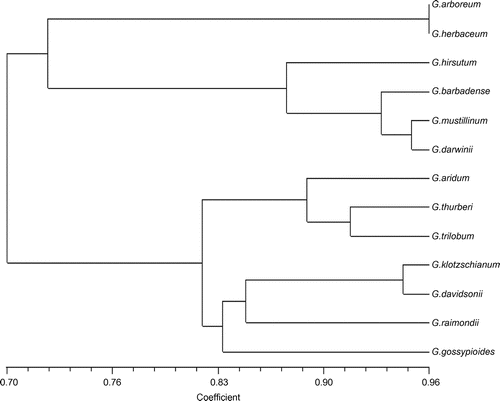
The variance of genetic similarity coefficients by SSR was between 0.66 and 0.97, and between 0.73 and 0.96 by RAPD. Due to their respective advantages, the combination analysis of SSR and RAPD markers proved to be more credible than sole marker analysis. Using SSR and RAPD polymorphisms, a dendrogram (Figure ) of 13 accessions was constructed with an unweighted paired group method using arithmetic averages (UPGMA) clustering algorithm. The dendrogram separated the 13 accessions into two main groups at a similarity coefficient value of 0.70. The first group consisted of A-genome and tetraploid cotton species. We can conclude that the relationship of tetraploid cotton species with A-genome species is nearer than D-genome species. In this group, G. arboreum and G. herbaceum, which have A-genomes, had the greatest similarity (0.962) and differed from tetraploid cotton species. In the tetraploid subgroup, G. hirsutum was the first one to be seperated from the others and G. barbadense was the second one. In addition, the relationship between G. mustillinum and G. darwinii is the closest comparing with other tetraploid. The second group consisted of D-genome cotton species. Within this group, G. aridum, G. thurberi and G. trilobum clustered together, while G. klotzschianum, G. davidsonii, G. raimondii and G. gossypioides were clustered into another group, where G. klotzschianum and G. davidsonii had higher similarity (0.945).
Relationship between diploid species and tetraploid species
On the basis of obtained RAPD and SSR binary data, a matrix of similarity coefficients between A- and D-genome species and tetraploid cotton species was calculated, and the results are shown in Table .
Table 2. The similarity coefficient result between tetraploid species and A- and D-genome species by RAPD and SSR markers.
According to the genetic similarity coefficients between tetraploid and A- and D-genome species, G. arboreum and G. herbaceum had higher similarity, but there was no significant difference in the genetic similarity between G. arboreum and G. herbaceum when compared with four tetraploid species. So G. arboreum and G. herbaceum are the possible closest living relatives of the ancestral A-genome donor and the position of them is equal on the course of formation of tetraploids. These two A-genome diploid species are phylogenetically equidistant from extant tetraploid cotton species. Among D-genome species, the genetic similarity coefficient of G. raimondii with four tetraploid species was the highest, so G. raimondii is the possible sole D-genome donor of tetraploid cotton species. The monophyletic origin of polyploid Gossypium species was testified by the present phylogenetic results.
Identification of the parental genomes of tetraploid cotton by GISH
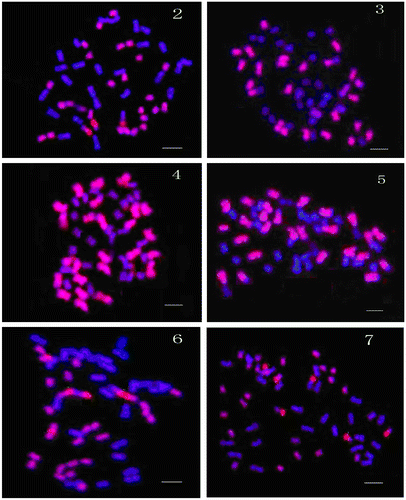
When the putative parental A-genome (G. arboreum or G. herbaceum) of tetraploid cotton was used as a probe, D-genome (G. raimondii) as a block, and AD-genome (G. hirsutum and G. barbadense) as a target, GISH images clearly discriminated two sets of chromosomes: 26 longer chromosomes from the A-genome revealed clear uniform red fluorescent signals; 26 shorter chromosomes with blue DAPI fluorescence were from the D-genome (Figures , , ). In another experiment with the same conditions, the D-genome (G. raimondii) was used as a probe, A-genome (G. arboreum or G. herbaceum) as a block, and AD-genome (G. hirsutum and G. barbadense) as a target, GISH images also clearly discriminated two sets of chromosomes. 26 shorter chromosomes coming from the D-genome revealed clear red fluorescent signals; 26 longer chromosomes with blue DAPI fluorescence were from the A-genome (Figures , , ). Some chromosomes showed both red and blue fluorescence signals at the chromosome arms and these probably represent reciprocal translocations between the parental genomes in the evolution process. Cronn et al. (Citation2002) also noticed acceleration of mutations compared to diploid cotton and Senchina et al. (Citation2003) found that polyploidy in cotton has been accompanied by a modest mutation-rate enhancement. We also used other D-genome species such as G. klotzschianum and G. davidsonii as a probe, but obtained fewer than 26 weak signals than those obtained by G. raimondii, and the present work supports the hypothesis that the donors of the parental genomes are G. arboreum or G. herbaceum and G. raimondii.
Figure 2-7 (Color online) GISH photographs of tetraploid cotton species (G. hirsutum and G. barbadense) with some diploid cotton species (G. arboreum, G. herbaceum and G. raimondii). (2) Target chromosomes of G. hirsutum hybridized with G. herbaceum as a probe and G. raimondii as a block (red fluorescent signals indicated those hybridized with probe and blue ones were the block, the same in other photographs). (3) Target chromosomes of G. hirsutum hybridized with G. arboreum as a probe and G. raimondii as a block. (4) Target chromosomes of G. barbadense hybridized with G. arboreum as a probe and G. raimondii as a block. (5) Target chromosomes of G. barbadense hybridized with G. raimondii as a probe and G. herbaceum as a block. (6) Target chromosomes of G. hirsutum hybridized with G. raimondii as a probe and G. herbaceum as a block. (7) Target chromosomes of G. hirsutum hybridized with G. raimondii as a probe and G. arboreum as a block. Bars in all figures = 5 μm.
Discussion
The discovery of PCR led to the development of the RAPD technique that involves the use of a single arbitrary primer in a PCR reaction and results in the amplification of several discrete DNA products (Devos and Gale Citation1992; Büscher et al. Citation1993). The earliest studies using RAPD in cotton were to differentiate between G. hirsutum varieties and to assess the introgression of wide hybridization from wild diploid species (Mergeai et al. Citation1998). Simple sequence repeats (SSRs) are abundant throughout the eukaryotic genome (Tautz and Renz 1989; Kijas et al. Citation1995) and numerous novel SSRs or microsatellite markers have been developed in Gossypium (Connell et al. Citation1998; Reddy et al. Citation2001; Han et al. Citation2004). The availability and abundance of microsatellite markers throughout the cotton genome make them particularly useful in genetic diversity studies (Reddy et al. Citation2001). In similar experiments carried out on other plant species, the superiority of the SSR marker system in comparison with the RAPD marker system is usually observed (Pejic et al. Citation1998; Rajora and Rahman Citation2003).
A two-technology approach combining RAPD and SSR data was earlier found to be more advantageous for elucidating genetic relationships among tetraploid alfalfa populations (Mengoni et al. Citation2000). The results of our work combining RAPD and SSR data not only confirm the previously phylogenetic analysis of cotton species (Wu et al. Citation2007; Wu et al. Citation2007), but also provide new insights into the phylogeny of the Gossypium genus.
Guan et al. (Citation2008) found that GISH was a relatively precise way of differentiating genome constitution in the recipient progenies at chromosome level. In this research, GISH was also found to be a powerful molecular cytogenetic method for determining the genome origin and organization of tetraploid cotton, if one parental gDNA from A- or D-genome is used as a labeled probe, together with an excess of another as a blocking DNA, and tetraploid cotton as target. There are no evident signal differences when using G. herbaceum and G. arboreum as probe, and G. hirsutum and G. barbadense as target. This showed that both G. arboreum and G. herbaceum are A-genome donors and the position of them is equal on the course of formation of tetraploids. Among D-genome species, only G. raimondii had clear strong fluorescent signals of 26 chromosomes while other D-genome species had fewer than 26 weak signals, so G. raimondii is the sole D-genome donor of tetraploid cotton species.
For several years allopolyploid Gossypium have been the subject of evolutionary investigations into the genomic mysteries of polyploidy. Despite many intensive studies of the tetraploid species of Gossypium, the phylogenetic relationships among these species have remained elusive. Earlier researchers suggested that the New World tetraploid species arose some 1–2 million years ago through the hybridization of an Old World taxon of the A-genome cytogenetic group, related to the present day species G. herbaceum and G. arboreum (2n = 2x = 26), with a taxon of the D-genome group, related to the New World species G. raimondii and G. gossipioides (2n = 2x = 26) (Wendel et al. Citation1992). AD-genome tetraploids combined an A-genome donated by the maternal diploid parent and a D-genome from the pollen parent (Galau and Wilkins Citation1989; Wendel Citation1989; Wendel and Cronn Citation2003). A recent study (Grover et al. Citation2012) demonstrated that the D-genome species G. raimondii and an A-genome species much like modern G. arboreum and G. herbaceum were involved in the creation of the polyploid species, and that this single combination gave rise to the polyploids. The present results also provide direct evidence for the monophyletic origin of polyploid Gossypium species.
Polyploid speciation, in which the entire genome is duplicated, is particularly frequent in plants, perhaps because polyploid plants often exhibit ecological differentiation, local dispersal, high fecundity, a perennial life history, and self-fertilization or asexual reproduction (Rieseberg and Willis Citation2007). Allopolyploidy has proved to be a powerful evolutionary factor that has played a decisive role in the evolution of most higher plants and accelerates genome evolution in two ways: revolutionary changes and evolutionary changes (Feldman and Levy Citation2009). Cai et al. (Citation2013) also confirmed polyploidy as the most significant evolutionary trend in chromosome number within Plukenetia species, a promising oilseed woody crop. Hybridization is frequent in many organismal groups. The origin of a new diploid species by means of hybridization requires the successful merger of differentiated parental species’ genomes. Rieseberg et al. (Citation2003) reported that hybridization facilitated ecological divergence in sunflowers. The genomic composition of the synthesized and ancient hybrids indicated that selection to a large extent governs hybrid species formation (Rieseberg et al. Citation1996). According to our study and others, the ancient tetraploid cotton species were formed possibly by hybridizing and chromosome doubling between archaic species related to G. arboreum or G. herbaceum and G. raimondii, then different tetraploid species might appear by further geographical isolation and genetic differentiation giving rise to five distant allotetraploid species, namely G. hirsutum, G. barbadense, G. mustelinum, G. darwinii, and G. tomentosum. In the present time, artificial tetraploids were synthesized by hybridizing A (G. arboretum, G. herbaceum) and D (G. raimondii) possible genomic diploid donor species and then doubling the chromosomes of F1 (AD) to simulate natural ancient tetraploid formation process. To study this process, the genomic composition of experimentally synthesized hybrid would be compared with that of an ancient hybrid species. We will focus on genomic evolution and interaction following plant speciation to further explore evolutionary mechanisms.
Acknowledgments
This work is supported by National Key Basis Research and Development Project (973 project) and National Natural Science Foundation of China (No. 31171599). The authors thank Associate Prof. Zhang Xian-yin for technical assistance that made this research possible, and for valuable suggestions on the manuscript.
References
- Büscher N, Zyprian E, Blaich R. 1993. Identification of grapevine cultivars by DNA analyses: Pitfalls of random amplified polymorphic DNA techniques using 10-mer primers. Vitis. 32:187–188.
- Cai ZQ, Zhang T, Jian HY. 2013. Chromosome number variation in a promising oilseed woody crop, Plukenetia volubilis L. (Euphorbiaceae). Caryologia. 66(1):54–58.
- Connell JP, Pammi S, Iqbal MJ, Huizinga T, Reddy AS. 1998. A high through-put procedure for capturing microsatellites from complex plant genomes. Plant Mol Biol Rep. 16(4):341–349.
- Cronn R, Small RL, Haselkorn T, Wendel JF. 2002. Rapid diversification of the cotton genus (Gossypium: Malvaceae) revealed by analysis of sixteen nuclear and chloroplast genes. Am J Bot. 89(4):707–725.
- Cronn R, Small RL, Wendel JF. 1999. Duplicated genes evolve independently after polyploid formation in cotton. Proc Natl Acad Sci USA. 96(25):14406–14411.
- Devos KM, Gale MD. 1992. The use of random amplified polymorphic DNA markers in wheat. Theor Appl Genet. 84(5–6):567–572.
- Feldman M, Levy AA. 2009. Genome evolution in allopolyploid wheat – a revolutionary reprogramming followed by gradual changes. J Genet Genomics. 36(9):511–518.
- Fryxell PA. 1992. A revised taxonomic interpretation of Gossypium L. (Malvaceae). Rheedea. 2:108–165.
- Galau GA, Wilkins TA. 1989. Alloplasmic male sterility in AD allotetraploid Gossypium hirsutum upon replacement of its resident A cytoplasm with that of the D species G. harknessii. Theor Appl Genet. 78(1):23–30.
- Grover CE, Grupp KK, Wanzek RJ, Wendel JF. 2012. Assessing the monophyly of polyploid Gossypium species. Plant Syst Evol. 298(6):1177–1183.
- Guan B, Wang K, Zhou BL, Guo WZ, Zhang TZ. 2008. Establishment of a multi-color genomic in situ hybridization technique to simultaneously discriminate the three interspecific hybrid genomes in Gossypium. J Integr Plant Biol. 50(3):345–351.
- Han ZG, Guo WZ, Song XL, Zhang TZ. 2004. Genetic mapping of EST-derived microsatellites from the diploid Gossypium arboreum in allotetraploid cotton. Mol Genet Genomics. 272(3):308–327.
- Kijas JM, Fowler JC, Thomas MR. 1995. An evaluation of sequence tagged microsatellite site markers for genetic analysis within Citrus and related species. Genome. 38(2):349–355.
- Li MX, Zhang ZP. 1998. Crop chromosomes and research technology. Beijing: Chinese Agricultural Press, 190–203.
- Liu B, Brubaker CL, Mergeai G, Cronn RC, Wendel JF. 2001. Polyploid formation in cotton is not accompanied by rapid genomic changes. Genome. 44(3):321–329.
- Mengoni A, Gori A, Bazzicalupo M. 2000. Use of RAPD and microsatellite (SSR) variation to assess genetic relationships among populations of tetraploid alfalfa. Medicago sativa. Plant Breed. 119(4):311–317.
- Mergeai G, Bi IVIV, Baudoin JP, DuJardin P. 1998. Use of random amplified polymorphic DNA (RAPD) markers to assist wide hybridization in cotton. In: Cotton, Bajaj YPS, editor. Berlin: 42:121–139.
- Otto SP, Whitton J. 2000. Polyploid incidence and evolution. Annu Rev Genet. 34(1):401–437.
- Paterson AH, Brubaker CL, Wendel JF. 1993. A rapid method for extraction of cotton (Gossypium spp.) genomic DNA suitable for RFLP or PCR analysis. Plant Mol Biol Rep. 11(2):122–127.
- Pejic I, Ajmone-Marsan P, Morgante M, Kozumplick V, Castiglioni P, Taramino G, Motto M. 1998. Comparative analysis of genetic similarity among maize inbred lines detected by RFLPs, RAPDs, SSRs, and AFLPs. Theor Appl Genet. 97(8):1248–1255.
- Rajora OP, Rahman MH. 2003. Microsatellite DNA and RAPD fingerprinting, identification and genetic relationships of hybrid poplar (Populus canadiensis) cultivars. Theor Appl Genet. 106(3):470–477.
- Reddy OU, Pepper AE, Abdurakhmonov I, Saha S, Enkins JN, Brooks T, Bolek Y, El-zik KM. 2001. New dinucleotide and trinucleotide microsatellite marker resources for cotton genome research. Crop Sci. 5(2):103–113.
- Rieseberg LH. 2008. Evolution: Replacing genes and traits through hybridization. Curr Biol. 19(3):119–122.
- Rieseberg LH, Raymond O, Rosenthal DM, Lai Z, Livingstone K, Nakazato T, Durphy JL, Schwarzbach AE, Donovan LA, Lexer C. 2003. Major ecological transitions in wild sunflowers facilitated by hybridization. Science. 301(29):1211–1216.
- Rieseberg LH, Sinervo B, Linder CR, Ungerer MC, Arias DM. 1996. Role of gene interactions in hybrid speciation: Evidence from ancient and experimental hybrids. Science. 272(3):741–745.
- Rieseberg LH, Willis JH. 2007. Plant speciation. Science. 317(17):910–914.
- Rohlf FJ, 1993. NTSYS-pc, numerical taxonomy and multivariate analysis system. Version 2.02h, Applied Biostatistical Inc, New York.
- Seelanan T, Schnabel A, Wendel JF. 1997. Congruence and consensus in the cotton tribe (Malvaceae). Syst Bot. 22(2):259–290.
- Senchina DS, Richard IA, Cronn RC, Liu B, Rong JK, Noyes DR, Paterson AH, Wing RA, Wilkins TA, Wendel JF. 2003. Rate variation among nuclear genes and the age of polyploidy in Gossypium. Mol Biol Evol. 20(4):633–643.
- Soltis DE, Albert AV, Leebens-Mack J, Bell CD, Paterson AH, Zheng CF, Sankoff D, dePamphilis CW, Wall PK, Soltis PS. 2009. Polyploidy and angiosperm diversification. Am J Bot. 96(1):336–348.
- Tautz D, Renz M. 1984. Simple sequences are ubiquitous repetitive components of eukaryotic genomes. Nucleic Acids Res. 12(10):4127–4138.
- Wang CY, Wang KB, Wang WK, Li MX, Song GL, Cui RX, Li SH, Zhang XD. 1999. Protocol of cotton FISH of somatic chromosomes with gDNA as probes. Acta Gossypii Sinica. 11(2):79–83.
- Wendel JF. 1989. New world tetraploid cottons contain old world cytoplasm. Proc Natl Acad Sci USA. 86(11):4132–4136.
- Wendel JF, Brubaker CL, Percival AE. 1992. Genetic diversity in Gossypium hirsutum and the origin of upland cotton. Am J Bot. 79(11):1291–1310.
- Wendel JF, Cronn RC. 2003. Polyploidy and the evolutionary history of cotton. Adv Agron. 78(1):139–186.
- Wood TE, Takebayashi N, Barker MS, Mayrosee I, Greenspoond PB, Rieseberg LH. 2009. The frequency of polyploid speciation in vascular plants. Proc Natl Acad Sci USA. 106(33):13875–13879.
- Wu YX, Daud MK, Zhu SJ. 2007. Phylogenetic diversity and relationship among Gossypium germplasm using SSRs markers. Plant Syst Evol. 268(1–4):199–208.
- Wu YX, Sun YQ, Chen CQ, Zhu SJ. 2007. Analysis of genetic relationship among cotton species (Gossypium spp.) by RAPD marker. Acta Agron Sin. 33(6):909–913.