Abstract
The onion plant (Allium cepa L.) is a suitable indicator plant for the determination of potential genotoxic agents in the samples taken from the environment. The genotoxic level of the agent under study is reflected by structural changes of the chromosomes and their changed numbers. The chromosomes under study are taken from the meristem cells of the young growing onion roots. A healthy normal onion cell has 16 (2n = 16) chromosomes. They are relatively large and so very appropriate for the detection of morphological changes. Prior to the chromosome study the root tip cells were immersed in a 0.1% aquatic solution of colchicine which stopped the mitotic cycle continuing beyond metaphase. The changes in morphology varied from a single distortion of a single chromosome up to several morphological changes observed on many chromosomes. We identified 15 categories of morphological aberrations which are classified into three groups: chromatid damage (CtD), centromere damage (CmD) and chromosome damage (CsD). CtD includes: single break chromatid, double break chromatid, isochromatid break, multiple break chromatid, gap chromatid, centric ring chromatid, acentric ring chromatid and triradial chromosomes. CmD includes: break centromere, gap centromere, single break centromere, double break centromere and multiple break centromere. CsD includes: ring chromosomes and dicentric chromosomes. Sometimes also the chromosome number changed which occurred as aneuploidy with monosomy 2n = 15 (2n =16 – 1) and euploidy – increased number of the basic chromosome number (2n = 6x – 8x). We identified also the translocation: t(3p–; 5p+).
Introduction
The use of chemicals that accumulate in the environment is increasing in Europe. These pollutants can enter spring and underground water used as drinking water. Although chemical analysis provides accurate measurements at the moment of sampling, as the repetition of sampling is often limited, we do not obtain a realistic impact on organisms over a longer period of time. Onion (Allium cepa L.) root meristem cells are very sensitive to genetic damage by chemicals, and the Allium test, involving the length of the root and chromosome aberration (CA) measurements, proved to be a suitable model system for measuring the environmental cytogenetic potential of pollutants (Al-Sabti Citation1989). The Allium test is very good indicator for analysing antiproliferative effects on plant medicinal extracts (Frescula et al. Citation2012).
Seven higher plant bioassays were reviewed by the US Environmental Protection Agency (EPA) Gene-Tox program in 1980. The Allium root tip CA assays were adopted by the International Program on Plant Bioassays (IPPB) for monitoring or testing environmental pollutants (Ma Citation1999; Ma et al. Citation2005). The metaphasic chromosome anomalies as detected in the Allium test procedure are not excluded to occur also in the human chromosomes when exposed to similar pollutants. There are many reports on the excellent correlation of the plant system with the mammalian system (Grant and Salamone Citation1994).
Monitoring chromosome morphology abnormalities in order to detect the degree of water toxicity is an old technique. In 1928 Stadler reported the effect of chemicals and physical agents on chromosome damage (Mulaszynska and Juchimiuk Citation2005). After the effect of colchicine on the mitosis of Allium had been discovered (Levan Citation1938) it was possible to determine the number of chromosomes and their morphology in A. cepa L. (Levan et al. Citation1964). The Allium test (Levan Citation1938, Citation1949) is based on the chromosome study of the meristem cells of the apical root cells of A. cepa L. in order to determine the influences of genotoxic substances in, for instance, benzene, naphalen, phenanthren and benzopyren. Moreover, the A. cepa system provides important information to evaluate the clastogenic and/or aneugenic effects of an agent on the genetic material (Leme and Marin-Morales Citation2009).
C-mitosis (without colchicine application) is stimulated by chemicals such as methyl mercury and nickel salts, and by many organic chemicals (Fiskesjö Citation1993). After such treatment a more detailed study of chromosome morphology is possible (Fiskesjö Citation1993). The diluted cytostatic chemical colchicine is used to stop the cell division process exactly in its metaphase. Thus in c-metaphase chromosome or chromatid break can be studied in detail (Fiskesjö Citation1993). So the colchicine pre-treatment enables the study of structural metaphase CAs. Metaphase chromosomes are chromosomes with the most condensed chromatin and can be therefore studied with an optical microscope. Allium cepa L. is an important bioindicator for environment poisons which influence mitosis (Fiskesjö Citation1985; Rank Citation2003; Bakare et al. Citation2012) and damage metaphase chromosomes (Al-Sabti and Kurelec Citation1985; Al-Sabti Citation1989; Kumar and Panneerselvam Citation2007; Ragunathan and Panneerselvam Citation2007; Panneerselvam et al. Citation2012).
The centromere is observed as a constriction in the chromosome and stains lighter than other parts of the chromosome. Its location on the chromosome is most important in determining the morphology of chromosomes, which are characterized as metacentric (m), submetacentric (sm), subtelocentric (st) or telocentric (t). The location of the centromere is described by the centromeric index using the rules of Levan et al. (Citation1964) and the Chicago Conference (Citation1966).
The complete sequence of stages from one cell division to the next is: G0, G1, S, G2 and M phase. The administration of genotoxic agents during the G0 and G1 phases of the cell cycle results in chromosome or chromatide damage prior to the mitosis. This induces the formation of the dicentric or ring chromosome; meanwhile administration in the S or G2 phase usually provokes the breakage of one or both chromatids.
Onion (Allium cepa L.) cells have eight pairs of relatively large chromosomes. This allows an easy detection of possible chromosome damage. In addition the chromosome morphology is very easily changed by chemicals. The Allium metaphase test, involving measurements of the length of the root and chromosome damage, proved to be a suitable model system for measuring the environmental cytogenotoxic potential of pollutants (Al-Sabti and Kurelec Citation1985). Therefore chromosome damage has become a relevant testing method. This biotest even introduces a new quality in the assessment study: chromosomes constitute the primary target for cytogenotoxically induced irreversibly damaged chromosome, damage or disturbance of cell division.
Onion is suitable for genotoxic studies because: (i) the root growth dynamics is very sensitive to pollutants; (ii) the mitotic phases are very clear in the onion; (iii) it has a stable chromosome number and stable karyotype; (iv) there is diversity in the chromosome morphology; (v) there is a clear and fast response to the genotoxic substances; and (vi) spontaneous chromosomal damages occur rarely. In this study we describe various chromosome and chromatid damages in the root meristem cells of onion, which represent biomarkers for different types of environmental pollution.
Material and methods
Onion preparation
Small onion (Allium cepa L.) bulbs of the same uniform size, weighing about 3–3.5 g, were denuded by removing the loose outer scales and scraped so that the root primordia were immersed into the different tested liquids (drinking quality water, river and lake water, industrial wastewater, rain water, snow melt water, river and lake sediments, soil, condensed water obtained from the atmosphere, and known chemicals).
Experimental procedure
The exposure time of the onion bulbs in each experiment was 72 hours at 22°C; they were protected against direct sunlight. In order to eliminate the influence of daylight rhythms the plants were exposed to contain artificial light of middle intensity. In an alternative version of our Allium metaphase test, the five onion bulbs were placed directly in the experimental water containers. The water sample under investigation was divided into three portions which were successively applied to the onion roots for 24-h periods. So each 24 h the roots obtained a fresh bath of the sample solution. After 72 hours the experiment was terminated and followed by macroscopic and microscopic tissue morphology investigation.
Chromosome preparations
The squash technique for onion root described by Al-Sabti and Kurelec (Citation1985) and Al-Sabti (Citation1989) was used for the preparation of chromosomes. Chromosome preparations were set up from root meristems containing actively growing cells by the following method: developing roots with bulbs were pre-treated with an 0.1% aquatic solution of colchicine for 3 hours at 21°C. After washing in distilled water for 20 min the terminal developing roots (2 mm) were fixed for 1 h in methanol:propionic acid mixture (3:1 or 1:1). Then they were macerated and stained in order to obtain a cellular suspension. This sample was stained with 0.5% aceto-carmine for 4–5 min at 60°C without hydrolysis, and squashed in aceto-carmine (Firbas and Al-Sabti Citation1995). The optical microscope used in the investigation was the Olympus–BX 41 (Olympus, Japan) with the PM 10 SP photo system. Typical magnifications used were 400× and 1000×.
Macroscopic parameters
Macroscopic parameters include the root length and other parameters such as usual root form, number, colour and turgidity after cultivation for 72 h.
Microscopic parameters
Therefore, additional colchicine treatment should only be used for the specific study of chromosome and chromatid (breaks) damage. and the study of of the chromosome shapes in their methaphasic state.
Level of genotoxicity
The level of genotoxicity is defined by the percentage of metaphase cells with damaged cells with damaged chromosomes related to all the cells inspected (typically 200).
Chromosome morphology, centromere position and karyotype
Chromosomes are categorized according to the position of the centromere. Arm ratio can be calculated by measuring the length of the short arm (p) and of the long arm (q) of the chromosome, and the centromeric index is as described by Levan et al. (Citation1964) and Chicago Conference (Citation1966). For numerical characterization of the karyotypes the parameters were calculated as in Genc et al. (Citation2013).
Control inhibition test
Toxicity and genotoxicity assays were performed with six concentrations of the test methyl methanesulphonate (MMS 4016, Sigma, USA) chemicals as control.
Results
Distribution genotoxicity (induction of chromosome damage in root cells) and general toxicity (root growth inhibition and malformation) in Allium cepa L. exposed to 100–0.001 ppm MMS are shown in Table and Figure A–F. Results for 10 mg l−1 MMS varied from 18.4% to 28.6% aberrant cells (Rank Citation2003). The normal metaphases and different types of chromosomal deviation are shown in Figures A, B, , A–U, , 6A, B, , A–D and Tables –. Samples were taken in rivers, springs, tarns, lowland lakes and tap water in Slovenia.
Table 1. Growth retardation toxicity and genotoxicity effects of MMS after 72 h of treatment at different concentrations.
Figure 1. Examples of series of onions cultivated for 72 h in different concentrations of MMS: (A) 100 ppm; (B) 0.001 ppm; (C) 10 ppm; (D) 0.01 ppm; (E) 1 ppm; (F) 0.1 ppm.
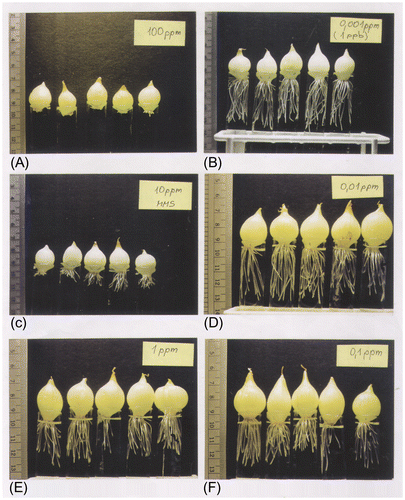
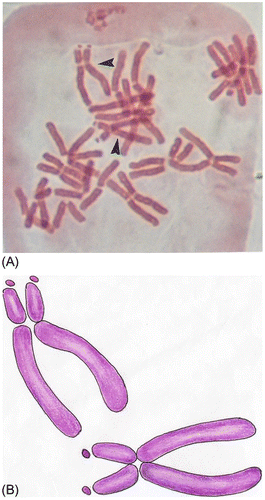
Figure 2. (A) Diploid metaphase chromosome; and (B) its karyotype from the root cells of onion (Allium cepa L.) containing 2n = 16 with 6 m, 8 sm and 2 st chromosomes (Figure b).
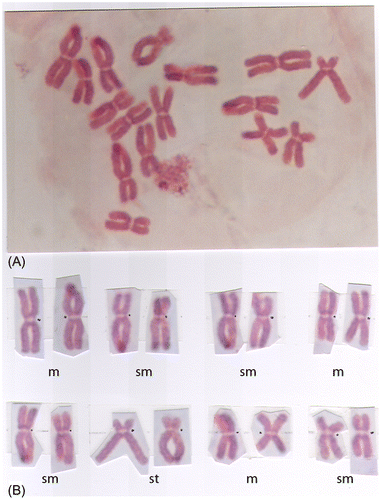
Figure 4. Chromatid and chromosome damage: (A, B) SBCt; (C) DBCt; (D) IctB; (E) MBCt; (F) CRCt; (G) ARCt; (H) TCs; (I) DCs; (J) RCs; (K) BCm; (L) GCe; (M) SBCe; (N) DBCe; (O) MBCe; (P) BCm+GCs; (R) BCm+SBCt+GCt; (S) BCm+ARCs+GCt; (T) BCm+IctB+BCt; (U) BCm+GCt+MBCt.
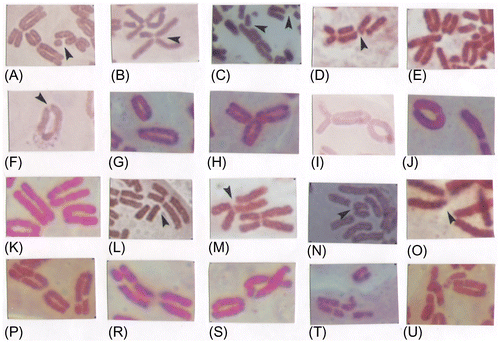
Figure 5. Monosomy (2n – 1) in the chromosome set of the onion (Allium cepa L.). Subtelocentric chromosome (arrow) is without homologue couple in chromosome set.
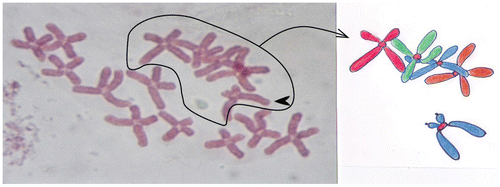
Table 2. Correlation between the genotoxicity level and the number of damaged chromosomes in chromosome set. Samples: environmental complex mixture as wastewater; river, spring, tarn and lowland lakes, to stain out to deposit positive, and drinking water in Slovenia; negative control = reverse osmosis tap water filtration; control= 10 ppm : methyl methanesulphonate (MMS).
1. Chromosome number and karyotype
Allium cepa L. has 16 monocentric chromosomes (2n =16) with basic number x = 8 (Figure A). Its karyotype contains six metacentric (m), eight submetacentric (sm) and two subtelocentric (st) chromosomes (Figure B). Two subtelocentric chromosomes contain satellites (Figure ), which are not always seen under a microscope. Telocentric chromosomes are not present.
2. Types of metaphase damage chromosome
The aberrations seen at metaphase are of three types: chromatid damage, chromosome damage, and chromatid type damage in the centromere region.
2.1. Chromatid damage
Single break chromatid (SBCt). SBCt can appear anywhere on the chromatid. The broken fragment can remain aligned with the chromatid (Figure A) or get displaced from it (Figure ).
Double break chromatid (DBCt)
DBCt involves two breaks, with the elimination of the chromosomal material between them (Figure C).
Isochromatid break (IctB)
IctB is the parallel breakage of the chromatid which is composed of two breaks in the same chromatid region. The broken fragments can also get displaced from the chromatid axis (Figure D).
Multiple break chromatid (MBCt)
In MBCt the broken fragments typically accumulate in a small group (Figure E).
Gap chromatid (GCt)
GCt is the result of the chromatid breakage and appears under the microscope as an unstained point in the chromatid. There can also appear several such unstained points. If gaps exist in both chromatids they are called isochromatid gaps (Figure K, P, T) and multiple gaps (Figure D, arrows).
Centric ring chromatid (CRCt)
CRCt is formed when the ends of the broken chromatid join and chromatid becomes circular in shape, containing the centromere (Figure F).
Acentric ring chromatid (ARCt)
ARCt appears when the displaced fragment of the chromatid becomes circular. It contains no centromere (Figure G).
Triradial chromosomes (TCs)
TCs are chromosomes with one centromere and three terminal chromatid ends (Figure H).
2.2. Chromosome damage
Dicentric chromosomes (DCs)
DCs appear with the simultaneous breakage of two chromosomes. These chromosomes join into the dicentric chromosome which contains two centromeres and an acentric fragment (Figure I).
Ring chromosomes (RCs)
RCs appear after the double breakage of the long and short chromosome part. The broken ends fuse, thus forming a cyclic chromosome and an acentric fragment (Figure J).
2.3. Centromere damage
Damages to the centromere belong in the chromatid damage class and are located near the centromere region of the chromosome. Here are some typical damage forms:
Break centromere (BCm)
BCm in the centromere region appears when the chromosome breaks into two parts. As this type of damage occurs often it can be inferred that the centromere part of the chromosome is the most sensitive to genotoxic substances. Such deformations can lead to the chromosome form designated the isochromosome (Figure K).
Gap centromere (GCm)
GCm represents a narrow chromatid breakage in the basal part of the chromatid. It appears as an unstained part of the chromosome (Figure L).
Single break centromere (SBCm)
SBCm is breakage of the basal part of the chromatid, by the displacement of the broken longer or shorter chromatid (Figure M).
Double break centromere (DBCm)
DBCm is the breakage and displacement of the basal parts of two chromatids (Figure N).
Multiple break centromere (MBCm)
MBCm arises when the chromosome breaks in the centromere region and then breaks into four chromatid fragments. MBCm occurs very rarely (Figure O).
3. Aberrations resulting in changes in the chromosome number
Polyploidy refers to a numerical change in a whole set of chromosomes. Organisms in which a particular chromosome is underrepresented (monosomy) or overrepresented are said to be aneuploidy. Monosomy in A. cepa is to represent without subtelocentric chromosome (2n = 15; 2n = 16 – 1) in the 6th pair (Figure ).
Euploidy (Polyploidy) A. cepa is to represent with six sets (6x) and eight sets (8x) to multiply (to increase) basic chromosome number. Hexaploid (6x) and Octaploid (8x) chromosomes are examples of euploidy (Figure ).
4. Chromosome reorganization/reconstruction
In a translocation, a segment from one chromosome is transferred to a nonhomologous chromosome or to a new site an the some chromosome. Translocation in onion A. cepa involves 3th and 5th chromosome. In a translocation, a segment arm (3p) is transferred to a 5th chromosome (Figure ).
Translocation in onion A. cepa is to transfer the chromosome arm (3p) from 3th chromosome on the 5th chromosome. Rearrangement novel karyotype is: 2n = 16; t: 3p–; 5p+ (Figure ).
5. The combined damage types on a particular chromosome
There are different types of chromatid damage in the centromere region and in chromosome chromatids. For example: BCm+GCt (Figure K), BCm+GCt (Figure P), BCm+SBCt+GCt (Figure R), BCm+ARCt+GCt (Figure S), BCm+DBCt+GCt (Figure T), and BCm+GCt+MBCt (Figure U).
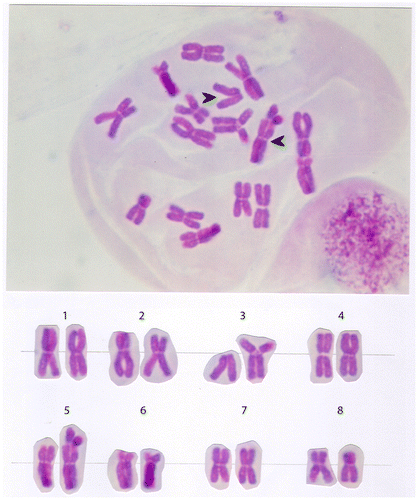
The number of damaged chromosomes increases with the level of the genotoxicity of the substance studied (Table , Figure A–D). The same holds for the number of deformations seen on a particular chromosome (Tables , ).
Figure 8. Different number chromosome damage in metaphase cells obtained from the meristem root-type cells of onion (Allium cepa L.): (A) one damaged chromosome; (B) four damaged chromosomes; (C) eight damaged chromosomes; (D) whole chromosome set is damaged.
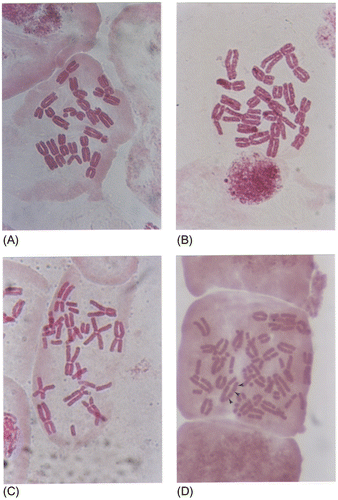
Table 3. Different subtypes of damage on a particular chromosome.
Table 4. The most frequent appearances of identical sub-types of chromosome damages on a single chromosome.
Discussion
The chromosome damage technique has become more and more important with increasing interest in the genotoxic effect of pollutants, and following recommendations to use onion chromosome as an indicator of mutagens in the environmental samples. CAs appear in two forms – the deviations of the metaphase chromosome number (numerical aberration) and the morphological deformations (structural aberration) of the chromosomes themselves.
We have shown several different types of chromosome and chromatid damage, resulting in numerous changes of chromosome morphology. We determined 15 types of structural chromosome damage, one translocation and two types of changed chromosome numbers. In the literature the Allium anaphase–telophase test has been most often performed without the application of the colchicine (Rank Citation2003). Genotoxic substances in the cells induce the aberration called c-mitosis, which is the result of damaged mitotic apparatus, and leads to metaphase chromosomes being freely distributed in the cell.
Meristem cells of Allium roots treated with the cytostatic colchicine show a much clearer picture of the metaphase chromosomes. The observations led us to the conclusion that there is a positive correlation between the level of genotoxicity of the substrate and the number of CAs – including one or several damages on a single chromosome up to damages on multiple chromosomes and even changed chromosome number. Our results also show that substrates with high genotoxic level induce a greater number of damaged chromosomes as well as more identical or different damages on the same chromosome. Low genotoxic levels induce less-damaged chromosomes as well as fewer damaged sites on a particular chromosome.
It has proved very convenient to apply colchicine which arrest dividing cells at metaphase (Al-Sabti and Kurelec Citation1985; Al-Sabti Citation1989; Kumar and Panneerselvam Citation2007; Ragunathan and Panneerselvam Citation2007; Palanikumar et al. Citation2011; Panneerselvam et al. Citation2012).
Rainfall and snowfall and petrochemical wastewater predominantly induced breaks, fragments, ring chromosomes and dicentric chromosomes (Al-Sabti Citation1989). Al-Sabti and Kurelec (Citation1985) took environmental samples from the river Sava and made a two phase analysis. In the first phase he was sampling just the water from the river. In the second phase he added to the river samples also the benzene and chloride. Both phases showed breaks, fragments, ring chromosomes and dicentric chromosomes, but these anomalies were more pronounced in the second phase. CA measurement in the Allium metaphase test is suitable for measuring the cytogenotoxic potential of chemicals present in waters. Cytogenetic effects of the food preservative potassium metabisulphite in Allium cepa root meristem cells induced CAs: breaks, gaps, multiple breaks and chromatid breaks (Kumar and Panneerselvam Citation2007). Sodium azide is a well-known potent mutagen, although it fails to induce CAs in root cell type such as break, gap, iso-chromatid break and exchange (Ragunathan and Panneerselvam Citation2007). CAs increase with increasing concentration of the test chemical and a longer period of treatment. Curcumin induces CA in A. cepa root tip cells in an insignificant manner when compared with an untreated control. Sodium azide alone induces CAs significantly with increasing concentrations. The total number of aberrations was significantly reduced in A. cepa L. root tip cells pretreated with curcumin. Curcumin has antimutagenic potential against sodium azide induced CAs in Allium cepa root meristem cells (Ragunathan and Panneerselvam Citation2007). Glycidol is used as a stabilizer in the manufacture of vinyl polymer-induced CAs in A. cepa L. root meristem cells. Different concentrations of glycidol induced cytological abnormalities such as breaks, gaps, exchange, multiple breaks and chromosome fragments (Panneerselvam et al. Citation2012). Increasing concentrations increased the number of CA.
To date, a variety of plant compounds have been studied extensively with regard to antimutagenic, anticarcinogenic, and antigenotoxic activity. Nevertheless, very few of them have been studied in terms of clastogenicity. The clastogenic activity of curcumin (the active component of turmeric) and aloin (the active component of aloe) had significant dose- and time-dependent clastogenic effects on the test plant system (Allium cepa L.). Cytological abnormalities, such as breaks, gaps, exchange, multiple breaks, and chromosome fragments, were scored. The frequency of CA increased as the concentration increased (Palanikumar et al. Citation2011).
Various types of structural chromosomal abnormalities can be cytologically divided into chromosome type and chromatid type. Chromosomal type damage are produced when breakage and reunion involves the whole chromosome. These lesions are produced in interphase (G1 and G0) before the DNA and chromosome material has been duplicated during the DNA synthesis period. This will lead to chromosome damage visible at the next mitosis. Chromatid type damage is produced by genotoxic chemical agents when cells are exposed in the S-phase or G2 states of interphase.
Very specific chromatid damage appears in the centromere region of the chromosome and on its chromatids. The centromere is a part of the chromosome. The most common damages are near to the centromere region or in the centromere region itself. This implies that the centromere region is most sensitive to genotoxic agents. Chromatid damages are therefore classified according to their location: damage in the centromere region and damage on the other chromosome parts. The scope of damage can include one to eight, sometimes even 12 chromosomes. All the chromosomes in the cell can even be damaged. The number of damaged chromosomes increases with the level of the genotoxicity of the substance studied, and the same holds for the number of deformations seen on a particular chromosome. Most agents shown to be carcinogenic and/or mutagenic to plants and animals have also been shown to be carcinogenic in man, so further investigations are necessary to identify contaminants of our ecosystem, and thus reveal more information on diseases such as cancer (Al-Sabti Citation1989).
Polyploidization refers to a numerical change in a whole set of chromosomes. It has already been shown that the influence of chemical or physical agents on the eggs immediately after fertilization could also be responsible for polyploidy induction in fish (Al-Sabti Citation1991).
Abnormal separation of sister chromatids caused by their failure to separate (move apart) properly during cell division. Cells with an extra chromosomes are referred to as trisomic (2n + 1), and cells which lack a chromosome as monosomic (2n – 1). Plant cytogenetics, using the Allium metaphase test, identifies the same chromosome damages as identified in human cytogenetics, including chromosome and chromatid damages, aneuplody, euploidy and translocation. These CAs cause clinical defects on the human body (Schauer Citation1981).
The cytogenetic effects of maleic hydrazine (MH) in a human lymphocyte culture result in chromatid gaps, chromatid breaks, isochromatid gaps and isochromatid breaks (Gokalp and Kaymak Citation2002). Genotoxicity studies of methyl methanesulphonate (MMS) induced in human lymphocyte culture chromosome damage: chromosome breaks, chromosome exchanges, chromatid breaks and chromatid exchanges (Slapšytė and Sukackaitė Citation2003). Equally induced, chemical chromosome damage in meristeme root cells of onion A. cepa.
The Allium metaphase test is also suitable for the detection of radiation, since radiation causes similar CAs as genotoxic substances (Al-Sabti Citation1989). So these two tests, for radioactive emissions and genotoxic substances, can be combined in a single testing session. A triradial three-arm chromosome configuration arises by the interaction of two chromosomes each having an isochromatide breakage. Many types of triradial chromosomes can be found in mammalian cells. The main reason for this type of damage is ionizing radiation that induces the formation of dicentric and ring chromosome (IAEA Citation1986; Hatayoglu and Orta Citation2007).
Genotoxic substance research is of great importance for the protection of the environment because it enables an insight into the influence of genotoxic substances on organisms. The goal of the research is to develop a valuable tool for the monitoring of the environment. The Allium metaphase test also shows us the source of the pollution. With the Allium and related genotoxic tests one can determine the influence of chemical substances on a healthy organism. This test also shows an excellent correlation with similar tests applied on mammals and other vertebrates. So the results can be extrapolated to man.
Using colchicine we are able to modify the basic Allium test on three levels: (i) an Allium general toxicity test by detecting elongation or inhibition and malformation of the roots of onion bulbs; (ii) an Allium anaphase-telophase test including an aberration in the cell division principle in the late anaphase and early telophase; and (iii) an Allium metaphase test used for study of the specific morphology (structure) of metaphase chromatids and chromosome damage.
References
- Al-Sabti K. 1989. Allium test for air and water borne pollution control. Cytobios. 58:71–78.
- Al-Sabti K. 1991. Handbook of genotoxic effects and fish chromosomes. Ljubljana: Institut Jožef Stefan.
- Al-Sabti K, Kurelec B. 1985. Chromosomal aberration in onion (Allium cepa) induced by water chloration by-products. B Environ Contam Tox. 34:80–88.
- Bakare AA, Adeyemi AO, Adeyemi A, Alabi OA, Osibanjo O. 2012. Cytogenotoxic effects of electronic waste leachate in Allium cepa. Caryologia. 65(2): 94–100.
- Chicago Conference. 1966. Standardization in human cytogenetics. Birth defects: Original Articles Series, Vol. II, No. 2. New York: The National Foundation.
- Firbas P, Al-Sabti K. 1995. Cytosistematic studies on the Charophyta in Slovenia. Arch Biol Sci. 47(1-2):45–54.
- Fiskesjö G. 1985. The Allium test as a standard in environmental monitoring. Hereditas. 102:99–112.
- Fiskesjö G. 1993. The Allium test – A potential standard protocol of assessment of environmental toxicity. In: Gorsuch JW, Dwyer FJ, Ingorsoll CG, La Point TW, editors. Environmental Toxicology and Risk Assessment, Vol. 2. Philadelphia: American Society for Testing and Materials. p. 331–345.
- Frescula VDS, Laughinghouse HD, Tedesco SB. 2012. Antiproliferative effect of the tree and medicinal species Luehea divaricata on the Allium cepa cell cycle. Caryologia. 65(1):27–33.
- Genc İ, Özhatay N, Cevri M. 2013. A karyomorphological study of the genus Allium (sect. Melanocrommyum) from Turkey. Caryologia. 66(1):31–40.
- Grant WF, Salamone MF. 1994. Comparative mutagenicity of chemicals selected for test in the International program on Chemical Safety’s collaborative study on plant systems for the collection of environmental mutagens. Mutat Res. 310(2):187–209.
- Gökalp FD, Kaymak F. 2002. The cytogenetic effect of maleic hydrazine in human lymphocyte culture. Trakya Üniversitesi Bilimsel Araştirmalar Dergisi B Serisi. 3(2):141–147.
- Hatayoglu SE, Orta T. 2007. Relationship between radiation induced dicentric chromosome aberrations and micronucleus formation in human lymphocytes. J Exp Clin Cancer Res. 26(2):229–234.
- International Atomic Energy Agency. 1986. Classification of chromosomal aberration. In: Biological dosimetry: Chromosomal aberration analysis for dose assessment. Technical Reports Series 260, p. 16–27. Vienna: IAEA.
- Kumar P, Panneerselvam N. 2007. Cytogenetic studies of food preservative in Allium cepa root meristem cells. Facta Universitatis Series: Med Biol. 14(2):60–63.
- Leme MD, Marin-Morales A. 2009. Allium cepa test environmental monitoring: A review on its application. Mutat Res. 682(1):71–81.
- Levan A. 1938. The effect of colchicine on root mitoses in Allium. Hereditas. 24:471–486.
- Levan A. 1949. The influence on chromosomes and mitosis of chemicals, as studied by the Allium test. Hereditas. 35(1):325–337.
- Levan A, Fredga K, Sandberg AA. 1964. Nomenclature for centromeric position on chromosomes. Hereditas. 52:201–220.
- Ma TH. 1999. The international program on plant bioassays and the report of the follow-up study after the hands-on workshop in China. Mutat Res. 426(2):103–106.
- Ma TH, Cabrera GL, Owens E. 2005. Genotoxic agents detected by plant bioassays. Rev Environ Health. 20(1):1–14.
- Mulaszynska J, Juchimiuk J. 2005. Plant genotoxicity: A molecular cytogenetic approach in plant bioassays. J Plant Genotox. 56:177–184.
- Palanikumar L, Ragunathan I, Panneerselvam N. 2011. Chromosome aberrations induced by curcumin and aloin in Allium cepa L. root meristem cells. Turk J Biol. 35:145–152.
- Panneerselvam N, Palinikumar L, Gopinathan S. 2012. Chromosomal aberration induced by Glycidol in Allium cepa. Int J Pharma Sci Res. 3(2):300–304.
- Ragunathan I, Panneerselvam N. 2007. Antimutagenic potential of curcumin on hromosomal aberation in Allium cepa. J Zhejiang Univ Sci B. 8(7):470–475.
- Rank J. 2003. The method of Allium anaphase-telophase chromosome aberration assay. Ekologija. 1:38–42.
- Schauer P. 1981. Humana genetika (Human genetics). Ljubljana: DDU Univerzum.
- Slapšytė G, Sukackaitė A. 2003. Genotoxicity studies of sodium selenite and its effect on methyl-methanesulphonate-induced chromosome damage. Biologija. 1:41–44.