Abstract
This study showed differences in the occurrence of blood cell alteration in triploid brook trout. The triploidisation was induced by hydrostatic shock pressure at 9500 psi for 5 min at different times after fertilisation (22.5, 27.5, 32.5, 37.5, 42.5, 47, 5, 52.5 and 62.5 min). The pressure shocks at 32.5 and 37.5 min after fertilisation caused the lowest share of pathologically altered blood cells in triploid fish.
Introduction
Triploidisation is a widely used method for producing sterile fish populations. The most effective method of production of triploid fish is application of pressure shocks (Pandian and Koteeswaran Citation1998). However triploid red tilapia was produced both by heat shocks and cold shocks (Pradeep, Srijaya, Bahuleyan et al. Citation2012; Pradeep, Srijaya, Papini et al. Citation2012). Selection of appropriate technical parameters for the experimental treatment of different fish species is not easy, and requires a lot of experience and skills to check the final result, for example ploidy verification in experimental groups of fish using cytogenetic or cytometric methods (Benfey et al. Citation1984; Johnstone and Lincoln Citation1986). In the red tilapia, identification of triploid individuals was conducted based on nuclear volume, cytoplasmic volume and nucleus surface area of erythrocytes (Pradeep et al. Citation2011). The most suitable method for ploidy verification in brook trout, Salvelinus fontinalis, seems to be an erythrocyte dimensions study from blood smears (Woznicki and Kuzminski Citation2002).
In triploid fish, produced by pressure or thermal shock, the alterations in erythrocytes, haemoglobin and red cell indices were observed (Benfey and Sutterlin Citation1984b; Benfey et al. Citation1984; Benfey Citation1999; Strunjak-Perovic et al. Citation2003; Wlasow et al. Citation2004; Dorafshan et al. Citation2008; Wang et al. Citation2010; Wlasow and Fopp-Bayat Citation2011). Triploidisation also influenced the changes in white blood cells, immunological defence reactions and stress response (Svobodová et al. Citation1998, Citation2001 Benfey and Biron Citation2000; Beyea et al. Citation2005; Maxime Citation2008; Fraser et al. Citation2012).
In the peripheral blood of triploid brook trout the percentage of red blood cells with divided nuclei were statistically significantly higher compared with the control group (19.1% versus 0.3%) (Wlasow et al. Citation2004). However, in this study the effect of triploidisation caused by shocks applied at 20 minutes after fertilisation was shown, but the influence of other technical parameters was not examined. Therefore the first purpose of the present study was to investigate the effect of differential time from fertilisation to pressure shock on the blood cell alteration in triploid brook trout. The second aim of the study was to estimate the minimal number of erythrocytes necessary for determination of percentage of red cells with divided nuclei in peripheral blood of brook trout triploids.
Material and methods
Fish used for this study were cultured at the Salmonid Research Department in Rutki (Institute of Inland Fisheries in Olsztyn, Poland). Both diploids (2n) and triploids (3n) came from the same lot of eggs. Triploid brook trout Salvelinus fontinalis (Mitchill) were obtained by using hydrostatic pressure shock of 9500 psi (65.5 × 103 kPa) on eggs at 10°C (Deeley and Benfey Citation1995). The pressure shock duration was 5 minutes, but time from fertilisation to shock varied in the experimental groups, and ranged from 22.5 min (group 3N-1) to 62.5 min. (group 3N-8, Table ).The diploid (control – 2N, 3N) received no pressure shock. Fish of the experimental groups were kept in separate tanks supplied with river water. During the experiment prophylactic chloramine baths were used twice a week. These baths are carried out during each rearing of fish at the Salmonid Research Department in Rutki (Institute of Inland Fisheries in Olsztyn, Poland) due to the presence of pathogens in the water supply hatchery and rearing system. Fish were fed granulate feed BioMar (Denmark). Survival from hatching to the collection of blood is shown in Table . Two months after hatching blood was collected from the caudal vessels to heparinised syringes. Propiscin (0.2% etomidate, Inland Fisheries Institute in Olsztyn, Poland) was used as an anaesthetic (0.5 ml l−1 of water for 10 min) in all experimental groups. Fish expected to be triploids (eight experimental groups, Table ) and diploids (20 specimens) were used for preparing blood smears. Smears were fixed in 95% methanol for 3 minutes, left to air dry and stained with 20% Giemsa solution for 15 minutes. The percentage of erythrocytes with divided nuclei was determined based on the observation of 300 cells from two slides for fish. For the study of white blood cells 200 cells were analysed, and the following forms have been studied: normal lymphocytes, Rieder’s lymphocytes (cells with divided nuclei), and neutrophil granulocytes (NGs) with 2, 3, 4 and ≥ 5 segments of nuclei. The identification was based on the leukocyte descriptions published by Lehmann and Stürenberg (Citation1975).
Table 1. Characteristics of diploid (2n) and triploid (3n) brook trout (Salvelinus fontinalis) used for blood cells analysis.
Simultaneously chromosome preparations (10 specimens from each experimental group) and the erythrocyte nuclei major axis measurements (all fish from each treatment) were done using procedure described by Woznicki and Kuzminski (Citation2002) in order to confirm fish ploidy. Differential cell counts were made under a 1000 × magnification.
Statistical analyses were performed on data from microscopic observations as the actual number of segmented nuclei in analysed preparations. In Tables and data were summarised in the form of percentages. All statistical analyses were evaluated at a significance level of 0.05. The chi-square test was used to evaluate the differences in proportion of “abnormal” to normal erythrocytes and leukocytes in triploid and diploid trout. Statistical differences between the observed number of red blood cells with segmented nucleus in the groups of 2N and 3N fish were calculated based on an alternative non-parametric analysis of variance – ANOVA Kruskal–Wallis (α = 0.05). In order to investigate how many nuclei should be counted to show the dependence of the number of nuclei divided by ploidy the Friedman ANOVA test was used. Data were collected from 71 fish, step by step as follows: 50 nuclei were observed and selected as normal and divided, and then the next 50 nuclei were observed and also selected as normal and divided (two or more parts), after that the cyclic analysis of next 50 nuclei was conducted. A total of 300 nuclei were counted. It was hypothesised that the results obtained for the count of 50, 100, 150, 200, 250 and 300 nuclei did not differ significantly and therefore the study of ploidy is sufficient to perform only counts of 50 nuclei.
Table 2. Occurrence of pathological forms of erythrocytes and lymphocytes in peripheral blood of diploid (2n) and triploid (3n) brook trout (Salvelinus fontinalis).
Table 3. Comparisons of the percentage of white blood cells forms in diploid (2n) and triploid (3n) brook trout (Salvelinus fontinalis).
Results and discussion
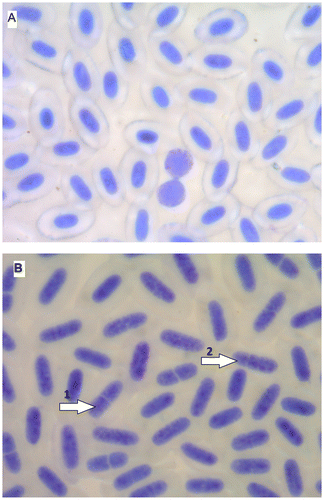
The diploid specimens of brook trout possessed 84 chromosomes, while triploid fish of this species possessed 126 chromosomes. The percentage of red blood cells with the segmented nuclei in triploids of brook trout was significantly higher than in diploid individuals (Table , Figure ). Similar observations in brook trout were published by Wlasow et al. (Citation2004) and in rainbow trout triploids (Johari et al. Citation2008). The phenomenon of division of nuclei in erythrocytes of triploid salmonids was signalled also by Benfey (Citation1999). According to Wang et al. (Citation2010) nuclear division in erythrocytes of triploids of rainbow trout is probably amitosis. The red blood cells of salmonids with the segmented nuclei are precursors of a stage before division into two smaller ones (Lehmann and Stürenberg Citation1975). This process is regarded as an indication of the compensation of disturbances in the body (for example, a reduced number of red blood cells are divided into smaller cells, to increase surface area and gas exchange of oxygen and carbon dioxide). Typically large triploid erythrocytes have less surface area to volume ratio than smaller diploid red cells, which means a negative influence of triploidisation on the transport of oxygen. There was an opinion that triploids may be more adapted for reduced oxygen content in the water than diploid fish (Benfey and Sutterlin Citation1984a). However, pathological changes observed in the blood indicate a possible reduction in the efficiency of production in triploid fish farming in unsuitable environmental conditions (Bernier et al. Citation2004).
Figure 1. (Color online) Blood of brook trout Salvelinus fontinalis: (A) diploid fish: erythrocytes and lymphocytes (two); (B) triploid fish: erythrocytes with divided nuclei (1, two parts; 2, three parts).
The study of indication the minimal number of erythrocytes nuclei that can determine percentage of red blood cells with divided nuclei of brook trout was conducted. The hypothesis that results of 50, 100, 150, 200, 250 and 300 calculations of nuclei do not differ from each other statistically was tested using the ANOVA Friedman test and the null hypothesis was not rejected due to no differences in the number of divided nuclei in 2N (n = 28, df = 5, F = 7.7444, p < 0.1709) and 3N (n = 44, df = 5, F = 3.2844, p < 0.6562). Therefore these results suggest that the calculation of 50 nuclei is sufficient to estimate the percentage of nuclei divided in one fish.
Pathological forms of lymphocytes were observed more frequently in the blood of triploid fish than in diploid (Table ). The profile of white blood cells was dominated by neutrophil granulocytes with the increased number of segments of nucleus (Table ). A similar characteristic of granulocytes occurred in triploids of brook trout (Wlasow et al. Citation2004) and Siberian sturgeon (Wlasow and Fopp-Bayat Citation2011). The phenomenon of hypersegmentation on nuclei in neutrophil granulocytes of triploid fish may be related to the segmentation of the predominance of ageing cells. Extending such a phenomenon may weaken the immune response, which is related to granulocytes as microphages.
In the present study the most advantageous variants of the experiment (with a minimum participation of pathological changes in red and white blood cells) were the groups that had been induced by shock pressure at 32.5 and 37.5 minutes post fertilisation.
Acknowledgements
The study was supported by the project no. 0804. 0809 of University of Warmia and Mazury in Olsztyn, Poland.
References
- Benfey TJ. 1999. The physiology and behavior of triploid fishes. Rev Fish Sci. 7(1):39–67.
- Benfey TJ, Biron M. 2000. Acute stress response in triploid rainbow trout (Oncorhynchus mykiss) and brook trout (Salvelinus fontinalis). Aquaculture. 184(1–2):167–176.
- Benfey TJ, Sutterlin AM. 1984a. Oxygen utilization by triploid landlocked Atlantic salmon (Salmo salar L.). Aquaculture. 42(1):69–73.
- Benfey TJ, Sutterlin AM. 1984b. The haematology of triploid landlocked Atlantic salmon, Salmo salar L. J Fish Biol. 24(3):333–338.
- Benfey TJ, Sutterlin AM, Thompson RJ. 1984. Use of erythrocyte measurements to identify triploid salmonids. Can J Fish Aquat Sci. 41(6):980–984.
- Bernier NJ, Brauner CJ, Heath JW, Randall DJ. 2004. Oxygen and carbon dioxide transport during sustained exercise in diploid and triploid chinook salmon (Oncorhynchus tshawytscha). Can J Fish Aquat Sci. 61(9):1797–1805.
- Beyea MM, Benfey TJ, Kieffer JD. 2005. Hematology and stress physiology of juvenile diploid and triploid shortnose sturgeon (Acipenser brevirostrum). Fish Physiol Biochem. 31(4):303–313.
- Deeley MA, Benfey TJ. 1995. Learning ability of triploid brook trout. J Fish Biol. 46(5):905–907.
- Dorafshan S, Kalbassi M R, Pourkazemi M, Amiri B M, Karimi SS. 2008. Effects of triploidy on the Caspian salmon Salmo trutta caspius haematology. Fish Physiol Biochem. 34(3):195–200.
- Fraser TWK, Rønneseth A, Haugland GT, Fjelldal PG, Mayer I, Wergeland HI. 2012. The effect of triploidy and vaccination on neutrophils and B-cells in the peripheral blood and head kidney of 0+ and 1+ Atlantic salmon (Salmo salar L.) post-smolts. Fish Shellfish Immun. 33(1):60–66.
- Johari SA, Kalbassi MR, Sourinezhad I, Wlasow T. 2008. Observation of red blood cell alterations in triploid rainbow trout (Oncorhynchus mykiss). Acta Sci Pol Pisc. 7(1–4):49–52.
- Johnstone R, Lincoln RF. 1986. Ploidy estimation using erythrocytes from formalin – fixed salmonid fry. Aquaculture. 55(2):145–148.
- Lehmann J, Stürenberg FJ. 1975. Haematologisch – serologische Substratuntersuchungen an der Regenbogenforelle (Salmo gairdneri Richardson). II. Beschreibung und Darstellung der wichtigsten Zellen in der Blutbildungsstätte und im peripheren Blutgefassystem. Gewässer und Abwässer. 55–56:1–123.
- Maxime V. 2008. The physiology of triploid fish: current knowledge and comparisons with diploid fish. Fish and Fisheries. 9(1):67–78.
- Pandian TJ, Koteeswaran R. 1998. Ploidy induction and sex control in fish. Hydrobiologia. 384(1-3):167–243.
- Pradeep PJ, Srijaya TC, Bahuleyan A, Renjithkumar CR, Jose D, Papini A, Chatterji AK. 2012. Triploidy induction by heat-shock treatment in red tilapia. Caryologia. 65(2):152–156.
- Pradeep PJ, Srijaya TC, Jose D, Papini A, Hassan A, Chatterji AK. 2011. Identification of diploid and triploid red tilapia by using erythrocyte indices. Caryologia. 64(4):485–492.
- Pradeep PJ, Srijaya TC, Papini A, Chatterji AK. 2012. Effects of triploidy induction on growth and masculinization of red tilapia [Oreochromis mossambicus (Peters, 1852) X Oreochromis niloticus (Linnaeus, 1758)]. Aquaculture. 344–349:181–187.
- Strunjak-Perovic I, Coz –Rakovac R, Popovic NT. 2003. Micronucleus occurrence in diploid and triploid rainbow trout (Oncorhynchus mykiss Walbaum). Vet Med-Czech. 48(8):215–219.
- Svobodová Z, Flajshans M, Kolarova J, Modra H, Svoboda M, Vajcova V. 2001. Leukocyte profiles of diploid and triploid tench, Tinca tinca L. Aquaculture. 198(1–2):159–168.
- Svobodová Z, Kolarova J, Flajshans M. 1998. The first findings of the differences in complete blood count between diploid and triploid tench, Tinca tinca L. Acta Vet Brno. 67(4):243–248.
- Wang B, Liu Y, Chen X, Fan Z. 2010. Amitosis-like nuclear division in erythrocytes of triploid rainbow trout Oncorhynchus mykiss. J Fish Biol. 76(5):1205–1211.
- Wlasow T, Fopp-Bayat D. 2011. The effect of thermal shock on morphological characteristics of blood cells in Siberian sturgeon (Acipenser baerii) triploidy. Acta Vet Brno. 80(2):215–218.
- Wlasow T, Kuzminski H, Woznicki P, Ziomek E. 2004. Blood cells alteration in triploid brook trout Salvelinus fontinalis (Mitchill). Acta Vet Brno. 73(1):115–118.
- Woznicki P, Kuzminski H. 2002. Chromosome number and erythrocyte nuclei length in triploid brook trout (Salvelinus fontinalis). Caryologia. 55(4):295–298.
