Abstract
Subgenus Seriphidium is one of the least karyologically studied groups in genus Artemisia. In this work, chromosome number and karyotypes of six taxa belonging to Seriphidium subgenus of genus Artemisia were investigated as detailed. Both chromosome number and karyological data of all taxa studied here were the first records from Turkey. Two of the taxa are new records to science (A. spicigera K. Koch. var. vanensis Kursat & Civelek var. nova and A. taurica Willd. var. pendulosa Kursat & Civelek var. nova) and another is a new record for the flora of Turkey (A. sieberi Bess. subsp. sieberi). All of the taxa studied except for A. spicigera K. Koch. var. spicigera were polyploid. Hypo-aneuploidy was observed in one taxon, A. santonicum L. subsp. santonicum with chromosome number 2n = 6x = 52. In addition, the simultaneous existence of B chromosome and aneusomy was recorded for A. taurica Willd. var. taurica, 2n = 4x = 36 + 0, 4B; 37 + 3, 4B.
Introduction
Artemisia L. is a main genus of the tribe Anthemideae and the largest genus of the family Asteraceae. The majority of the members of this genus have a high economic value (Chehregani et al. Citation2010; Hayat et al. Citation2010). The genus comprises around 600 taxa at the specific or subspecific level, according to various authors (Ling Citation1991a, 1991b, 1995a, 1995b; Bremer and Humphries Citation1993; Vallès and McArthur Citation2001; Kadereit and Jeffrey Citation2007; Vallès et al. Citation2011). Because of the high number of taxa, numerous taxonomists are trying to solve the problem of its classification and phylogeny but its natural classification still has not been achieved (McArthur et al. Citation1981; Torrell et al. Citation1999; Vallès et al. Citation2003; Kurşat, Civelek, et al. Citation2011; Kurşat, Türkoğlu, et al. Citation2011). Moreover, Artemisia is a taxonomically complex genus because some species have different morphological forms and others closely resemble each other. These traits make it quiet difficult to correctly identify a sample without detailed morphological review (Hayat, Khan, et al. Citation2009). The genus is currently divided into five main groups [Artemisia, Absinthium (Mill.) Less., Dracunculus (Besser) Rydb., Seriphidium Besser and Tridentatae (Rydb.) McArthur] but subgeneric classification is subject to rearrangements in the light of recent molecular studies (Torrell et al. Citation1999; Vallès et al. Citation2003). Although these five large groups are usually recognized within the genus, treated as sections or subgenera depending on the authors consulted (Shishkin and Bobrov Citation1995; Vallès et al. Citation2003; Hayat, Ashraf, et al. Citation2009 and references therein), a comprehensive infrageneric classification has also not yet been established (Vallès et al. Citation2011 and references therein).
Subgenus Seriphidium comprises approximately 140 species with distributions mainly in arid regions of central, southwest and western Asia, Middle East, northern Africa, and Europe (Watson et al. Citation2002). According to Bremer and Humphries (Citation1993), Seriphidium is distinguished from other Artemisia species by morphologic characters. Based on these characters, this section is sometimes recognized as a distinct genus (Bremer and Humphries Citation1993; Ling Citation1991a, 1995b). However, Watson et al. (Citation2002) asserted that molecular data do not support the segregation of Seriphidium from within the boundaries of Artemisia. Hence, they have integrated Seriphidium with Artemisia again. However, the global classification system for the systematic relationships of the taxon within the genus Artemisia are still debated.
Over the last five or six decades, many karyological and cytological studies have been performed in the genus Artemisia (Kawatani and Ohno Citation1964; Torrell et al. Citation1999; Vallès and McArthur Citation2001; Vallès, Torrell and Garcia-Jacas Citation2001; Vallès, Torrell, Garcia-Jacas and Kapustina Citation2001; Kreitschitz and Vallès Citation2003; Rabiei et al. Citation2003; Hoshi et al. Citation2006; Pellicer, Garcia, Garnatje, Dariimaa, et al. Citation2007; Pellicer, Garcia, Garnatje, Hidalgo, et al. Citation2007; Naseri et al. Citation2009; Chehregani and Hajisadeghian Citation2009; Chehregani et al. Citation2010; Pellicer et al. Citation2010; Tabur et al. Citation2011, 2012; Dolatyari et al. Citation2013; Jalili et al. Citation2013). Chromosome number is the most basic of all karyological and cytogenetic data, but is still a source of relevant information in plant systematics and evolution (Stebbins Citation1971; Stace Citation2000; Stuessy Citation2009; Garbari et al. Citation2012). Despite its relevance, the chromosome number is only known for around 25% of angiosperms (Stuessy Citation2009). The Asteraceae are one of the families in which major efforts towards chromosome number and genome size determination have been made (Watanabe et al. Citation2007; Semple and Watanabe Citation2009; Meng et al. Citation2012; Mousavi et al. Citation2013; Olanj et al. Citation2013; Khaldi et al. Citation2014). In particular, hypotheses on basic chromosome numbers in Asteraceae have been hampered by a lack of understanding of which genera were basal within tribes and which tribes were basal within the family. For example, Cronquist (Citation1981) reported that Asteraceae had a range of base numbers from x = 2 to x = 19+ and suggested that perhaps x = 9 was ancestral. Earlier, Solbring (Citation1977) had also concluded x = 9 was the ancestral base number of the family based on an analysis of habit and frequency of chromosome number. Among the Asteraceae genera, Artemisia is one of the most complex genera from this viewpoint (Vallès et al. Citation2011). The chromosome number data of more than one-third of Artemisia species still remain unknown or doubtful and very scarce karyotypic studies have been conducted (Nasari et al. Citation2009; Tabur et al. Citation2011, 2012, Dolatyari et al. Citation2013). In the genus Artemisia, the chromosome number in diploid level is most often 2n = 18 or 16; the highest chromosome number known is 144 (Pellicer, Garcia, Garnatje, Hidalgo, et al. Citation2007). The most common basic chromosome numbers are x = 8 and 9, although x = 7, 10, 13, and 17 have also been reported by some researchers (Vallès et al. Citation2005; Chehregani and Hajisadeghian Citation2009; Matoba and Uchiyama Citation2009).
On the other hand, polyploidy has been recognized as a common phenomenon in the genus, which also has aneuploidy (Kawatani and Ohno Citation1964). Polyploidy has played a central role in the evolution and speciation in higher plants, which are estimated from 35 to 80% of all species (Stebbins Citation1971; Soltis and Soltis Citation2000). Therefore, cytological study provides essential information to examine phylogenetic relationships and speciation in higher plants (Hoshi et al. Citation2006). Both basic chromosome numbers in Artemisia (x = 8 and 9) show polyploidy, with levels up to 12x for x = 9 and 6x for x = 8 (Vallès and McArthur Citation2001). Clearly, dysploid–polyploid complexes have played a major role in the karyological evolution of Artemisia. In Anthemideae, and particularly in Artemisia and its relatives, both changes in the basic chromosome number and polyploidy seem to occur mostly in the later stages of their evolutionary history (Vallès and McArthur Citation2001). In Artemisia, dysploidy decreases are common (Semple and Watanabe Citation2009) but subgenera Seriphidium and Tridentatae have only one basic chromosome number (x = 9) (Kawatani and Ohno Citation1964). Chromosome numbers and karyological features of many species belong to subgenus Seriphidium are yet unknown or scarce.
For these reasons, new karyological studies are still necessary to unravel the conflicts. Karyological studies including karyotype analysis are welcome as a possible contribution to the systematics of large and difficult genera such as Artemisia. The aims of the present study were to perform an extensive karyological survey on Artemisia genus, to discover the chromosome numbers of several representatives of subgenus Seriphidium from different Anatolian localities and to identify or verify chromosome numbers and karyological characters of the subgenus Seriphidium in light of the new cytogenetic data.
Material and methods
Herbarium vouchers of all taxa studied are deposited in the herbarium of Department of Biology, Faculty of Science, Fırat University, Turkey (FUH). The locations, collector(s) and dates are shown in Table .
Table 1. Provenance of the populations of the Seriphidium subgenera.
Root-tip meristems were obtained by germinating achenes on one layer of wet filter paper at 20 ± 1°C in an incubator. The germinated achenes (0.5–1 mm) were pretreated with saturated paradichlorobenzene for 4 h at room temperature. Materials were fixed in a solution of ethanol and glacial acetic acid (3:1) overnight at room temperature and stored at 4°C in 70% ethanol until used. Hydrolysis was carried out with 1 N HCl at 60°C for 15–18 min, stained in Feulgen for 1–1.5 h at room temperature and squashed and mounted in a drop of 45% acetic acid (Sharma and Gupta Citation1982). The best metaphase plates were photographed at a magnification of 1000 × with a digital camera (Olympus C-5060) mounted on an Olympus CX41 microscope (Olympus Optical Co. Ltd. Japan).
For each accession, average chromosome measurements were calculated on 10 metaphase plates. The quantitative values were obtained from chromosome character measurements. These were chromosome number, total length (C), long-arm length (L), short-arm length (S), arm ratio (r = L/S), centromeric index (I = 100 × S/C), relative length (RL = total length (C) / total haploid length × 100), and chromosome type. Thereafter, chromosome morphologies and karyograms were constructed. Karyotype formula was determined by chromosome morphology based on the centromeric position (Levan et al. Citation1964). Karyotype asymmetry was estimated using the mean centromeric index, the ratio of the shortest-to-longest pairs, and the intrachromosomal asymmetry (A1) and interchromosomal asymmetry (A2) indices (Romero Citation1986). To assess the existence of previously published chromosome counts in the taxa studied, we used the most common indexes of plant chromosome numbers (Vallès, Torrell and Garcia-Jacas Citation2001; Vallès, Torrell, Garcia-Jacas and Kapustina Citation2001 and references therein), previous publications (Vallès et al. Citation2005 and references therein), as well as online chromosome number databases, the chromosome number databases ‘Index to Plant Chromosome Numbers’ (Missouri Botanical Garden, http://mobot.mobot.org/W3T/Search/ipcn.html) and ‘Index to Chromosome Numbers in the Asteraceae’ (Watanabe Citation2014).
Results and discussion
During recent taxonomic revision of the genus Artemisia L., Kurşat (Citation2010), Kurşat, Civelek, et al. (Citation2011) and Kurşat, Türkoğlu, et al. (Citation2011) observed that there were differences between the classifications of subgenus Seriphidium in the Flora Europaea and Flora of Turkey. In the present study, we have followed the nomenclature in the Flora Europaea adopted by Tutin and Persson (Citation1976). The classification at the genus level of Asteraceae is very dynamic: every year at least 10 new genera are described and many more are resurrected or moved into synonymy. The infrageneric classification of Artemisia is also an unresolved problem. However, many studies continue to make important contributions to a global classification of Artemisia, and also Asteraceae (Bremer Citation1994; Kadereit and Jeffrey Citation2007; Kurşat, Civelek, et al. Citation2011; Kurşat, Türkoğlu, et al. Citation2011).
Two of the taxa studied here are new records to science (A. spicigera K. Koch. var. vanensis Kursat & Civelek var. nova and A. taurica Willd. var. pendulosa Kursat & Civelek var. nova) and one other taxon is a new record for the flora of Turkey: A. sieberi Bess. subsp. sieberi (Kurşat Citation2010). Kurşat, Civelek, et al. (Citation2011) reported that the description of A. herba-alba Asso. in Flora of Turkey (Davis Citation1975) was problematic and that the Turkish specimens were the same as the specimens called A. sieberi Bess., which were collected from Siberia, Caucasia, Central Asia and Kazakhstan. Thus, they determined that A. herba-alba was not found in Turkey and the specimens given as A. herba-alba in Flora of Turkey (Davis Citation1975) were actually A. sieberi subsp. sieberi which is a new record for Turkey. Recently, a new subspecies record for the flora of Turkey has been also reported: A. santonicum subsp. patens (Neilr.) K. M. Perss., which collected from northwest of Turkey (Kurşat, Türkoğlu, et al. Citation2011). Therefore, A. santonicum L. is now represented as two subspecies in Turkey.
The somatic chromosome numbers (2n) of the taxa studied and previous reports with their references are present in Table . Mean numerical karyological data of the taxa studied belonging to subgenus Seriphidium are shown in Table . Karyotypic characters of three taxa could not be obtained due to difficulties in their germination and the lack of enough metaphase plates (A. santonicum L. subsp. santonicum, A. spicigera K. Koch var. vanensis Kursat & Civelek var. nova, A. sieberi Bess. subsp. sieberi). The somatic metaphase chromosomes of all taxa studied are displayed in Figure and haploid idiogram and karyograms of other three taxa are present in Figure . Both the chromosome number and karyotype characters of the all taxa studied here are new reports for the Anatolian populations. One taxon was diploid with 2n = 2x = 18, three taxa were tetraploid with 2n = 4x = 36 and two taxa were hexaploid with 2n = 6x = 54, 52. The chromosomes were all median (m) or submedian (sm) according to the classification system of Levan et al. (Citation1964).
Table 2. Somatic chromosome numbers of Artemisia (subg. Seriphidium) studied in the Anatolian populations, with indication of previous reports in every taxon if any.
Table 3. Mean numerical karyological data of the taxa studied belonging to subgenus Seriphidium.
Figure 1. (Color online) Micrographs of the somatic metaphase chromosomes of taxa studied belong to Seriphidium subgenus of Artemisia. (a) A. santonicum subsp. santonicum (2n = 6x = 52, hypo-aneuploid); (b–e) A. taurica var. taurica (2n = 4x = 36, 36 +4B, 37 + 3B, 37 + 4B; from Polatlı); (f) A. taurica var. taurica (2n = 4x = 36; from Şereflikoçhisar); (g) A. taurica var. pendulosa (2n = 6x = 54); (h) A. spicigera var. spicigera (2n = 2x = 18); (i) A. spicigera var. vanensis (2n = 4x = 36); (j) A. sieberi subsp. sieberi (2n = 4x = 36). Karyotypic characters of three taxa (a, i and j) could not be obtained due to difficulties in their germination and the lack of enough metaphase plates. Arrows indicate B chromosomes. Scale bar = 5 μm.
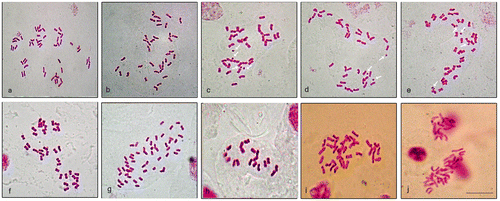
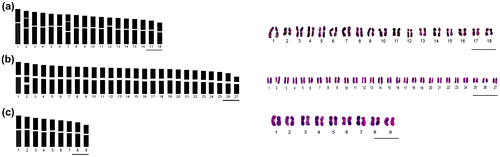
Figure 2. Haploid idiogram and karyogram of (a) A. taurica var. taurica; (b) A.taurica var. pendulosa; and (c) A. spicigera var. spicigera. Karyotypic characters of A. santonicum subsp. santonicum, A. spicigera var. vanensis and A. sieberi subsp. sieberi could not be obtained due to difficulties in their germination and the lack of enough metaphase plates. Scale bars in all figures = 5 μm.
A. santonicum L. subsp. santonicum (2n = 6x = 52)
A. santonicum subsp. santonicum is distributed in the Central Anatolia region, at an altitude between 938 and 1000 m (Kurşat, Türkoğlu, et al. Citation2011). Numerous authors have reported the existence of different two ploidy levels for this species, including 2n = 2x = 18 and 2n = 4x = 36 (Watanabe Citation2014 and references therein). However, the taxon has been represented as A. santonica in all of these references. In our study, the chromosome number of A. santonicum L. subsp. santonicum was identified as 2n = 6x = 52 (Figure a) and its basic number is also x = 9. As it is widely known, subgenus Seriphidium displays only one basic chromosome number, x = 9 (Vallès and McArthur Citation2001). According to our data, this hexaploid level is the first report for this taxon. We believe that the count (2n = 6x = 52, not 54) must have been a result of hypo-aneuploid decreases. Aneuploidy is a common phenomenon in Artemisia (Vallès and McArthur Citation2001; Pellicer, Garcia, Garnatje, Dariimaa, et al. Citation2007; Sánchez-Jiménez et al. Citation2009), and in subgenus Seriphidium (Dolatyari et al. Citation2013).
A. taurica Willd. var. taurica (2n = 4x = 36 + 0, 4B; 37 + 3, 4B)
For this taxon we report the same chromosome number (2n = 4x = 36) in two studied populations (Table ). Our count is the fourth record for this taxon; three previous counts are 2n = 18, 36 (Kawatani and Ohno Citation1964), 2n = 18 (Pellicer et al. Citation2010) and 2n =36 (Probatova et al. Citation2010) on plants from Russia. However, the taxon has been evaluated as A. taurica by these three authors. In the Polatlı population, a variable chromosome number (2n = 4x = 36 + 0, 4B; 37 + 3, 4B) was counted (Figures a), indicating aneusomy – implying different chromosome numbers in cells of the same meristem – and the presence of B chromosomes. In another population (Şereflikoçhisar), 2n = 4x = 36 chromosomes were assigned (Figure f), in this case with no evidence for aneusomy and B chromosomes. To date, aneusomy was known to be present in five Artemisia species: A. verlotiorum Lamotte (2n = 48, 50, 52; Martinoli and Ogliotti Citation1970; Vallès Citation1987), A. maritima L. (2n = 50, 54, 56; Persson Citation1974), A. negrei A. Ouyahya (2n =56, 58,64, 65; Ouyahya and Viano Citation1988), A. dracunculus L. (2n =87, 88, 89, 90; Kreitschitz and Vallès Citation2003) and A. quettensis Podlech (2n = 49, 51, 54; Dolatyari et al. Citation2013). Aneusomy is most frequent in polyploidy cytotypes and in plants with vegetative multiplication (Vallès et al. Citation2011). In this study, B chromosomes and detailed karyological features of the taxon have been reported for the first time. Our work is also the first report of chromosome number of the taxa for the Turkish populations. The karyotype formula of the taxon is determined as 30m + 2mSat + 2Sm + 2SmSat (Table ). In addition, the simultaneous existence of B chromosomes and aneusomy in one species is reported for the second time for Artemisia genus. One previous report has been presented for A. quettensis by Dolatyari et al. (Citation2013).
A. taurica Willd. var. pendulosa Kursat & Civelek var. nova (2n = 6x = 54)
A. taurica Willd. var. pendulosa Kursat & Civelek var. nova is a new record to science (Kurşat Citation2010). Therefore, both chromosome number and karyological features of the taxon are presented the first time in this study. Our data have shown that the chromosome number of this taxon is 2n = 6x = 54 (Figures b), and its karyotype formula is 50m + 2mSat + 2Sm. This new hexaploid level again supports polyploidization as an evolutionary factor contributing to speciation in the genus. It is determined that all the chromosomes of this taxon were median, excluding the 27th chromosome, which has a submedian centromere. Also, a satellite connecting with short arm has been observed in chromosome 2 (Table ).
A. spicigera K. Koch. var. spicigera (2n = 2x = 18)
The chromosome number of this taxon was found as 2n = 2x = 18 (Figure h). Six reports, all on Iranian material, have been published so far: 2n = 18, 36 (Saedi et al. Citation2006); 2n = 36 (Rabiei et al. Citation2003; Naseri et al. Citation2009); 2n = 36, 72 (Atri et al. Citation2009); 2n = 18, 36, 45, 54, and 72 (Chehregani et al. Citation2010) and 2n = 36 (Dolatyari et al. Citation2013). However, the taxon has been evaluated at species level by all of these authors. Our count is the first report on Turkish material. The karyological data on the taxon is limited, with only two records (Naseri et al. Citation2009; Dolatyari et al. Citation2013). The karyotype formulae of A. spicigera at tetraploid level (2n = 4x = 36) are given as 32m + 4sm by Naseri et al. (Citation2009) and as 32m + 4sm, 28m +2msat + 5sm + 1smsat and 30m + 4sm + 2 st in three different populations by Dolatyari et al. (Citation2013). In our study, the karyotype formula of this taxon at diploid level is determined as 18m. The karyological data presented here are the first report at the diploid level for the taxon (Figure c, Table ).
A. spicigera K. Koch. var. vanensis Kursat & Civelek var. nova (2n = 4x = 36)
A. spicigera K. Koch. var. vanensis Kursat & Civelek var. nova is also a new record to science (Kurşat Citation2010) and our work is the first report of the chromosome number of the taxon (2n = 4x = 36, Figure i). However, karyological characters of the taxon could not be obtained due to difficulties in their germination. The tetraploid chromosome number reported here could be indicative of a speciation process, since polyploidy is a very powerful evolutionary mechanism in Artemisia (Vallès et al. Citation2011 and references therein). In this case the karyological differentiation may be phenotypically reflected and this phenomenon could be explained morphological differences between var. spicigera and var. vanensis. Moreover, polyploidy particularly seems to some extent frequent in var. spicigera, which forms a polyploidy series on Iranian material (Saedi et al. Citation2006; Rabiei et al. Citation2003; Atri et al. Citation2009; Naseri et al. Citation2009; Chehregani et al. Citation2010).
A. sieberi subsp. sieberi (2n = 4x = 36)
This taxon was designated as A. herba-alba Asso. in early botanical references to Iran and neighboring countries (Boissier Citation1875). In Flora Iranica, Podlech (Citation1986) used this name as a synonym of A. sieberi. Mozaffarian (Citation2008) followed this opinion, but proposed extensive research to solve the correct delimitation of these taxa. In a recent report, it was recorded that the species designated as A. herba-alba in Flora of Turkey (Davis Citation1975) is actually A. sieberi subsp. sieberi (Kurşat, Civelek, et al. Citation2011). Thus, the chromosome number of this taxon under A. sieberi subsp. sieberi name was identified as 2n = 4x =36 (Figure j). In our literature review, we recorded that this count is the first from Turkey, the fourth report as A. sieberi. The previous count is a meiotic one (n = 9) on plants from Pakistan (Khatoon and Ali Citation1993) and another previous two counts are 2n = 36 (Dolatyari et al. Citation2013) and 2n = 18, 36 (Jalilia et al. Citation2013) from Iran. Karyological features of the taxon could not be obtained due to difficulties in their germination. The only report related to karyological features of species is presented as 28m + 8sm (Dolatyari et al. Citation2013).
In general, our results confirm the existence of a single basic chromosome numbers in Seriphidium subgenus. All the taxa studied here have the basic chromosome number of x = 9, which is predominant in the Asteraceae (Kawatani and Ohno Citation1964). Dolatyari et al. (Citation2013) reported that Seriphidium is the subgenus of Artemisia less resolved in the molecular and phylogenetic studies. Also, they emphasized that high polyploidy percentages, frequent existence of B-chromosomes, aneuploidy and aneusomy are other cytological mechanisms that can compensate for the higher uniformity in chromosome numbers due to the lack of dysploidy in Seriphidium.
Polyploidy is one of the main evolutionary mechanisms in the Asteraceae family (Semple and Watanabe Citation2009) and has been widely recognized as particularly active in Artemisia (McArthur et al. Citation1981), with more than half the taxa in Artemisia genus having been estimated as having at least some polyploid populations (Vallès et al. Citation2011). Polyploid species can exhibit higher ecological tolerance than their progenitor species. Thus all the taxa studied here except one showed examples of polyploidy (three tetraploid and two hexaploid). The chromosome number of A. santonicum subsp. santonicum is the first report of the hexaploid level (2n = 6x = 52 as aneuploid). Moreover, the simultaneous existence of B chromosomes and aneusomy in one species were observed in one taxon, which is a rare phenomenon (A. taurica var. taurica, 2n = 4x = 36 + 0, 4B; 37 + 3, 4B).
This work presents detailed chromosomes complements of three taxa with rare known or unknown karyological features to date. These taxa exhibited very similar karyotypes, with chromosome pairs barely distinguishable from one another. The chromosomes of taxa studied were median (m) or submedian (sm). A. taurica var. taurica had a pair of satellites connecting to the short arm of chromosome 2 and 7. A. taurica var. pendulosa had also a satellite connecting to the short arm of chromosome 2 (see Table , Figure b).
The results allow us to compare the additional karyomorphological parameters using A1 and A2 indices, which do not depend on chromosome number or chromosome size (Romero Citation1986). The results for A1 and A2 values of taxa studied are shown in Table . Stebbins (Citation1971) suggested that asymmetrical karyotypes are more advanced than symmetrical ones in relation to phylogeny and evolutionary processes. Karyotype symmetry is an important indicative of evolutionary traits. That is, karyotypes tend to be more asymmetric (at inter- and intrachromosomal levels) especially in polyploid taxa, which is in agreement with the evolutionary trend. Likewise, it supports this opinion that A1 and A2 indexes of two polyploidy taxa studied here are higher than diploid ones. Moreover, correlations between karyological parameters show that mean chromosome length is positively correlated with mean arm ratio and A1 asymmetry index, suggesting more asymmetrical karyotypes in taxa with longer karyotypes (Table ). Tabur et al. (Citation2012) reported that the A2 index in particular indicates differences between two basic chromosome numbers of Artemisia, and species with x = 9 had smaller A2 values than species with x =8. Meanwhile, all the taxa examined in the present research belong in the Seriphidium subgenus, having only one basic chromosome number (x = 9). Therefore, A2 indexes of none of the taxa studied here were significantly different from each other (0.10–0.12). This shows that the A1 parameter is more useful for estimation of evolutionary relationships among some subgenera or sections of genus Artemisia. In contrast, Meng et al. (Citation2012) asserted that the changes in ploidy levels and karyotype asymmetry in genus Leontopodium are not necessarily coincident. They suggest not only that polyploidy and dysploidy play important roles during karyotype evolution and speciation, but also that ecological selection should be an alternative important factor for karyotype asymmetry.
Conclusion
The existence of species with extensive morphological similarities and the abundance of polyploids in the Artemisia have led to misunderstandings of the relationships between species. Therefore, more investigations in karyomorphology, molecular cytogenetics, and molecular phylogenetics are necessary to clarify and justify the Artemisia species’ taxonomical relationships, evolution and polyploidization mechanisms. This is a work centered on the Seriphidium subgenus of genus Artemisia, and it aims to improve understanding of what kind of karyological characters there are at a subgeneric level. The karyological characters are essential for drawing significant conclusions on the relative closeness and distance of the various taxa. Karyological studies (from chromosome counts to molecular cytogenetics) and molecular studies (combining different techniques in as many taxa as possible) will also be effective tools to improve the systematic definition and to solve the systematic and evolutionary paradox in Artemisia and its subgenera. We believe that such comprehensive studies will contribute to future reviews, monographic, and floristic studies.
Acknowledgment
The authors thank the Scientific and Technological Research Council of Turkey (TÜBİTAK) for financial support of project TÜBİTAK-106T559.
References
- Atri M, Chehregani A, Jalali F, Yousefi S. 2009. New chromosome counts in some species of the genus Artemisia L. (Asteraceae) from Iran. Cytologia. 74(4):443–448.
- Boissier E. 1875. Artemisia.In Flora Orientalis, vol 3. Genéva: H. Georg. p. 360–376.
- Bremer K. 1994. Asteraceae: Cladistics and classification. Portland, Oregon: Timber Press.
- Bremer K, Humphries CJ. 1993. Generic monograph of the Asteraceae-Anthemideae. Bull Brit Mus Nat Hist Bot. 23(2):71–177.
- Chehregani A, Atri M, Yousefi S, Jalali F. 2010. Polyploidy variation in some species of the genus Artemisia L. (Asteraceae) in Iran. Caryologia. 63(2):168–175.
- Chehregani A, Hajisadeghian S. 2009. New chromosome counts in some species of Asteraceae from Iran. Nord J Bot. 27(3):247–250.
- Cronquist A. 1981. An integrated system of classification of flowering plants. New York: Columbia University Press.
- Davis PH. 1975. Flora of Turkey and the East Aegean Islands. vol 5. Edinburgh: Edinburgh University Press.
- Dobes C, Vitek E. 2000. Documented chromosome number checklist of Austrian vascular plants. Vienna: Verlag des Naturhistorischen Museums Wien; p. 642.
- Dolatyari A, Vallès J, Naghavi MR, Fazeli SAS. 2013. Karyological data of 47 accessions of 28 Artemisia (Asteraceae, Anthemideae) species from Iran, with first new reports for Iranian populations and first absolute counts in three species. Plant Syst Evol. 299(8):1503–1518.
- Garbari F, Bedini G, Peruzzi L. 2012. Chromosome numbers of the Italian flora. From the Caryologia foundation to present. Caryologia. 65(1):62–71.
- Hayat MQ, Khan MA, Ashraf M, Jabeen S. 2009. Ethnobotany of genus Artemisia L. (Asteraceae) in Pakistan. Ethnobot Res Applicat. 7(1):147–162.
- Hayat MQ, Ashraf M, Khan MA, Mahmood T, Ahmad M, Jabeen S. 2009. Phylogeny of Artemisia L.: recent developments. Afr J Biotechnol. 8(11):2423–2428.
- Hayat MQ, Ashraf M, Khan MA, Yasmin G, Shaheen N, Jabeen S. 2010. Palynological study of the genus Artemisia (Asteraceae) and its systematics implications. Pak J Bot. 42(2):751–763.
- Hoshi Y, Matoba H, Kondo K. 2006. Physical mapping of ribosomal RNA genes in the genus Artemisia L. (Asteraceae). Caryologia. 59(4):312–318.
- Jalili A, Rabie M, Azarnivand H, Hodgson JG, Arzani H, Jamzad Z, Asri Y, Hamzehee B, Ghasemi F, Hesamzadeh Hejazi SM, Abbas-Azimi R. 2013. Distribution and ecological consequences of ploidy variation in Artemisia sieberi in Iran. Acta Oecologica. 53(1):95–101.
- Kadereit JW, Jeffrey C, editors. 2007. Flowering plants. Eudicots. Asterales. In: KKubitzki, editor. The families and genera of vascular plants. Vol. VIII. Berlin: Springer. p. 131–139.
- Kawatani T, Ohno T. 1964. Chromosome numbers in Artemisia. Bull Natl Inst Hygien Sci. 82:183–193.
- Khaldi S, Hidalgo O, Garnatje T, El Gazzah M. 2014. Karyological and genome size insights into cardoon (Cynara cardunculus L., Asteraceae) in Tunisia. Caryologia. 67(1):57–62.
- Khatoon S, Ali SI. 1993. Chromosome atlas of the angiosperms of Pakistan. Karachi: Department of Botany, University of Karachi.
- Kreitschitz A, Vallès J. 2003. New or rare data on chromosome numbers in several taxa of the genus Artemisia (Asteraceae) in Poland. Folia Geobot. 38(3):333–343.
- Kurşat M. 2010. The taxonomic revision of Artemisia L. (Asteraceae) genus growing in Turkey [PhD thesis]. [Elazığ (Turkey)]: Fırat University, Science.
- Kurşat M, Civelek Ş, Türkoğlu İ, Tabur S. 2011. Artemisia sieberi Bess. subsp. sieberi: a new record for Turkey and a delete record for Turkey Artemisia herba-alba Asso. (Asteraceae). Pak J Bot. 43(4):1819–1821.
- Kurşat M, Türkoğlu İ, Civelek Ş, Tabur S. 2011. A new subspecies record for the flora of Turkey: Artemisia santonicum L. subsp. patens (Neilr.) K.M.Perss. (Asteraceae). Turk J Bot. 35(1):89.
- Kuzmanov BA, Georgieva S. 1983. IOPB Chromosome number reports. LXXXI. 32(4):663–669.
- Levan A, Fredga K, Standberg AA. 1964. Nomenclature for centromeric position on chromosomes. Hereditas. 52(2):201–220.
- Ling YR. 1991a. The Old World Seriphidium (Compositae). Bull Bot Res Harbin. 11(4):1–40.
- Ling YR. 1991b. The Old World Artemisia (Compositae). Bull Bot Res Harbin. 12(1):1–10.
- Ling YR. 1995a. The New World Artemisia L. In: Hind DJN, Jeffrey C, Pope GV, editors. Advances in Compositae systematics. Kew: Royal Botanic Gardens. p. 255–281.
- Ling YR. 1995b. The Old World Seriphidium (Besser) Fourr. In: Hind DJN, Jeffrey C, Pope GV, editors. Advances in Compositae systematics. Kew: Royal Botanic Gardens. p. 283–291.
- Majovsky J, Murin A, Ferakova M, Hindakova T, Schwarzola T, Uhrikova A, Vachova M, Zaborsky J. 1987. Karyotaxonomicky prehl’ad flory Slovenska [Karyotaxonomical an overview on the flora of Slovenkia]. Bratislava: Veda vydavotel’stvo Slovenskej Academia Vied.
- Martinoli G, Ogliotti P. 1970. Ricerche citotassonomiche in Artemisia vulgaris L. ed Artemisia verlotorum Lamotte. G Bot Ital. 104(5):373–387.
- Matoba H, Uchiyama H. 2009. Physical mapping of 5S rDNA, 18S rDNA and telomere sequences in three species of the genus Artemisia (Asteraceae) with distinct basic chromosome numbers. Cytologia. 74(2):115–123.
- McArthur ED, Pope CL, Freeman DC. 1981. Chromosomal studies of subgenus Tridentatae of Artemisia: evidence for autopolyploidy. Am J Bot. 68(5):589–605.
- Meng Y, Nie ZL, Sun H, Yang YP. 2012. Chromosome numbers and polyploidy in Leontopodium (Asteraceae: Gnaphalieae) from the Qinghai-Tibet Plateau of SW China. Caryologia. 65(2):87–93.
- Moore DM. 1982. Flora Europaea, check-list and chromosome atlas. London: Cambridge University Press; p. 1–423.
- Mousavi SH, Hassandokht MR, Choukan R, Sepahvand N, Khosrowchali M. 2013. Cytological study of chromosome and genome composition of Iranian lettuce (Lactuca sativa L.) accessions. Eur. J Exp Biol. 3(1):303–311.
- Mozaffarian V. 2008. Compositae: Anthemideae & Echinopeae. In: Assadi M, Maassoumi AA, Mozaffarian V, editors. Flora of Iran, No 59. Tehran: Research Institute of Forests and Rangelands. p. 443.
- Naseri HR, Azarnivand H, Jafari M. 2009. Chromosomal evolution in some Iranian Artemisia L. using numerical analysis of karyotypes. Cytologia. 74(1):55–64.
- Olanj N, Sonboli A, Riahi H, Osaloo SK. 2013. Karyomorphological study of nine Tanacetum taxa (Asteraceae, Anthemideae) from Iran. Caryologia. 66(4):321–332.
- Ouyahya A, Viano J. 1988. Recherches cytogénétiques sur le genre Artemisia L. au Maroc. Bol Soc Brot. 1(2):105–124.
- Pellicer J, Garcia S, Canela MA, Garnatje T, Korobkov AA, Twibell JD, Vallès J. 2010. Genome size dynamics in Artemisia L. (Asteraceae): following the track of polyploidy. Plant Biol. 12(5):820–830.
- Pellicer J, Garcia S, Garnatje T, Dariimaa S, Korobkov AA, Vallès J. 2007. Chromosome numbers in some Artemisia (Asteraceae, Anthemideae) species and genome size variation in its subgenus Dracunculus: Karyological, systematic and phylogenetic implications. Chromosome Bot. 2(1):45–53.
- Pellicer J, Garcia S, Garnatje T, Hidalgo A, Korobkov AA, Dariimaa S, Vallès J. 2007. Chromosome counts in Asian Artemisia L. (Asteraceae) species: from diploids to the first report of the highest polyploidi in the genus. Bot J Linn Soc. 153(3):301–310.
- Persson K. 1974. Biosystematic studies in the Artemisia maritime complex in Europe. Opera Bot (Lundensi). 35:1–188.
- Podlech D. 1986. Artemisia. In: Rechinger K, editor. Flora Iranica. Graz (Austria): Academische Druck-u. p. 158–223.
- Probatova NS, Korobkov AA, Gnutikov AA, Rudyka EG, Kotseuba VV, Seledets VP. 2010. IAPT/IOPB chromosome data 10. Taxon. 59(6):1934–1938.
- Rabiei M, Jalili A, Ghamari ZA. 2003. Studying on ploidy level of five Artemisia species from the north of Iran. Iran J Rangeland Forest Plant Breed Genet Res. 11(4):429–441.
- Romero ZC. 1986. A new method for estimating karyotype asymmetry. Taxon. 35(3):526–530.
- Saedi K, Jalili A, Azamivand H, Ghamari Zare A. 2006. Karyotypic studies of Artemisia L. species in west Azerbaijan province, Iran. Pajouhesh Sazandegi. 67:2–10.
- Sánchez-Jiménez I, Pellicer J, Hidalgo O, Garcia S, Garnatje T, Vallès J. 2009. Chromosome numbers in three Asteraceae tribes from Inner Mongolia (China), with genome size data for Cardueae. Folia Geobot. 44(3):307–322.
- Semple JC, Watanabe K. 2009. A review of chromosome numbers in the Asteraceae with hypotheses on chromosome bas number evolution. In: Funk VA, Susanna A, Stuessy T, Bayer R, editors. Systematics, Evolution and Biogeography of the Compositae. Vienna: International Association for Plant Taxonomy; p. 21–32.
- Sharma PC, Gupta PK. 1982. Karyotypes in some pulse crops. Nucleus. 25:181–185.
- Shishkin BK, Bobrov EG, editors. 1995. Flora of the USSR. vol XXVI. Dehra Dun: Bishen Singh, Mahendra Pal Singh and Koeltz Scientific Books.
- Solbring OT. 1977. Chromosomal cytology and evolution in the family Compositae. In: Heywood VH, Harborne JB, Turner BL, editors. The biology and chemistry of the Compositae. vol 1. London: Academic Press; p. 267–281.
- Soltis PS, Soltis DE. 2000. The role of genetic and genomic attributes in the success of polyploids. Proc Natl Acad Sci USA. 97(13):7051–7057.
- Stace CA. 2000. Cytology and cytogenetics as a fundamental taxonomic resource for the 20th and 21st centuries. Taxon. 49(3):451–477.
- Stebbins GL. 1971. Chromosomal evolution in higher plants. London: Arnold.
- Stuessy TF. 2009. Plant taxonomy. The systematic evolution of comparative data. 2nd ed. New York: Columbia University Press.
- Tabur S, Civelek Ş, Öney S, Yılmaz ŞB, Kurşat M. 2011. Chromosome numbers and karyotypes of some taxa of genus Artemisia (Asteraceae, Anthemideae) subgenus Dracunculus (Bess.) Rydb. Caryologia. 64(3):335–342.
- Tabur S, Civelek Ş, Öney S, Yılmaz-Ergün ŞB, Kurşat M, Türkoğlu İ. 2012. Chromosome counts and karyomorphology of some species of Artemisia (Asteraceae) from Turkey. Turk J Bot. 36(3):235–246.
- Torrell M, Garcia-Jacas N, Susana A, Vallès J. 1999. Infrageneric phylogeny of the genus Artemisia L. (Asteraceae, Anthemideae) based on nucleotide sequences of nuclear ribosomal DNA internal transcribed spacers (ITS). Taxon. 48(4):721–736.
- Tutin TG, Persson KM. 1976. Artemisia L. In: Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine DH, Walters M, Webb D, editors. Flora Europaea. vol 4. Cambridge: Cambridge University Press; p. 178–186.
- Vallès J. 1987. Aportación al conocimiento citotaxonómico de ocho táxones Ibéricos del género Artemisia L. (Asteraceae, Anthemideae). An Jard Bot Madrid. 44(1):79–96.
- Vallès J, Garcia S, Hidalgo O, Martín J, Pellicer J, Sanz M, Garnatje T. 2011. Biology, genome evolution, biotechnological issues, and research including applied perspectives in Artemisia (Asteraceae). Adv Bot Res. 60(7):349–419.
- Vallès J, Garnatje T, Garcia S, Sanz M, Korobkov AA. 2005. Chromosome numbers in the tribes Anthemideae and Inuleae (Asteraceae). Bot J Linn Soc. 148(1):77–85.
- Vallès J, McArthur ED. 2001. Artemisia systematics and phylogeny: cytogenetic and molecular insights. Ogden, Utah: US Department of Agriculture Forest Service, Rocky Mountain Research Station; p. 67–74.
- Vallès J, Torrell M, Garcia-Jacas N. 2001. New or rare chromosome counts in Artemisia L. (Asteraceae, Anthemideae) and related genera from Kazakhstan. Bot J Linn Soc. 137(4):399–407.
- Vallès J, Torrell M, Garcia-Jacas N, Kapustina LA. 2001. New or rare chromosome counts in the genera Artemisia L. and Mausolea Bunge (Asteraceae, Anthemideae) from Uzbekistan. Bot J Linn Soc. 135(4):391–400.
- Vallès J, Torrell M, Garnatje T, Garcia-Jacas N, Vilatersana R, Susanna A. 2003. The genus Artemisia and its allies: phylogeny of the subtribe Artemisiinae (Asteraceae, Anthemideae) based on nucleotide sequences of nuclear ribosomal DNA internal transcribed spacers (ITS). Plant Biol. 5(3):274–284.
- Watanabe K. 2014. Index to chromosome numbers in Asteraceae; [updated 2014 Jan]. http://www.lib.kobe-u.ac.jp/infolib/meta_pub/G0000003asteraceae_e
- Watanabe K, Yahara T, Hashimoto G, Nagatani Y, Soejima A, Kawahara T, Nakazawa M. 2007. Chromosome numbers and karyotypes in Asteraceae. Ann M Bot Gard. 94(3):643–654.
- Watson LE, Bates PL, Evans TM, Unwin MM, Estes JR. 2002. Molecular phylogeny of subtribe Artemisiinae (Asteraceae), including Artemisia and its allied and segregate genera. BMC Evol Biol. 2(17):1–12.
