Abstract
Fritillaria stribrnyi Velen. (Liliaceae) is an endangered plant that naturally grows only in southern Bulgaria and Turkey in Europe. In this study, microsporogenesis, microgametogenesis, in vitro pollen germination and spermatogenesis were investigated in F. stribrnyi using a light microscope. Young flower buds and mature pollen grains, collected from natural habitats (in Edirne, Turkey), were used as materials. Microsporogenesis was investigated by the usual acetic-orcein squash method. Microgametogenesis slides were prepared with the paraffin method. Pollen fertility was estimated by lactophenol-aniline blue staining. For pollen germination, pollen grains were collected by immediately dehiscing anthers and then incubating in basic germination medium at 25°C for 24 h. Generally, the phases of meiosis were regular, although there were a few abnormalities such as laggard chromosomes in meiosis I and asynchronization in phases of meiosis II. The meiotic division resulted in the formation of isobilateral types of tetrads. The first mitotic division in pollen was observed clearly, and generative and vegetative cells were formed regularly. The germination percentage of pollen grains was found to be 84%, and the lengths of tubes were measured as 1896.22 ± 760.50 μm. F. stribrnyi has an elegant flower, and, therefore, its cultivation is possible in the garden as an ornamental plant. This is the first embryological study of F. stribrnyi, and the results of this study of the reproduction biology of this endangered plant will be useful for in vitro, in situ and ex situ conservation and genetic studies.
Introduction
Liliaceae is one of the larger families of flowering plants, containing about 250 genera and approximately 3500 species. The family is represented by 35 genera and 461 species in the Flora of Turkey (Davis Citation1984; Davis et al. Citation1988; Güner et al. Citation2000). The family has genera that are ornamental plants such as Fritillaria, Lilium and Tulipa.
Fritillaria L. genus is represented in northern temperate regions by seven subgenera and 165 taxa. The genus has 35 species (41 taxa) in Turkey, 21 species of which are endemic (Tekşen and Aytaç Citation2011). It contains 25 taxa in Greece, six species in Bulgaria, and 22 taxa in Russia (Tomovic et al. Citation2007; Tekşen and Aytaç Citation2008). Many species of the genus, which are wild growing, are cultivated for their attractive flowers. Bulbs of some species have been exported from Turkey for over 100 years (Ulug et al. Citation2010). Turkey is very rich with respect to both the total number of species and endemic species of the genus because the Mediterranean region is a center of diversity for the genus Fritillaria (Tomovic et al. Citation2007).
Fritillaria stribrnyi Velen. grows in a limited region of southern Bulgaria and northwest Turkey. This species is distributed naturally only in European Turkey (Edirne, Kırklareli, Istanbul and Canakkale) (Rix Citation1984; Başak Citation1991). The conservation status of the species, according to the Turkish Red Data Book, is “Endangered” (EN) (Ekim et al. Citation2000) in Turkey, while it is “Critically Endangered” in Bulgaria, according to Petrova and Vladimirov (Citation2009).
Studies related to the Fritillaria genera that grow in Turkey are very limited. They are usually taxonomical, caryological, morphological and anatomical studies (Başak Citation1991; Bozbek Citation2007; Özler and Pehlivan Citation2007; Tekşen and Aytaç Citation2008; Tekşen et al. Citation2010; Tekşen and Aytaç Citation2011). Recently, micropropagations of F. imperialis and F. persica, which are economically important and ornamental species, were made via plant tissue culture methods (Dilik Citation2006; Ulug et al. Citation2010). There are a few taxonomical, caryological and palinological papers on F. stribrnyi (Başak Citation1991; Tekşen and Aytaç Citation2008; Tekşen et al. Citation2010). The species has 2n = 24 chromosomes (Başak Citation1991) and monosulcate pollen grains (Tekşen et al. Citation2010). As a result of the literature survey, embryological studies on Fritillaria tenella, F. persica, F. imperialis, F. messanensis, F. pudica, F. japonica, F. ussuriensis and F. liliacea species have been performed (Davis Citation1966; Noda Citation1975; Yu-fen and Jia-heng Citation1990). However, there are no data on the embryology of F. stribrnyi.
The species is represented by a low number of small populations in Turkey, and it is facing a very high risk of extinction caused by human pressures in the wild. The habitat of the species is generally under Paliurus spina-christi in meadows and pastures. Their habitats are rapidly declining due to the destruction of pastures. This work is part of an ongoing project on the reproduction biology and conservation biology of F. stribrnyi. The aim of the study is to provide a comprehensive description of the meiosis and pollen mitosis in anther loculi, in vitro pollen germination in liquid germination medium, and spermatogenesis (mitosis II) in germinated pollen tubes of F. stribrnyi, which is an endangered species.
Material and methods
Flower buds and pollen grains of Fritillaria stribrnyi were used as materials. Materials were collected from the natural habitat in pastures of Üyüklütatar village in Edirne Province (European Turkey).
Microsporogenesis
Flower buds of different sizes were taken from healthy plants at 10:00–10:30AM in February–April 2011, and they were immediately fixed in the field in 3:1 (v/v) ethanol:glacial acetic acid solution. The buds were incubated in fixative at room temperature for 24 h, and then they were transferred to 70% ethanol and stored in a refrigerator until use. For meiosis stages, anthers of different sizes were squashed in a drop of acetic orcein after hydrolysis in 1 N HCl for 10 min at 60°C. Prepared slides were stored in a freezer at –18°C. Cover slips were removed from frozen slides. To make permanent slides, both the slides and cover slips were placed first in ethyl alcohol and then in xylene prior to mounting with entellan.
Pollen mitosis I
The anthers were dissected from fixed flower buds and dehydrated by a gradually increasing ethyl alcohol series. They were then embedded in paraffin (58°C) after the process of paraffin infiltration in xylene (Jensen Citation1962). Paraffin-embedded samples were sectioned by a Leica rotary microtome (RM 2255) (Leica Biosystems, Nussloch, Germany) in a thickness of 10 μm, and sections were stained Alcian blue (pH 2.5) – Nuclear fast red (K.M. Lyons Lab/UCLA; https://www.mcdb.ucla.edu/Research/Lyons/Protocols_files/ Alcianblue_NFR_paraffin_lyonslab.PDF). The slides were dehydrated by a gradually increasing ethyl alcohol series and, then they were passed through an ethyl alcohol-xylene series and transferred to xylene prior to mounting with entellan.
Pollen viability
The pollen grains were collected from flowers soon after the dehiscence of anthers. For pollen viability tests, pollen grains were stained with a drop of lactophenol-aniline blue solution for 30 min at room temperature. Stained pollen grains were considered fertile, and unstained pollen grains were considered sterile. A total of 3000 pollen grains, both stained and unstained, were counted in 10 randomly chosen regions, and pollen viability was calculated.
In vitro pollen germination
Anthers of F. stribrnyi were collected one day before anthesis and were placed in Petri dishes at room temperature for 24 h. After a day, the pollen from the dehisced anthers was put in test tubes containing 1 ml of liquid germinating medium consisting of 10% sucrose, 0.01% H3BO3, 0.03% Ca(NO3)2.4H2O, 0.02% MgSO4.4H2O and 0.01% KNO3 for 24 h at 25°C. Samples were then fixed with an acid-alcohol mixture (1 glacial acetic acid: 3 ethyl alcohol) following 24 h inoculation. The germinating pollens were transferred to slides and stained with acetic-orcein or lactophenol-aniline blue. Slides were mounted in glycerin-gelatin to make permanent preparations (Jensen Citation1962). Measuring 30 randomly selected zones on slides for 3000 pollen grains determined the in vitro germination percentages. The length of pollen tubes was measured on in vitro germinated pollen grains. Pollen was considered germinated when the pollen tube was equal to or longer than the diameter of the pollen grains. Pollen tube lengths were measured using Image-Pro Plus, version 5.1 (Media Cybernetics Citation2005). For tube length measurements, normal pollen tubes on 30 randomly selected fields from 10 slides were considered. The experiment was repeated three times.
Spermatogenesis in germinated pollen tubes
The division of the generative cell in germinated pollen tubes was examined on slides stained with acetic-orcein. All observations were carried out using an Olympus BH2 light microscope (Tokyo, Japan), and images were captured by a ProgRes C12 digital camera (Jenoptik, Jena, Germany).
Results
F. stribrnyi is a rare, wild plant that has elegant flowers and is facing a very high risk of extinction in the wild. Microsporogenesis, microgametogenesis, pollen viability, in vitro pollen germination and spermatogenesis within the pollen tube are described in detail in the following sections.
Microsporogenesis and pollen mitosis I
The microspore mother cells (MMCs) (Figure a) undergo two consecutive meiotic divisions and form an isobilateral microspore tetrad. Each MMC passes through prophase I (leptotene, zygotene, pachytene, diplotene and diakinesis, shown respectively in Figure b–f), metaphase I (Figure g), anaphase I (Figure h) and telophase I (Figure i). The cytokinesis that occurs is the successive type, and the dyads are formed at the end of meiosis I (Figure j). Callose begins to accumulate around the microsporocytes from the leptotene stage. Meiosis II in each dyad via prophase II (Figure k), metaphase II (Figure l), anaphase II (Figure m) and telophase II (Figure n) results in isobilateral tetrads (Figure o). The phases of meiosis in F. stribrnyi are generally regular, but there are a few abnormalities in 3% of cases such as lagging chromosomes in metaphase I (Figure a) and telophase I (Figure b) and asynchrony in the stages of meiosis II (Figure c, d).
Figure 1. (Color online) Microsporogenesis in Fritillaria stribrnyi stained with acetic-orcein. (a) Microspore mother cells; (b) leptotene; (c) zygotene; (d) pachytene; (e) diplotene; (f) diakinesis; (g) metaphase I; (h) anaphase I; (i) telophase I; (j) dyad; (k) prophase II; (l) metaphase II; (m) anaphase II; (n) telophase II; (o) isobilateral tetrad. Scale bars = 20 μm.
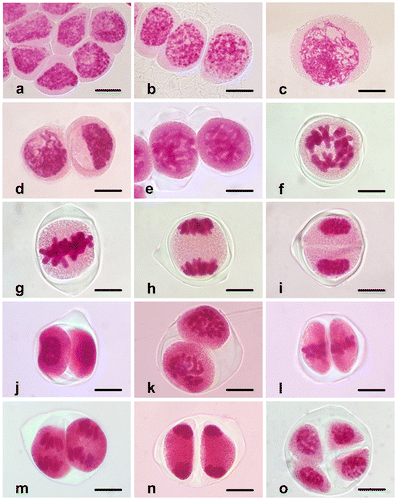
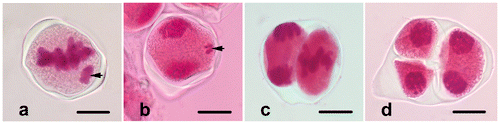
Figure 2. (Color online) Abnormalities in Fritillaria stribrnyi during microsporogenesis. (a) Lagging chromosome in metaphase I (arrow); (b) lagging chromosome in telophase I (arrow); (c, d) asynchrony in the stages of meiosis II. Scale bars = 20 μm.
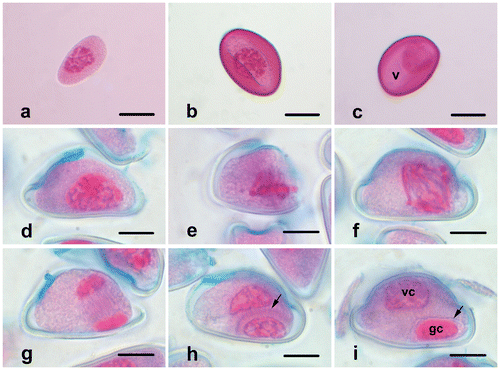
When the microspores just release from the tetrad, they have dense cytoplasm and centrally placed and irregularly shaped nuclei (Figure a). Later, the exine develops, and the germination aperture occurs on the uni-nucleate microspore (Figure b). Many small vacuoles appear in the cytoplasm. They fuse into a large, central vacuole and push the cytoplasm together with the nucleus against the microspore margin (Figure c). The nucleus is located in a position opposite the aperture. Then, young pollen grains undergo pollen mitosis I (prophase, Figure d), and the mitotic spindle forms asymmetrically at the metaphase stage (Figure e). Following the anaphase (Figure f) and the telophase (Figure g) stages, a curved phragmoplast forms between nuclei (Figure h), and the cytokinesis occurs asymmetrically. Finally, a generative cell with dense chromatin and smaller cytoplasm and a vegetative cell with loose chromatin and larger cytoplasm form (Figure i). Pollen grains are 2-celled when released from the anther.
Figure 3. (Color online) Microgametogenesis in Fritillaria stribrnyi.(a) Microspore just released from the tetrad; (b) microspore with developed exine and aperture; (c) vacuolated microspore; (d) prophase; (e) metaphase; (f) anaphase; (g) telophase; (h) phragmoplast formation at the end of telophase (arrow); (i) vegetative and generative cells in pollen grain (arrow shows the wall of generative cell) (gc, generative cell; v, vacuole; vc, vegetative cell). Scale bars = 20 μm.
Pollen viability and in vitro pollen germination
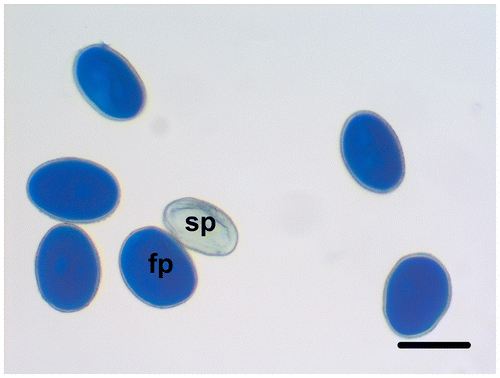
The fertile pollen grains of F. stribrnyi are well-stained with lactophenol aniline blue solution, while the sterile pollen grains are shriveled and empty, and they are not stained (Figure ). The percentage of fertile pollen grains is 92.72%.
Figure 4. (Color online) Fertile and sterile pollen grains of Fritillaria stribrnyi stained with lactophenol-aniline blue solution (fp, fertile pollen; sp, sterile pollen). Scale bar = 50 μm.
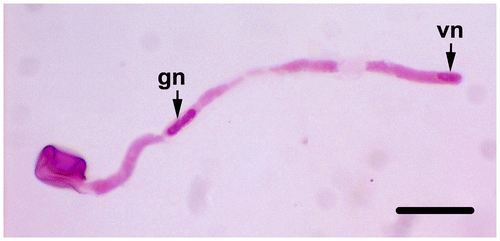
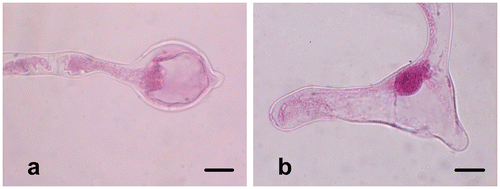
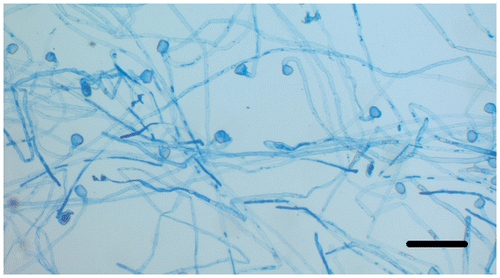
In vitro pollen germination observations are performed on germinated pollen grains that are stained with aceto-orcein or lactophenol-aniline blue following incubation at 25°C for 24 h. Pollen grains begin to germinate rapidly after contact with the liquid germination medium. After 10 min, the pollen tube is over twice the size of the pollen grain in equatorial diameter. The germination rate of pollen grains is calculated as 84%, and the pollen tube length is measured as 1896.22 ± 760.50 μm after 24 h incubation (Figure ). The vegetative nucleus enters first into the pollen tube and then the generative nucleus enters into the observed pollen tubes (Figure ). Pollen tubes are generally regular, but some pollen tubes produce swollen or branched tube tips (Figure b). The rate of these abnormalities is 4%.
Figure 5. (Color online) Pollen tubes germinated at 25°C for 24 h and stained with lactophenol-aniline blue solution. Scale bar = 200 μm.
Spermatogenesis in germinated pollen tubes
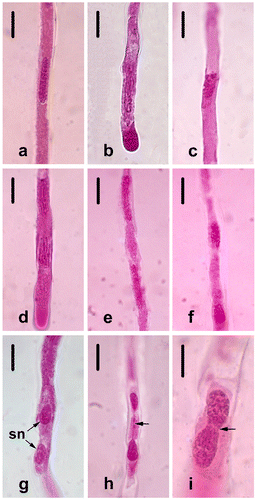
Spermatogenesis (pollen mitosis II) always occurs in pollen tubes after tube formation in in vitro germination medium. While the generative cell in bicellular mature pollen grains is ellipsoid-shaped (Figure i), it is long and cylindrical-shaped with blunt ends in the pollen tube (Figure ). In the pollen tubes, which are fixed and stained after 24 h, all the stages of mitosis are easily observed. At prophase, the chromatin begins to condense in the nucleus (Figure a), then the nuclear envelope disappears and the chromosomes lie in the generative cell cytoplasm (Figure b). At metaphase, a typical equatorial plate forms, and the chromosomes arrange at the plate (Figure c). During anaphase, the sister chromatids regularly separate and move toward opposite poles as individual chromosomes in the tube (Figure d). At telophase, the chromosomes begin to uncoil, and the nuclear envelope reforms around each chromosome group (Figure f). Eventually, two sperm nuclei occur inside the pollen tube (Figure g). Although spermatogenesis is generally regular, chromosome bridges of different thicknesses are seen in some tubes at telophase (1%) (Figure i).
Figure 8. (Color online) Spermatogenesis of Fritillaria stribrnyi in in vitro germinated pollen tube. (a) Prophase; (b) prometaphase; (c) metaphase; (d) anaphase; (e) telophase; (f) the end of telophase; (g) two sperm nuclei; (h) telophase bridge (arrow); (i) telophase bridge (arrow) Scale bars: (a–h) 20 μm; (i) 10 μm.
Discussion
This study reports microsporogenesis, pollen mitosis I, pollen viability, in vitro pollen germination and spermatogenesis within pollen tubes of F. stribrnyi, which is facing a very high risk of extinction caused by human pressures in the wild. For successful sexual reproduction in plants, microsporogenesis, microgametogenesis and pollen germination is very important. Therefore, these events have been at the center of interest for botanists.
The phases of meiosis in F. stribrnyi are generally regular, but there are a few abnormalities in 3% of cases such as the lagging chromosomes in the stages of meiosis I and asynchrony in the stages of meiosis II. Similar abnormalities are seen in Gagea stipitata (Liliaceae) (Koul et al. Citation1976), Gagea lutea (Bohdanowicz et al. Citation2005), Tulipa clusiana (Liliaceae) (Wafai and Koul Citation1982), Bellevalia edirnensis (Hyacinthaceae) (Dane Citation2006), Leucojum aestivum (Amaryllidaceae) (Ekici and Dane Citation2012) and Allium senescens (Liliaceae) (Zhang et al. Citation2012). These abnormalities can be caused by genetic or environmental factors. Bohdanowicz et al. (Citation2005) report that the dividing microsporocytes of Gagea lutea are affected by cold treatment (4°C) and drying in a laboratory, and these conditions increase abnormalities in the stages of microsporogenesis as compared to cold and drying in natural conditions. Shiva Kameshwari and Muniyamma (Citation2001) explain that there are more meiotic disturbances of the tetraploid species Chlorophytum elatum (2n = 28) (Liliaceae) than the diploid species C. heynei and C. laxum (2n = 14). Cytokinesis of meiosis in F. stribrnyi is successive, and the tetrads show isobilateral (or tetragonal) arrangement such as in most of the monocotyledons and the other Liliaceae members (Davis Citation1966; Yu-fen and Jia-heng Citation1990; Furness and Rudall Citation1999). However, tetrahedral tetrads together with isobilateral tetrads are reported in Tulipa clusiana (Wafai and Koul Citation1982) and Gagea stipitata (Koul et al. Citation1976). In three species of Chlorophtum (Liliaceae) – C. elatum, C. heynei and C. laxum – only isobilateral tetrads were seen by Shiva Kameshwari and Muniyamma (Citation2001).
Asymmetric cell division, which is dependent on the formation of a typical hemispherical phragmoplast, is very important for normal differentiation of the vegetative and generative cells in pollen mitosis I. The free microspores released from tetrads in F. stribrnyi divide asymmetrically to give rise to a large vegetative cell and a small generative cell. Before the division, the cytoplasm becomes vacuolated, and the microspore nucleus migrates towards the edge of the pollen. According to Ekici and Dane (Citation2004), this region, to which the nucleus migrates during pollen mitosis, determines the location of generative cells and is controlled genetically. These events are similarly seen in the other monocotyledones such as Tulipa clusiana (Liliaceae) (Wafai and Koul Citation1982), Oryza sativa (Poaceae) (Dane and Meric Citation2005), Bellevalia edirnensis (Hyacinthaceae) (Dane Citation1999), Agapanthus praecox (Agapanthaceae) (Zhang et al. Citation2010) and Leucojum aestivum (Amaryllidaceae) (Ekici and Dane Citation2012).
The disturbances in the course of microsporogenesis in F. stribrnyi are few, and thus the pollen fertility rate is high (92.72%). Li et al. (Citation2003) suggest that the abnormalities in meiosis are the major cause of abortive pollen in Lilium davidii var. unicolor (Liliaceae). As in most Liliaceae members (Davis Citation1966), including Fritillaria ussuriensis (Yu-fen and Jia-heng Citation1990), mature pollen grains are two-celled when released from the anther of F. stribrnyi. In Allium senescens (Liliaceae), a few three-celled pollen grains are found along with the usual two-celled, as observed by Zhang et al. (Citation2012).
The pollen grains of F. stribrnyi begin to germinate rapidly in liquid medium. The germination rate is very high at the end of 24 hours (84%). It is has been shown that the content of the germination medium is very important for F. stribrnyi. There are many studies related to in vitro pollen germination of Liliaceae members in solid or liquid media. The germination rate in fertile genotypes of Allium sativum is about 80% in solid medium containing 15% sucrose (Shemesh Mayer et al. Citation2013). The pollen germination rate of Ornithogalum caudatum (Liliaceae) is the greatest in 10% sucrose, and no germination is observed in 30% sucrose concentration (Goss Citation1962). The in vitro germination rates of Asparagus species (Liliaceae) is the greatest in 10% sucrose concentration for diploid A. racemosus and 30% for tetraploid A. densiflorus (Dhoran et al. Citation2011). Dhoran et al. (Citation2011) report that the genotype has an important role in in vitro pollen germination.
The average tube length is 1896.22 ± 760.50 μm after 24 h incubation. The pollen tubes of F. stribrnyi can grow about 79 μm per hour within 24 h. Aksoy et al. (Citation2013) report that the average pollen tube length in Amsonia orientalis is 1678 μm after 12 h in the germination medium. The tube length of germinating pollen is measured as approximately 95.25 ± 38 μm in Hypericum perforatum and 165.92 ± 53 μm in Hypericum rumeliacum 4 h after inoculation (Arda et al. Citation2006).
The spermatogenesis of F. stribrnyi is easily observed in germinated pollen tubes after 24 hours. At the end of prophase, the nuclear membrane disappears, and the chromosomes are arranged in metaphase plate. Anaphase and telophase stages are generally regular, although there are a few chromosome bridges (1%). Two sperm nuclei that are surrounded by a little cytoplasm occur at the end of telophase. There are many papers on generative cell division of the Liliaceae members, such as some Lilium (Cooper Citation1936) and Tulipa species (Upcott Citation1936; Johnston Citation1941), Gagea lutea (Zhang et al. Citation1995), Hyacinthus orientalis (Del Casino et al. Citation1992), Ornithogalum virens (Banas et al. Citation1996) and Convallaria majalis (Del Casino et al. Citation1999). Liliaceae members exhibit different properties related to the formation of the metaphase plate during the division of the generative cell. Lilium (Cooper Citation1936) and Tulipa species (Upcott Citation1936; Johnston Citation1941), Gagea lutea (Zhang et al. Citation1995) and Ornithogalum virens (Banas et al. Citation1996) have a distinct metaphase plate, while in Hyacinthus orientalis (Del Casino et al. Citation1992) and Convallaria majalis (Del Casino et al., Citation1999), a typical metaphase plate seems lacking. For Fritillaria genus, this paper is the first report on generative cell division in growing pollen tubes.
The present study aims to explain the reproduction biology of F. stribrnyi, an endangered species which is facing a very high risk of extinction caused by human pressures in the wild, and the results of this study provide useful data for further in vitro, in situ and ex situ conservation programs for this species.
Acknowledgment
This study was supported by The Scientific Research Fund of Trakya University, Turkey [Project No: TUBAP – 2009 / 100].
References
- Aksoy Ö, Kızılırmak S, Akdeniz GB. 2013. Investigation of mitosis, microsporogenesis and pollen germination in the critically endangered plant Amsonia orientalis (Apocynaceae). Caryologia. 66(3):282–288.
- Arda H, Meric C, Unal S. 2006. In vitro pollen germination in Hypericum perforatum L. and Hypericum rumeliacum Boiss. Acta Biol Hun. 57(1):97–103.
- Banas M, Tirlapur UK, Charzynska M, Cresti M. 1996. Some events of mitosis and cytokinesis in the generative cell of Ornithogalum virens L. Planta. 199(2):202–208.
- Başak N. 1991. The genus Fritillaria (Liliaceae) in European Turkey. Bot Chron. 10:841–844.
- Bohdanowicz J, Szczuka E, Swierczynska J, Sobieska J, Koscinska-Pajak M. 2005. Distribution of microtubules during regular and disturbed microsporogenesis and pollen grain development in Gagea lutea (L.) Ker.-Gaw. Acta Biol Cracov Ser Bot. 47(2):89–96.
- Bozbek H. 2007. Some anatomical and morphological studies on several Fritillaria taxa grown in Kütahya and Eskişehir [Master thesis]. Kütahya: Dumlupınar University.
- Cooper DC. 1936. Development of the male gametes of Lilium. Bot Gaz. 98(1):169–177.
- Dane F. 1999. A study of pollen mitosis and pollen morphology in hexaploid (2n = 24) Bellevalia edirnensis Özhatay & Mathew. Turk J Biol. 23(3):357–368.
- Dane F. 2006. Cytological and histological studies on reproductive system of hexaploid Bellevalia edirnensis Özhatay & Mathew (Hyacinthaceae). Acta Biol Hun. 57(3):339–354.
- Dane F, Meric C. 2005. Cytological and embryological studies of anther in rice (Oryza sativa) cv. ‘Rocca’. Acta Bot Hung. 47(3–4):257–272.
- Davis GL. 1966. Systematic embryology of angiosperms. New York, NY: John Wiley.
- Davis PH (ed). 1984. Flora of Turkey and the East Aegean Island. Vol. 8, pp. 67–358. Edinburgh: Edinburgh University Press.
- Davis PH, Mill RR, Tan K, editors. 1988. Flora of Turkey and the East Aegean Island. Vol. 10, pp. 221–226. Edinburgh: Edinburgh University Press.
- Del Casino C, Bohdanowicz J, Lewandowska B, Cresti M. 1999. The microtubule organization during generative-cell division in Convallaria majalis. Protoplasma. 207(3–4):147–153.
- Del Casino C, Tiezzi A, Wagner VT, Cresti M. 1992. The organization of the cytoskeleton in the generative cell and sperms of Hyacinthus orientalis. Protoplasma. 168(1–2):41–50.
- Dhoran VS, Nathar VN, Gudadhe SP. 2011. Pollen viability and in-vitro germination in diploid and tetraploid Asparagus L. Int J Curr Res. 3(6):74–77.
- Dilik M. 2006. Micropropagation of crown impetial (Fritillaria imperialis L.) and (F. persica L.) cv. Adıyaman [Master thesis]. Ankara: Ankara University.
- Ekici N, Dane F. 2004. Polarity during sporogenesis and gametogenesis in plants. Biologia, Bratislava. 59(6):687–696.
- Ekici N, Dane F. 2012. Microsporogenesis, pollen mitosis and in vitro pollen tube growth in Leucojum aestivum (Amaryllidaceae). In: Mworia JK, editor. Botany. InTech: Rijeka, Croatia; p. 173–190.
- Ekim T, Koyuncu M, Vural M, Duman H, Aytaç Z, Adıgüzel N. 2000. Red data book of Turkish plants (Pteridophyta and Spermatophyta). Turkish Association for the Conservation of Nature–Van Centennial University. Ankara: Barıscan Ofset. [ In Turkish].
- Furness CA, Rudall PJ. 1999. Microsporogenesis in monocotyledons. Ann Bot. 84(4):475–499.
- Goss JA. 1962. In vitro germination of Ornithogalum caudatum pollen. Trans Kans Acad Sci. 65(3):310–317.
- Güner A, Özhatay N, Ekim T, Başer KHC, editors. 2000. Flora of Turkey and the east Aegean Island. vol 11. Edinburgh: Edinburgh University Press; p. 222–265.
- Jensen WA. 1962. Botanical histochemistry – principles and practice. San Francisco and London: W.H. Freeman and Company.
- Johnston GW. 1941. Cytological studies of male gamete formation in certain Angiosperms. Amer J Bot. 28(4):306–319.
- Koul AK, Wafai BA, Wakhlu AK. 1976. Studies on the genus Gagea. III. Sporogenesis, early embryogeny and endosperm development in hexaploid Gagea stipitata. Phytomorphology. 26(3):255–263.
- Li X, Chen LM, Du J, Liang WF, Xing HT. 2003. Observations on abnormal meiosis of pollen mother cells in Lilium davidii var. unicolor. Acta Bot Boreal-Occid Sin. 23(9):1796–1799.
- Media Cybernetics. 2005. Image-Pro Plus, version 5.1 for Windows. Silver Spring, MD: Media Cybernetics.
- Noda S. 1975. Achiasmate meiosis in Fritillaria japonica group. I. Different modes of bivalent formation in the two sex mother cells. Heredity. 34(3):373–380.
- Özler H, Pehlivan S. 2007. Comparison of pollen morphological structures of some taxa belonging to Asparagus L. and Fritillaria L. (Liliaceae) from Turkey. Bangladesh J Bot. 36(2):111–120.
- Petrova A, Vladimirov V, editors. 2009. Red List of Bulgarian vascular plants. Phytol Balcan. 15(1):63–94.
- Rix EM. 1984. Fritillaria L. In: Davis PH, editor. Flora of Turkey and the East Aegean Islands. vol 8. Edinburgh: Edinburgh University Press; p. 284–302.
- Shemesh Mayer E, Winiarczyk K, Blaszczyk L, Kosmala A, Rabinowitch HD, Kamenetsky R. 2013. Male gametogenesis and sterility in garlic (Allium sativum L.): Barriers on the way to fertilization and seed production. Planta. 237(1):103–120.
- Shiva Kameshwari MN, Muniyamma M. 2001. Cytomorphological study in three species of the genus Chlorophytum Ker Gawl. (Liliaceae). Taiwania. 46(4):307–317.
- Tekşen M, Aytaç Z. 2008. Fritillaria mughlae (Liliaceae), a new species from Turkey. Ann Bot Fenn. 45(2):141–147.
- Tekşen M, Aytaç Z. 2011. The revision of the genus Fritillaria L. (Liliaceae) in the Mediterranean region (Turkey). Turk J Bot. 35(5):447–478.
- Tekşen M, Aytaç Z, Pınar NM. 2010. Pollen morphology of the genus Fritillaria L. (Liliaceae) in Turkey. Turk J Bot. 34(5):397–416.
- Tomovic G, Vukojicic S, Niketic M, Zlatkovic B, Stevanovic V. 2007. Fritillaria (Liliaceae) in Serbia: Distribution, habitats and some taxonomic notes. Phytol Balcan. 13(3):359–370.
- Ulug BV, Korkut AB, Sisman EE, Ozyavuz M. 2010. Research on propagation methods of Persian Lily bulbs (Fritillaria persica Linn.) with various vegetative techniques. Pak J Bot. 42(4):2785–2792.
- Upcott M. 1936. The mechanics of mitosis in the pollen tube of Tulipa. P Roy Soc Lond B Biol Sci. 121(822):207–220.
- Wafai BA, Koul AK. 1982. Analysis of breeding systems in Tulipa. II. Sporogenesis, gametogenesis and embryogeny in tetraploid T. clusiana. Phytomorphology. 32(4):289–301.
- Yu-fen L, Jia-heng S. 1990. The studies on embryology in Fritillaria ussuriensis Maxim. Acta Bot Sin. 32(7):499–504.
- Zhang H-Q, Bohdanowicz J, Pierson ES, Li Y-Q, Tiezzi A, Cresti M. 1995. Microtubular organization during asymmetrical division of the generative cell in Gagea lutea. J Plant Res. 108(3):269–276.
- Zhang D, Zhuo L-H, Shen X-H. 2010. Sporogenesis and gametogenesis in Agapanthus praecox Willd. ssp. orientalis (Leighton) Leighton and their systematic implications. Plant Syst Evol. 288(1–2):1–11.
- Zhang J, Liu XR, Zhang FX, Liu JX. 2012. Microsporogenesis and development of the male gametophyte in Allium senescens L. (Liliaceae) in China. Plant Syst Evol. 298(9):1619–1624.