Abstract
Detailed karyotype, genome size and RAPD marker analysis were employed to assess genetic diversity in Taro (Colocasia esculenta var antiquorom Schott.). Karyotype analysis revealed genotype specific chromosomal characteristics and structural alterations in chromosome with variations of ploidy from 2n = 2x = 28 (cv. Mothan, cv. Muktakeshi, cv. Sree Kiran, cv. Sree Pallavi, cv. Sunajhili) to 3n = 3x = 42 (cv. Banky, cv. DP-25, cv. Duradin, cv. H-3, cv. Telia) in the genome. Highly significant variations in the genomic length, volume and total form (TF) % were noted at variety level. Total genomic chromosome length varied from 46.96μm in cv. Sree Kiran to 100.49μm in cv. Duradin. Total genomic chromosome volume varied from 18.22μm3 in cv. Sunajhili to 38.22μm3 in cv. Duradin. Total form percentage was varied from 24.94% in cv. Sree Kiran to 39.04% in cv. H-3 confirming near metacentric to metacentric chromosomes in the karyotype. Significant variations in the 4C DNA content noted among the cultivars that ranged from 7.24 pg in cv. Sree Kiran to 18.24 pg in cv. Duradin; accordingly, genome size varied from ~7095 to 17875 Mbp. High genome size in all the triplod varieties with 3x = 42 chromosomes could be due to the presence of extra set of chromosomes in the genome or high amount of repetitive DNA. The variation in the genome size at the variety level might be attributed to loss or addition of highly repetitive sequences in the genome. Amplification of genomic DNA in 10 genotypes using Operon primers yielded 230 amplified DNA fragments, ranging in size from 200 to 2500bp out of which 79 bands were polymorphic. A total of 8 unique RAPD bands were observed among 10 taro genotypes that revealed primer wise polymorphism ranged from 16.66 to 47.36% with an average polymorphic percentage of 34.34%. Whereas, among the cultivars the polymorphic percentage varied from 3.70% between cv. DR-25 & cv. Duradin and cv. Telia & cv. H-3 to 41.94% between cv. Mothan & cv. Muktakeshi. Genetic similarity based on Jaccard’s coefficient varied from 0.54 to 0.96, indicating wide genetic variability among the varieties based on RAPD markers. Similarity measures and cluster analysis generally reflected the expected trends in relationships of diploid and triplod taro varieties. Dendrogram obtained from the genetic distances among the varieties could be useful for breeders to choose the diverse parents for breeding programme aimed at varietal improvement.
Introduction
Taro (Colocasia esculenta var. antiquorom Schott.), a members of the family Araceae is a traditional root crop of the tropics grown for its edible corms and leaves and is believed to be one of the earliest cultivated root crops in the world (Kuruvilla and Singh Citation1981). Food and Agriculture Organization (FAO) reported that taro production has doubled over the past decade (FAOSTAT Citation2000) and is now the fifth most-consumed root vegetable worldwide. Cultivated types are mostly diploid (2n = 2x = 28) with some triploids having 2n = 3x = 42 chromosomes. Two major taxonomical varieties are found i.e. dasheen type (Colocassia esculenta cv. esculentus) which has large central corm with sucker and stolons and the second is the eddoe type (Colocasia esculenta cv. antiquorom) which has small central corm and large number of small cornels (Purseglove Citation1972). Taro is the major food crop for Melanesian and Polynesian people, and is grown vegetatively, rarely from seed, for both domestic consumption and export. There are growing concerns over the narrow genetic base of taro cultivars in the Pacific islands, particularly with the outbreak of taro leaf blight (Phytophthera colocasiae) in Samoa and American Samoa in 1993–1994. This has led to the initiation of several breeding programs with the aim of broadening the genetic base of breeding populations, in addition to selection for resistance to taro leaf blight. Some of the studies using molecular techniques, specifically isozymes (Lebot and Aradhya Citation1991) and RAPDs (Irwin et al. Citation1998) were reported which have indicated that the Oceanian cultivars, particularly the Polynesian cultivars, showed very little diversity and have stressed the importance of broadening the base of existing breeding programs.
Molecular characterization of taro germplasm and early progeny selection with highly desirable traits for development of an efficient breeding program is important to speed the integration of new genetic material into elite germplasm. In addition, DNA marker for taro germplasm will contribute to knowledge of the genetic relationships between accessions of the wild and cultivated genepool, and hence facilitate the breeding of taro cultivars to satisfy market needs and to respond against diverse biotic (e.g., taro leaf blight) and abiotic (e.g., drought) challenges. Microsatellites have proven to be particularly useful for inbreeding crops with low levels of intraspecific diversity (Roder et al. Citation1995) and are increasingly useful for root crops that are frequently vegetatively propagated such as cassava (Chavarriaga-Aguirre et al. Citation1998; Roa et al. Citation2000), sweet potato (Buteler et al. Citation1999), and yam (Terauchi and Konuma Citation1994).
Cultivar identification and techniques to assess cultivar homogeneity are important for seed production, germplasm maintenance, crop certification and registration. The new cultivars obtained from the restricted gene pool are likely to be genetically quite similar and hence difficult to differentiate morphologically. Therefore, genetic identification of cultivars and varieties are useful in maintaining germplasm and planning of breeding programme for new cultivar production to chatter the demand of this crop to grow in different agroclimatic environment. Karyotype analysis provides valuable information related to the mechanisms of genome evolution. There are few cytological studies in taro because of large and relatively numerous chromosomes (Wilkinson Citation1994). Somatic chromosome study on Colocasia esculenta, were reported from time to time (Kurakubo Citation1940; Ito Citation1942; Rao Citation1947; Delay Citation1951; Sharma and Das Citation1954; Mookerjee Citation1955; Pfitzer Citation1957; Fukushima et al. Citation1962; Yen and Wheeler Citation1968; Vijaya Bai et al. Citation1971; Kawahara Citation1978; Kuruvilla et al. Citation1981; Coastes et al. Citation1988; Okada and Hambali Citation1989; Petersen Citation1989; Sreekumari and Mathew, Citation1991b, Citation1991c; Kokubugata and Konishi Citation1999), but cytological data are mainly associated with different populations of C. esculenta for its variation of chromosome numbers. Recently Yang et al. (Citation2003) also reported three diploid (2n = 28) species of Colocasia like C. gongii, C. gaoligongensis and C. gigantea from Yunnan, China. However, no detail information on karyotypes of Indian taro cultivers is available for breeding purpose.
Genome size is an important character of fundamental significance that provides useful data in many cytotaxonomic and evolution studies (Price Citation1976). It plays an important role in tolerance/ resistance to low temperature and in response to ozone depletion or to the effect of global warming (Bennett and Leitch Citation1995). DNA markers generated from randomly amplified polymorphic DNA (RAPD) is used to assess and characterize genetic variation among plant genotypes of interest at DNA level (Williams et al. Citation1990), which is not necessarily expressed as differences in phenotype. DNA polymorphism in Indian taro was reported earlier using microsatellite markers (Mace and Godwin Citation2002), AFLP (Quero-Garcia et al. Citation2004) and RAPD (Lakhanpaul et al. Citation2003) without any detailed chromosomal number, karyotype and DNA content analysis which are very basic and important information for breeding as well as molecular biological work in crop improvement programme. DNA content report is also very meager in taro. In the present investigation, an attempt has been made to utilize above mentioned techniques for assessment of genetic variation in ten draught resistant varieties of taro (Colocasia esculenta var. antiquorom) and subsequently interpret their phylogeny and affinities for identification of breeding partner in the crop improvement programme of this important minor tuber crop.
2. Materials and methods
Ten varieties of Colocasia esculenta var antiquorom Schott. advanced draught resistant breeding lines were obtained from the Central Tuber Crop Research Institute, Bhubaneswar India (Table ) and were grown in the experimental green house of Orissa University of Agriculture and Technology, Bhubaneswar.
Table 1. Somatic chromosome number, karyotypic parameters and 4C DNA content of ten varieties of C. esculenta var. antiquorom.
2.1. Karyotype analysis
For chromosome preparation, root tips from the sprouted tubers were pre-treated in saturated solution of pDB (para-dichlorobenzene) with aesculine for 3h at 18°C followed by overnight fixation in 1:3 acetic acid:ethanol. Chromosomes were stained in 2% aceto-orcine after cold hydrolysis in 5N HCl for 5 min. The root tips were then squashed in 45% propionic acid. Ten well scattered metaphase plates from each genotype were selected for karyotype analysis. The genomic chromosome length and volume of a karyotype were determined following the method of Das and Mallick (Citation1993). The total genomic chromosome length was ascertained by adding the length of haploid set of chromosomes in the karyotype and the total genomic chromosome volume of karyotype was calculated by applying the formula πr2h, where ‘r’ and ‘h’ represents the radius and length of the chromosome, respectively. The form % (F%) of individual chromosomes was calculated following the method of Levan et al. (Citation1964), and the total form percentage (TF%) was the average of the sum total of F% of a karyotype. The mean values of total genomic chromosome length and total genomic chromosome volume with standard error were calculated.
2.2. 4C nuclear DNA content and genome size
For Feulgen cytophotometric estimation of 4C DNA content, ten fixed root-tips from each genotypes were fixed in 1:3 acetic acid:ethanol for overnight in room temperature, hydrolysed in 1N HCl for 12 min at 60°C. Hydrolysed root tips were washed in distilled water and stained in Schiff’s reagent for 2h at 14°C. Each root-tip squash was prepared in 45% acetic acid. In situ nuclear DNA content was estimated from metaphase chromosomes using a Nikon Optiphot microscope fitted with a microspectrophotometer using monochromatic light at 550 nm following the method of Sharma and Sharma (Citation1980), with ten scorings made from each slide. In situ DNA content were obtained on the basis of optical density, which was converted to picograms (pg) using the 4C nuclear DNA values (67.1 pg) for Allium cepa cv. Deshi (Van’t Hof Citation1963) as a standard. The genome size of different genotypes was calculated from their 4C DNA values and according to their ploidy level. Genome size = (4C DNA value / ploidy level) pg × 980 Mbp = value in pg × 980 Mbp. To find out the significant differences in chromosome length, volume and genome size among different species, if any, analysis of variation (ANOVA) test (Sokal and Rohlf Citation1973) was performed.
2.3. Isolation of DNA
Genomic DNA was isolated from young expanding leaves using the method of (Saghai and Maroof et al. Citation1984). Cigars leaves (2g) were ground to fine powder in liquid nitrogen and suspended in 20 ml of CTAB (Cetryl trimethyl ammonium bromide) buffer containing 2% CTAB, 100 mM Tris-HCl, pH 8, 20 mM EDTA, 1.4 M NaCl, and 1% β-mercaptoethanol. The suspension was incubated at 60°C for 1h in a water bath. The DNA was extracted in chloroform:isoamyl alcohol (24:1) and centrifuged at 10000 × g for 20 min at 10°C. The aqueous phase was transferred to a new sterile 50ml tube and the DNA was precipitated with a double volume of chilled iso-propanol, hooked out and dried in vacuum concentrator after rinsing DNA in 70% ethanol. Dried DNA was dissolved in a minimum amount of T10E1 buffer (10 mM Tris-HCl, 1mM EDTA; pH 8.0). Isolated DNA was further purified by treating with RNAse at 37°C for 2h followed by chloroform:isoamyl (1:24): phenol (1:1), chloroform:isoamyl (1:24) and subsequently ethanol precipitation in the presence of 0.3M sodium acetate (pH 5.2). The DNA was spooled out, washed in 70% ethanol, air dried and dissolved in T10E1 buffer and the DNA concentration was estimated using Versaflour TM Fluorometer (Bio-Rad, USA) using Hoechst 33258 as the fluorimetric dye. The DNA was diluted to a final concentration of 25 ng μl−1 using T10E1 buffer, to use as template for RAPD analysis.
2.4. PCR amplification and gel electrophoresis
RAPD profiles were generated by using single decamer random oligonucleotide primers (Operon Technologies, Alameda, USA) in polymerase chain reaction (PCR) following the standard protocol of Williams et al. (Citation1990). Each reaction mixture (25μl) for PCR amplification, was prepared with 25ng genomic template DNA, 200μM each of dNTP, 25ng primer, 0.5 unit Taq DNA Polymerase (Bangalore Genei Pvt. Ltd., Bangalore, India) and 10 × PCR assay buffer (50 mM KCl, 10 mM Tris-HCl, 1.5 mM MgCl2, pH 9.0). The PCR reaction was carried out in a Gene Amp PCR 2400 thermal cycler (Perkin Elmer, USA) programmed for 45 cycles using 20 primers each of OP-A, OP-D and PO-N series. The first cycle was programmed for 5 min at 94°C for denaturation, 1 min at 37°C for primer annealing and 2 min at 72°C for DNA polymerisation. In the next 45 cycles the period of denaturation was maintained at 1 min while the primer annealing and DNA polymerization was same as in the first cycle. An additional cycle of 15 min at 72°C was used for primer extension. The amplified samples were stored at 4°C and separated by electrophoresis in 1.5% agarose gel in 1×TAE buffer for 3h at 55V. Gene Ruler 100bp DNA ladder plus (MBI Fermantas, Lithuania) was used as marker to determine the size of the amplicons. Amplified products were visualized by staining the gel with ethidium bromide and image was captured in an image analyser Gel Doc-G 700 (Bio-Rad, USA) for documentation and analysis. Only those amplification products that appeared consistently in three replications were scored for further analysis.
2.5. RAPD data analysis
In RAPD analysis, the presence or absence of the bands was taken into consideration and the difference in the intensity of the band was ignored. Index of diversity was calculated from the frequency of a RAPD bands within each genotypes. From RAPD data a binary matrix was obtained. The matrix was elaborated utilizing the multivariate analysis NTSYS-pc (Rohlf Citation1993). The binary matrix was transformed in a similarity matrix using the Jaccard’s coefficient. The cluster analysis was carried out using the UPGMA (Unweighted pair group mean average) method (Sneath and Sokal Citation1973).
3. Results
3.1. Chromosome characteristics and 4C DNA content
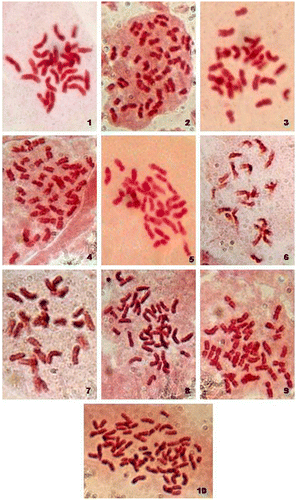
Detailed analysis of somatic chromosomes of ten varieties of C. esculenta var. antiquorom of the family Araceae showed somatic chromosome number 2n = 2x = 28 chromosome in cv. Mothan, cv. Muktakeshi, cv. Sree Kiran, cv. Sree Pallavi, cv. Sunajhili and 2n = 3x = 42 chromosome in cv. Banky, cv. DP-25, cv. Duradin, cv. H-3, cv. Telia (Figs. , Table ). On the basis of the size of the chromosome and the position of the constrictions, a number of chromosome types were found common with the genotypes studied though they differed from each other in the minute structural details of the karyotype. A general description of the representative types of chromosomes is given below.
Figures 1–10. (Color online) Somatic metaphase chromosomes of 10 cultivars of taro (C. esculenta var. antiquorom) (×1942). 1 = cv. Muktakeshi (2n = 28), 2 = cv. Banky (2n = 42), 3 = cv. Sree Kiran (2n = 28), 4 = cv. Telia (2n = 42), 5 = cv. Sree Pallavi (2n = 28), 6 = cv. Mothan (2n = 28), 7 = cv. Sunajhili (2n = 28), 8 = cv. H-3 (2n = 42), 9 = cv. DP-25 (2n = 42), 10 = cv. Duradin (2n = 42).
Type A: Chromosomes are large to medium sized with two constrictions in nearly median to median and nearly sub median to sub median in position respectively.
Type B: Large to medium sized chromosomes with one constrictions comprised with median to nearly median primary constriction.
Type C: Chromosomes are medium to small with sub median to nearly median primary constrictions.
Type D: Chromosomes are comparatively smaller to Type ‘C’ chromosome with nearly sub median to sub median primary constrictions.
Though all the four types of chromosomes were present only in cv. Banky out of the studied varieties whereas Types A, B and C chromosomes were present in rest of the varieties (Table ). The karyotype formula of all the genotypes revealed definite differences in the chromosome structure. Type A chromosomes were present in all the genotypes where as Type D were only present in cv. Banky. Furthermore, dose differences in Type A, B and C chromosomes in all the genotypes were found. Type C chromosomes were the most numerous in all the genotypes that varied from 14 in cv. Sree Kiran and cv. Sree Pallavi to 26 in cv. DP-25. The number of Type B chromosomes were not varied that much as compared to Type C; cv. Muktakesi, cv. Mothan and cv. Sunajhili possessed 10 numbers whereas cv. Banky, cv. Sree Kiran and cv. Sree Pallavi showed 12 numbers and cv. Telia, cv. H-3 and cv. Duradin had 18 numbers. The highest number of Type C chromosomes found in the genotype DP-25 a triploid. Detailed analysis of the somatic compliments and the different genomic characteristics showed genotype specific variations in chromosome structure (Table ). The total genomic chromosome length varied from 46.96μm in cv. Sree Kiran to 100.49μm in cv. Duradin and the total genomic chromosome volume varied from 18.22μm3 in cv. Sunajhili to 38.22μm3 in cv. Duradin. The centromeric index in the chromosomes of all the genotypes varied from 24.94% in cv. Sree Kiran to 38.472% in cv. Muktakeshi. ANOVA analysis showed significant variations in chromosome length, volume and TF% among the studied taro varieties.
4C DNA content varied from 7.24 p.g. in cv. Sree Kiran to 18.24 p.g. in cv. Duradin. In diploid varieties (2n = 28) the minimum nuclear DNA obtained 7.24 p.g. in cv. Sree Kiran and maximum 9.25 p.g. in cv. Muktakeshi whereas among triploid varieties (3n = 42) DNA content varied from 15.22 p.g. in cv. Banky to 18.24 p.g. in cv. Duradin. However, DNA content per chromosome ranged from 0.258 in cv. Sree Kiran to 0.434 in cv. Duradin. Accordingly, the calculated genome length also varied from 7095.2 Mbp to 17875.2 Mbp (Table ).
3.2. RAPD analysis
RAPD analysis of the studied 10 varieties of taro were carried out using 60 random decamer primers belong to 3 set viz. OP-A, OP-D, OP-N. Out of the total 60 primers used, 14 most informative ones were selected (Table ). Figs. and depicted the RAPD profiles of 10 varieties amplified by OPD-08 and OPN-11 respectively that showed distinct RAPD profile of each varieties. Each RAPD marker locus was expressed as two alleles: presence or absence of the band. A Total of 142 amplification products were obtained out of which 79 were polymorphic (69.91%) (Table ). Similarity index values calculated after combining the effects of two most useful primers ranged from 0.58 to 0.96 and the percentage of polymorphism among the genotypes varied from 3.70% between cv. Telia and cv. H-3 and cv. Duradin and cv. DP-25 to 41.94% in between cv. Muktakeshi and cv. Mothan (Table ). The amplified bands ranged in each primers varied from 300bp to 2500bp. RAPD profiles of 10 genotypes shared a number of common bands for all the primers. The most frequent monomorphic bands in the cultivars were at 500bp and 650bp amplified with OPC-07 and 900bp and 1000bp in OPC-18. The polymorphic band of 500bp, 700bp, 1200bp and 2200bp were present in all the genotypes but not in cv. Muktakeshi in OPC-07 primer. The polymorphic band of 800bp was present only in cv. Mothan, cv. Sunajhilli, cv. H-3 in OPC-07 primer discriminating other varieties. The polymorphic band of 1600bp in OPC-18 was present in all the varieties except cv. Muktakeshi.
Table 2. RAPD primers, their nucleotide sequence and number of amplicons generated from ten varieties of taro.
Figures 11a & 11b. RAPD amplification profiles of the 10 cultivars of taro using primer OPD-08 and OPN-11 respectively with marker DNA (M) Gene Ruler 100bp DNA ladder plus (MBI Fermentas, Lithuania). 1 = cv. Muktakeshi, 2 = cv. Banky, 3 = cv. Sree Kiran, 4 = cv. Telia, 5 = cv. Shree Pallavi, 6 = cv. Mothan, 7 = cv. Sunajhilli, 8 = H-3, 9 = DP-25, 10 = cv. Duradin.
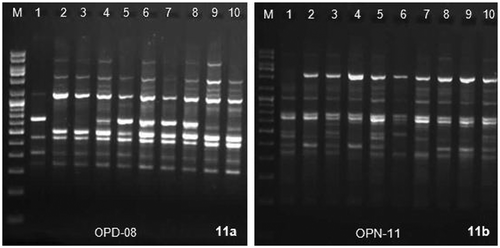
Table 3. DNA polymorphism (%) among ten varieties of C. esculenta var. antiquorom as revealed by RAPD markers.
3.3. Cluster analysis
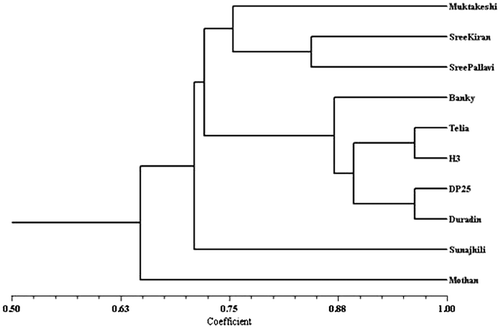
The dendrogram obtained on the basis of the genetic distances radically separated the 10 genotypes Cluster I having only cv. Mothan and Cluster II with rest of the cultivars. First cluster sub-cluster I with cv. Mothan and rest 8 cultivars out of them all the 5 triploid cultivars grouped together and 3 (cv. Muktakeshi, cv. Sree Kiran and c. Sree Pallavi) out of 5 diploid cultivars formed a group. It was revealed from the dendrogram that cv. DP-25 and cv. Duradin as well as cv. Telia and cv. H-3 having closure genetic affinity than the triploid cv. Banky (Fig. ).
4. Discussion
4.1. Karyotype, chromosome length, and genome size
Detailed karyotype analysis in 10 cultivars of C. esculenta var. antiquorom revealed some interesting facts at inter-varietal level. The chromosome number (2n = 2x = 28) was constant in cv. Muktakeshi, cv. Mothan, cv. Sree Kiran, cv. Sree Pallavi and cv. Sunajhili and (2n = 3x = 42) was constant in rest of the studied cultivars (Table ) having triploid set of chromosomes in the root tip cells. Type of chromosomes and the number of secondary constricted chromosomes varied significantly with the genotypes. A, B and C types of chromosomes were common in all the genotypes, with high variability in terms of chromosomes in each category. However, Type D chromosomes, very short sub-median type, was only present in a triploid cv. Banky (Table , Fig. ) Furthermore, in respect of karyotype formula, there were no differences between cv. Sree Kiran and cv. Sree Pallavi having 2n = 28 chromosomes. In contrast, the genotype chromosome length and volume varied significantly. Detailed karyotype analysis revealed the number of secondary constricted chromosomes i.e. Type A chromosomes were varied from 2 to 6. Median chromosomes (Type B) were comparatively less in number as compared to sub-median chromosomes (Type C) in both diploid whereas of median chromosomes in triploid varieties. Type B obtained in triploid varieties was 18 except cv. DP-25 (10 numbers) and cv. Banky (12 numbers) whereas in diploid varieties it was 10 in number except cv. Sree Kiran and cv. Sree Pallavi having 12 numbers. The variation of Type B and Type C chromosomes were not pronounced among diploid varieties cv. Muktakeshi, cv. Mothan and cv. Sunajhili as well as cv. Sree Kiran and cv. Sree Pallavi but a significant variation was observed among triploid varieties (Table ). Total F% analysis showed symmetric karyotype having median to nearly median chromosomes with a moderate fluctuation of F% values from 33.29% to 41.02% in cv. Sree Pallavi to cv. Banky respectively except cv. Sree Pallavi with 24.56%. The sifting of median chromosome of genome to highly sub-median chromosomes in cv. Sree Pallavi as compared to the other studied varieties might be due to the break and reunion of the more chromosomes in evolution for stabilization of these vegetatively propagated varieties. The gradual alterations and shifting of TF% values might be due to the chromosomal alteration in the genome. The structural alterations in the chromosome morphology as well as variations of secondary constricted chromosomes in the genotypes might be due to duplication of chromosomes or translocations between the chromosomes with or without secondary constrictions at a very early stage of evaluation (Das Citation2008).
Total chromosome length and volume differed markedly among the genotypes. Minute observations showed a proportional increase in chromosome length with an increase in chromosome volume. A correlation coefficient of 0.69 was found between the total chromosome length and total chromosome volume suggesting a high interdependence between them at the varietal level. These facts indicate the predetermined genetic control of chromosome coiling. Evidently, differences in chromosome length or chromosome volume were due to differential condensation and spiralization of the chromosome arms. In addition, the genotype specific compaction of DNA threads along with nucleosomes or the additional gene sequences with altered non-histone proteins in the chromosome played an important role in the chromosomal architecture of the genotypes (Chattopadhyay and Sharma Citation1990). Previous cytological studies on Colocasia indicated some confusion concerning the basic chromosome number of the genus, and some different chromosome numbers were estimated, such as 2n = 28, 36 and 42, and x = 7, 12 and 14 were suggested as the basic chromosome number of Colocasia by some previous researchers (Rao Citation1947; Dely Citation1951; Darlington and Waylie Citation1955; Coates et al. Citation1988; Okada et al. Citation1989). But, cultivated varieties of C. esculenta var. antiquorom possess the same chromosome number, 2n = 2x = 28 and 2n = 3x = 42 which is in accordance to chromosome behaviours in meiosis as suggested by Vijaya Bai et al. (Citation1971) and Okada et al. (Citation1989); the basic chromosome number of Colocasia is x = 14. The populations of C. esculenta with 2n = 42 chromosomes are triploid, 3x = 42 which is in accordance with Yang et al. (Citation2003) for 2n = 28. The fact that plants with 42 chromosomes are sterile is one of evidences of x = 12 were suggested as a basic chromosome number of Colocasia based on the observations of Rao (Citation1947) and Dely (Citation1951). However, none of the more recent studies on Colocasia have confirmed x = 12 as a base number. It therefore seems that, the plants observed with a base number of 12 were either misidentified as Colocasia species, or that the chromosome counts were inaccurate. Three species studied by Yang et al. (Citation2003) i.e. C. gongii, C. gigantean and C. esculenta were diploid with 2n = 2x = 28. C. esculenta is the only species in Colocasia with various chromosome number and various basic chromosome number. The varieties with chromosome number of 2n = 42 were triploid with a basic chromosome number of x = 14 but were not hexaploid with a basic chromosome number of x = 7. The chromosome number of varieties cultivated of C. esculenta may vary due to long history and the various conditions of cultivation. Differences in the numbers of median and sub-median chromosomes as well as satellite-chromosomes among species were reported by Yang et al. (Citation2003) in interspecific level as well as varietal level of C. esculenta which showed diversity not only in the chromosome number but also in karyotypes as reported earlier (Sreekumari and Mathew Citation1991a). So, karyotypes cannot be compared between C. gongii, C. gigantean and C. esculenta. It is very necessary to study other species in Colocasia for revealing phylogenetic relationships of whole genus. Chromosome number reported from root tip cells revealed that diploids (2n = 28) and triploids (2n = 42) occur in Indian taros in almost equal proportion. The frequency of the ploidy types showed clear difference in ploidy-wise distribution in the different zones of the country. Although both the types occur in all the regions, the diploids predominate in South India over the triploids while the triploids convincingly out-numbered the diploids in the north (Sreekumari and Mathew Citation1991a). Several factors are known to influence the frequency of polyploids in different eco-geographical regions. Polyploids in general have larger dimensions and greater adaptability which apparently enable them to thrive better in a wide range of higher latitudinal and altitudinal zones. As in the case of Indian taros, Zhang and Zhang (Citation1990) also observed a greater percentage of triploid forms in higher altitude regions of China. Initial screening of the germless accessions for tuber yield revealed the superiority of triploids compared to diploid accessions in several characters such as plant height, tillering habit, number and size of leaves, corm and cormel yield. The corm and cormel yield showed very promising and impressive increase in the higher ploidy types. This implies that for selecting high yielding types in taro, it is desirable to consider the triploids rather than diploids (Sreekumari and Thankamma Pillai Citation1993). The same was found to be true in another tuber crop viz. cassava which showed significant increase in tuber yield and starch content in the artificially produced triploids which might be due to triploidy per se (Sreekumari and Thankamma Pillai Citation1994).
4.2. Diversification in genome size
The genome size varied significantly from ~7095 Mbp in cv. Sree Kiran to ~17875 cv. Duridan. The triploid varieties had a much larger genome size as compared to diploid varieties which might be due to the extra set of chromosomes as well as large chromosome size as revealed in the karyotype. Since, per chromosome DNA content variation was not changed that much, the possibilities of atopolyploidy origin of these varieties might be not evidently strong enough through c-mitosis rather than allopolyploid origin. The variability in the genome size in different genotypes might be attributed to the loss or addition of many repeats in the genome through alterations in the micro- and macro-environment during evolution in the selection of new cultivars (Price et al. Citation1980). The correlation coefficient between total chromosome length and genome size showed significant correlation (r = 0.521). This clearly suggests that the genome size is positively correlated with the total chromosome length. Such variations are in agreement with the findings of other workers (Das Citation2008). The analysis of genome size at the cultivar level in repeated experiments revealed the stable genome size in each genotype. On the other hand the genome size differed significantly among the genotypes. Flavell et al. (Citation1997) reported that differences in genome size depend on the repetitive DNA amount. We agree that variability of genome size can be attributed to loss or addition of highly repetitive DNA sequences rather than the AT- or GC-rich sequences in a genome (Martel et al. Citation1997) which reached a certain level and got stabilized during micro-evolution and gradual selection.
4.3. RAPD analysis
The amplified pattern of DNA with Operon primers revealed genetic distance between Indian taro varieties. Frequency of polymorphic loci was estimated in various potato genotypes considering three criteria for primer selection (1) reproducibility (2) number of polymorphic loci per assay and (3) levels of polymorphism detected in a specific group of genotypes. Some of the analyzed taro varieties had a characteristic RAPD pattern consisting of 6 to 10 major fragments with their size ranging from 300bp to 2500bp. Similarity index values ranged from 0.58 to 0.96 with standard deviation of 0.12 indicating a wide genetic base of the taro germplasms used in the analysis. The percentage of polymorphism among the genotypes varied from 3.7% to 41.94% (Table ). As the polymorphisms were detected as the presence or absence of a particular band, RAPDs scored were dominant markers. Therefore, the genotypes heterozygous or dominant homozygous for a particular locus showed similar bands and thus didn’t show polymorphism. Thus, RAPD used here did not detect possible changes in allele frequencies except when the allele detected as the RAPD band was completely lost (Bamberg et al. Citation2001). Monomorphic bands of the same molecular weight present in all the genotypes at 500bp and 650bp amplified with OPC-07 and 900bp and 1000bp in OPC-18 could be the specific marker for C. esculenta var. antiquorom. The polymorphic band of 500bp, 700bp, 1200bp and 2200bp were present in all the genotypes but not in cv. Muktakeshi in OPC-07 primer. The polymorphic band of 800bp was present only in cv. Mothan, cv. Sunajhilli, cv. H-3 in OPC-07 primer discriminating other varieties. The polymorphic band of 1600bp in OPC-18 was present in all the varieties except cv. Muktakeshi. These specific markers obtained by RAPD amplification can be sequenced and specific primers can be designed to amplify only the band of interest that can then be used as cultivar specific markers, which could be potentially used in identification purposes. The genetic variability obtained in the Indian taro genotypes are also in accordance with the findings of Lakhanpaul et al. (Citation2003) using RAPD markers.
The dendrogram obtained on the basis of the genetic distances radically separated the 10 genotypes Cluster I having only cv. Mothan and Cluster II with rest of the cultivars. First cluster sub-cluster I with cv. Mothan and rest 8 cultivars out of them all the 5 triploid cultivars grouped together and 3 (cv. Muktakeshi, cv. Sree Kiran and c. Sree Pallavi) out of 5 diploid cultivars formed a group. It was revealed from the dendrogram that cv. DP-25 and cv. Duradin as well as cv. Telia and cv. H-3 having closure genetic affinity than the triploid cv. Banky. However, out of the five diploid studied sofar showed less genetic closeness (Fig. ). Although cv. Mothan and cv. Sunajhili had 2n = 28 chromosomes but they might be of different ancestral origin than the diploids cv. Muktakeshi, cv. Shree Kiran and cv. Shree Pallavi. This cluster analysis could be helpful in plant breeding to make informed decisions regarding selection of diverse parents from inter clusters for breeding programme in order to maximize the expression of heterosis for any desired character of agronomic importance.
Mace and Godwin (Citation2002) reported microsatellites from an enriched library of C. esculenta using the precloning enrichment technique of Edwards et al. (Citation1996), revealed polymorphism among the different accessions of C. esculenta from the Pacific island region and Southeast Asia that suggests the abundance of multiple copies of some microsatellites in the taro genome may be quite low in comparison with other species. The low number of alleles detected by each microsatellite marker, on average only 3.2/locus, could also suggest that there is only a low degree of SSR locus duplication in the taro genome, particularly in contrast to other re-cent studies on microsatellite diversity in cassava (Chavarriaga-Aguirre et al. Citation1998) and poplar (van der Schoot et al. Citation2000), which revealed on average 7.5 and 13 alleles/locus, respectively. The most abundant repeat motif in C. esculenta was AC/GT. This contrasts with previous surveys carried out on microsatellite abundance in plant genomes, where AT repeat types were found to be the most predominant, followed by AG/TC repeats, and finally AC/GT repeats (Powell et al. Citation1996). Another more recent study using RAPDs (Irwin et al. Citation1998) also revealed that the Melanesian and Indonesian taros are far more diverse than the cultivars from Polynesia. Consequently, to differentiate between clonal varieties of taro, a highly discriminatory molecular marker technique is required. The microsatellites isolated by Mace and Godwin (Citation2002) meet this requirement, because although they amplify fewer loci compared with other techniques such as RAPDs and AFLPs, they do have a higher information content, i.e., more than two alleles per locus. Therefore, SCAR marker development from RAPD markers of 10 cultivars of diploid and triploid could have large resolving power with the ability to discriminate between cultivars. Moreover, these markers also can be developed as probes for FISH analysis in determining the cultivars in chromosomal level.
Acknowledgement
The germplasm obtained from Central Tuber Crop Research Institute, Bhubaneswar, India for this research is highly acknowledged.
References
- Bamberg JB, Kiru SD, del Rio AH. 2001. RAPD comparison of reputed duplicate populations in the Russion and US potato genebanks. Am J Potato Res. 78:365–369.
- Bennett M, Leitch I. 1995. Nuclear DNA amounts in angiosperms. Ann Bot. 76:113–176.
- Buteler MI, Jarreti RL, LaBonte DR. 1999. Sequence characterisation of microsatellites in diploid and polyploid Ipomoea. Theor Appl Genet. 99:123–132.
- Chattopadhyay D, Sharma AK. 1990. Chromosome studies and microspectro-photometric estimation of nuclear DNA in different strains of Coriandum sativum L. Cytobios 64:43–51.
- Chavarriaga-Aguirre P, Maya MM, Bonierbale MW, Kresovich S, Fregene MA, Tohme J, Kochert G. 1998. Microsatellites in Cassava (Manihot esculenta Crantz): discovery, inheritance and variability. Theor Appl Genet. 97:493–501.
- Coates DJ, Yen DE, Gaffey PM. 1988. Chromosome variation in Taro, Colocasia esculenta: Implications for origin in the Pacific. Cytologia. 53:551–560.
- Darlington CD, Waylie AP. 1955. Chromosome Atlas of Flowering Plants. Landon, UK, George Allen and Unwin Ltd.
- Das AB, Mallick R. 1993. Nuclear DNA chromosome changes within the tribe Ammineae. Cytobios. 74:197–207.
- Das AB. 2008. Assessment of genetic diversity and phylogenetic analysis of ‘Star Cactus’ (Astrophytum) through chromosome and RAPD markers. Cytologia. 73:179–188.
- Delay J. 1951. Nombres chromosomiques chez les phanéorgames. Rev Cytol Biol Végétales. 12:1–368.
- Edwards KJ, Barker JHA, Daly A, Jones C, Karp A. 1996. Microsatellite libraries enriched for several microsatellite sequences in plants. BioTechniques. 20:758–760.
- FAOSTAT. 2000. FAO statistical database: agricultural production of primary crops. Available from http://apps.fao.org/default.htm. Accessed July 2001.
- Flavell RB, Rinpau J, Smith DB. 1997. Repeated sequence DNA relationships in four cereal genomes. Chromosome. 63:205–222.
- Fukushima E, Iwasa S, Tokumasu S, Iwamasa M. 1962. Chromosome numbers of the taro varieties cultivated in Japan. Chromosome Information Service 3:38–39.
- Irwin SV, Kaufusi P, Banks K, de la Pefta R, Cho JJ. 1998. Molecular characterisation of taro (Colocasia esculenta) using RAPD markers. Euphytica. 99:183–189.
- Ito T. 1942. Chromosomen und Sexualität von der Araceae I. Somatische Chromosomenzahleneiniger Arten. Cytologia. 12:313–325.
- Kawahara T. 1978. Chromosome number of Taros in Nepal and India. Chromosome Information Services. 24:4–5.
- Kokubugata G, Konishi T. 1999. Implication of a basic chromosome number of x = 14 in seven cultivars of two varieties of Colocasia esculenta by fluorescent in situ hybridization using rDNA probe. Cytologia. 64:77–83.
- Kurakubo Y. 1940. Über die chromosomenzahlen von Araceae-Arten. Bot Zool. 8:1492.
- Kuruvilla KM, Singh A. 1981. Karyotypic and electrophoretic studies on taro and its origins. Euphytica. 30:405–412.
- Lakhanpaul S, Velayudhan KC, Bhat KV. 2003. Analysis of genetic diversity in Indian taro [Colocasia esculenta (L.) Schott] using random amplified polymorphic DNA (RAPD) markers. Genet Resour Crop Ev. 50:603–609.
- Lebot V, Aradhya KM. 1991. Isozyme variation in taro (Colocasia esculenta (L.) Schott.) in Asia and Oceania. Euphytica. 56:55–66.
- Levan A, Fredyak Sandberg A. 1964. Nomenclature for centromeric position on chromosome. Heredity 52:201–220.
- Mace ES, Godwin ID. 2002. Development and characterization of polymorphic microsatellite markers in taro (Colocasia esculenta). Genome 45:823–832.
- Martel E, Denay D, Siljakyakovtev S, Brown S, Sarr A. 1997. Genome size variation and basic chromosome number in pearl millet and fourteen related Pennisetum species. J Hered. 88:139–143.
- Mookerjee A. 1955. Cytology of different species of aroids with a view to trace the basis of their evolution. Caryologia. 7:221–291.
- Okada H, Hambali GG. 1989. Chromosome behaviors in meiosis of the inter-specific hybrids between Colocasia esculenta (L.) Schott and C. gigantean Hook. f. Cytologia. 54:389–393.
- Petersen G. 1989. Cytology and systematic of Araceae. Nord J Bot. 9:119–166.
- Pfitzer P. 1957. Chromosomenzahlen von Araceen. Chromosoma 8:436–440.
- Powell W, Morgante CA, Hanafey M, Vogel J, Tingey S, Rafalski A. 1996. The comparison of RFLP, RAPD, AFLP and SSR (microsatellite) markers for germplasm analysis. Mol Breeding 2:225–238.
- Price HJ. 1976. Evaluation of DNA content in plants. Bot Rev. 42:27–52.
- Price HJ, Bachman K, Cihambers KL, Riggs J. 1980. Detection of intraspecific variation in nuclear DNA content of Microseris douglasii. Bot Gaz. 141:195–198.
- Purseglove JK. 1972. Tropical crops. Monocotyledons I: Longman Press, London, U.K.
- Quero-Garcia J, Noyer JL, Perrier X, Marchand JL, Lebot V. 2004. A germplasm stratification of taro (Colocasia esculenta) based on agro-morphological descriptors, validation by AFLP markers. Euphytica 137:387–395.
- Rao NS. 1947. A note on the chromosome number of Colocasia antiquorum Schott. Curr Sci. 16:229.
- Roa AC, Chavarriaga-Aguirre P, Duque MC, Maya MM, Bonierbale MW, Iglesias C, Tohme J. 2000. Cross-species amplification of cassava (Manihot esculenta) (Euphorbiaceae) microsatellites: allelic polymorphism and degree of relationship. Am J Bot. 87:1647–1655.
- Roder MS, Plaschke J, Konig SU, Borner A, Sorells ME, Tanksley SD, Canal MW. 1995. Abundance, variability and chromosomal location of microsatellites in wheat. Mol Gen Genet. 246:327–333.
- Rohlf FJ. 1993. Ntsys-PC. Numerical taxonomy and multivariate analysis system Version 1.80-Setauket, NY, Exeter Software.
- Saghai-Maroof MA, Soliman KM, Jorgensen RA, Allard RW. 1984. Population Biology Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal location, and population dynamics. Proc Natl Acad Sci. USA. 81:8014–8018.
- Sharma AK, Das NK. 1954. Study of karyotypes and their alteration in Aroids. Agronomial Lusitania. 16:23–48.
- Sharma AK, Sharma A. 1980. Chromosome Techniques: theory and practice. 3rd ed. Butteruorths, London.
- Sneath PHA, Sokal R. 1973. Numerical Taxonomy. Freeman, San Francisco.
- Sokal PR, Rohlf FJ. 1973. Introduction to Biostatistics. Freeman, San Francisco.
- Sreekumari MT, Mathew PM. 1991a. Distribution of diploid and triploid taro in India. J Root Crops 18:132–133.
- Sreekumari MT, Mathew PM. 1991b. Karyomorphology of five morphotypes of Taro (Colocasia esculenta (L) Schott). Cytologia 56:215–218.
- Sreekumari MT, Mathew PM. 1991c. Karyotypically distinct morphotypes in Taro (Colocasia esculenta (L) Schott). Cytologia 56:399–402.
- Sreekumari MT, Mathew PM. 1993. Meiosis in triploid taro (Colocasia esculenta (L) Schott). J Cytol Genet. 28:7–11.
- Sreekumari MT, Thankamma Pillai PK. 1994. Breeding barriers in taro (Colocasia esculenta (L.) Schott). J Root Crops. 20:60–63.
- Terauchi R, Konuma A. 1994. Microsatellite polymorphism in Dioscorea tokoro, a wild yam species. Genome 37:794–801.
- van der Schoot J, Pospíšková M, Vosman B, Smulders MJM. 2000. Development and characterization of microsatellite markers in black poplar (Populus nigra L.). Thoer Appl Genet. 101(1–2): 317–322.
- Van’t Hof J, Sparrow AH. 1963. A relationship between DNA content, nuclear volume, and minimum mitotic cycle time. Proc Natl Acad Sci. USA 49:897–902.
- Vijaya Bai VK, Magoon ML, Krishnan R. 1971. Meiosis and pollen mitosis in diploid and triploid Colocasia antiquorum Schott. Genetica 42:187–198.
- Wilkinson MJ. 1994. Genome Evolution in Potatoes. In: Bradshaw and Mackay. Ed. Potato Genetics. University Press. pp. 43–70.
- Williams JGK, Kubelik AR, Livak KJ, Rafalski JA, Tingey SV. 1990. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucl Acids Res. 18:6531–6535.
- Yang Z, Yi T, Li H, Gong X. 2003. A cytological study on three species of Colocasia (Araceae) from Yunnan. Caryologia 56:323–327.
- Yen DE, Wheeler JM. 1968. Introduction of taro into the pacific: the indications of chromosome numbers. Ethnology 7:259–267.
- Zhang G, Zhang D. 1990. The relationship between geographic distribution and ploidy level of taro, Colocasia esculenta. Euphytica 47:25–27.