Abstract
In this paper, four endemic Centaurea species were investigated in terms of their chromosome numbers and karyomorphology. The chromosomal counts confirmed the results of previous reports, that the genus Centaurea has different basic chromosome numbers. Centaurea yaltirikii and Centaurea demirizii are diploid taxa. Centaurea yaltirikii has 2n = 18 chromosomes, and therefore has a basic chromosome number of nine, which is common in the genus Centaurea. A local endemic species, Centaurea demirizii has 2n = 16 chromosomes and a different basic chromosome number of x = 8, and this basic number is relatively rare for Centaurea. Centaurea leptophylla and Centaurea saligna are tetraploid with 2n = 36 chromosomes. We found predominance of chromosomes being metacentric and submetacentric. The results increase our karyological knowledge of these species. Moreover, this paper gives short taxonomic and morphologic notes to complement the information provided by Flora of Turkey with regards to the studied species.
Introduction
The family Asteraceae shows a high variety of chromosome numbers. Its variability is very helpful in studies of cytotaxonomy and karyotype evolution, particularly with regard to the ploidy level and basic chromosome number (Charanarsri et al. Citation1973; Weiss-Schneeweiss et al. Citation2003; Uysal et al. Citation2009; Uysal and Köse Citation2009). Earlier karyomorphologic data were used to evaluate the numerical changes in chromosomes of Asteraceae and other families (Carr et al. Citation1999; Ayodele Citation1999; Martin et al. Citation2009; Yıldız et al. Citation2009; Gunjan and Roy Citation2010; Tabur et al. Citation2012). The importance of karyology in Centaurea had been previously suggested by Guinochet and Foissac (Citation1962), Tonjan (Citation1980), Garcia-Jacas and Susanna (Citation1992), Susanna et al. (Citation1995), Garcia-Jacas et al. (Citation1996), and Wagenitz and Hellwig (Citation1996).
Centaurea is a large genus with nearly 250 species belonging to the tribe Cardueae (Susanna and Garcia-Jacas Citation2009). Centaurea shows great morphological diversity and comprises many species (Bovina and Polevova Citation1998; Petit et al. Citation2001). The distribution of the genus is mainly Eurasian, with a center confined to the eastern Mediterranean-Turanian region (Wagenitz Citation1955). The traditional classification of Centaurea, which is a nonmonophyletic taxon, is problematic (Garcia-Jacas et al. Citation2000, 2001; Susanna and Garcia-Jacas Citation2007, Citation2009). Centaurea has one of the highest rates of endemism in Turkey, with 112 endemics among 181 total species, 18 endemics among 32 subspecies, and 16 of the 28 varieties (Uysal Citation2012).
Floral and achene micromorphology, pollen morphology, karyology and phylogenetic studies,were conducted on Centaurea (Garcia-Jacas and Susanna Citation1992; Susanna et al. Citation1995; Garcia-Jacas et al. Citation1996, Citation2000, Citation2001; Wagenitz and Hellwig Citation1996; Routsi and Georgiadis Citation1999). Additionally, taxonomical revisions and remarks have been implemented by different researchers within the last decade (Uysal Citation2006; Garcia-Jacas et al. Citation2006; Mráz et al. Citation2011). All of the above-mentioned approaches were carried out to solve and elucidate the taxonomic issues of the genus. Despite these efforts, there are still some taxonomic problems to be solved in Centaurea. For instance, some Centaurea species are taxonomically cited as “species incertae sedis” in Flora of Turkey, due to descriptions based on only one type or inadequate specimens.
Centaurea demirizii is a local endemic species distributed in the Doğubeyazit distinct of Ağrı Province, in eastern Anatolia. The species is very similar to its relatives located in the same section, especially in terms of the capitulum. The capitulum is truncate at the base both in C. demirizii and C. glastifolia. Unlike the related species, the capitulum of C. pteurocaula is clearly narrowed towards to base by attenuation at the junction with the peduncle. However, the habit is quite different and the characters of the pappus are aberrant for the Chartolepis (Cass) DC. section, to which the species belongs. Unlike the other members of Chartolepis, the species has creeping stem and scabrous achene. Another local endemic species, C. leptophylla, is known from only one type locality near the town of Yusufeli in Artvin Province, in northeastern Anatolia. The taxonomic position of this species was not identified in the first revision of Flora of Turkey, because the species had apparently not been collected since 1843 and was only imperfectly known. Centaurea saligna, a regional endemic, only occurs in several provinces of eastern Anatolia (Uysal Citation2012). According to IUCN (Citation2001) criteria (version 3.1), we confirmed that its threat category should be assigned as VU [Vulnerable, criteria B1c (I, ii, iii, iv) and C2a (I)] as reported before (Ekim et al. Citation2000). Taxonomically, the species, located in the Cheirolepis section, has some differences compared to other close relatives, by its head structure and with hyaline appendage borders. Finally, another regional endemic species, Centaurea yaltirikii is only known from two localities on the south of the Düzce districts, in the western Black Sea region, similar to Centaurea drabifolioides and Centaurea cheirolepidoides in terms of general characters.
Reports on the chromosome numbers within the genus Centaurea were recently completed for the Turkish species (Uysal et al. Citation2009; Uysal and Köse Citation2009; Dydak et al. Citation2009; Meriç et al. Citation2010, and references therein), but there is very limited information about their karyomorphologies (Siljak-Yakovlev et al. Citation2005; Martin et al. Citation2009; Koçyiğit and Bona Citation2013). Hence, we aimed to contribute to the karyomorphology of the genus and solve the taxonomical problems of the analyzed Centaurea species.
Material and methods
Plant materials belonging to the genus Centaurea were collected from several localities of Turkey (Table ).
Table 1. List and localities of the studied Centaurea species.
Mature seeds were selected and periodically germinated for chromosomal analyses. Chromosome counts were made on somatic metaphases using the squash technique. Root meristems from germinating achenes were used. Samples were pretreated with 0.002 M 8-hydroxyquinoline at 4°C for 8 h. The material was fixed with Carnoy for 24 h at low temperatures (+4°C). Before staining, the material was hydrolyzed with 5 N HCl for 1 h at room temperature, stained with 1% aceto-orcein and mounted in 45% acetic acid. Slides were made permanent in Euparal by means of Bowen’s method (1956). At least 10 metaphases were examined per taxa; the best metaphase plates were photographed (100×) with a digital camera (Olympus DP-72, Selçuk University, Molecular Plant Biology), mounted on an Olympus BX53 microscope. Idiograms and karyotyping analyses were carried out using a new program called KAMERAM 2.9.4.0 (ITU Teknokent, Argenit İstanbul, Turkey), a chromosomal analyses software developed by a multidisciplinary team together with the Computer Engineering Department (Istanbul Technical University). We took into account seven different asymmetry indices to analyze the karyomorphologies of the endemic Centaurea species using KAMERAM. Chromosome nomenclature followed that proposed by Levan et al. (Citation1964), with the symbols m and sm designating metacentric and submetacentric chromosomes, respectively. The intrachromosomal asymmetry index (A1) and the interchromosomal asymmetry index (A2) proposed by Romero Zarco (1986) were used. Karyotype asymmetry was also calculated according to the indexes suggested by Paszko (Citation2006) (Table ).
Table 2. Karyotype formula according to Levan et al. (Citation1964) and characteristics of the studied Centaurea taxa.
Results
Section: Pseudoseridia
Centaurea yaltirikii N. Aksoy, H. Duman & A. Efe
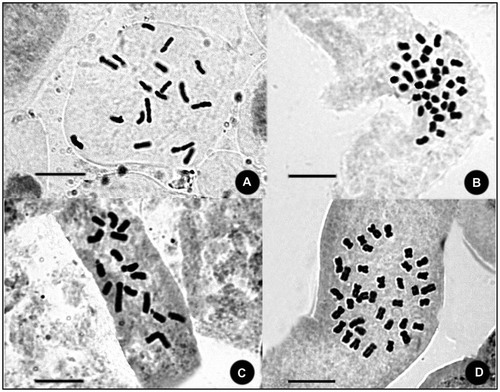
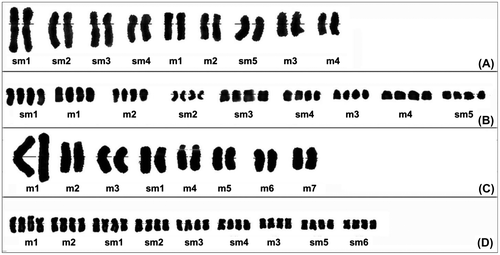
This species is a member of the Pseudoseridia section, which is characterized by very conspicuous yellow flowers, simple appendages, and comprises different types of pappus hairs on achenes, which range from plumose to scabrous. The species is closely related to Centaurea drabifolioides and C. cheirolepidoides in terms of its morphology. However, the species can be distinguished by broadly oblong-lanceolate and asymmetrically decurrent stem leaves at first glance. As with similar species, it has simple appendages with a few cilia, ending in a distinct spinule at the margins. The chromosome number and morphology are first reported in this paper. Its chromosome number is 2n = 18 (Figure A). The karyotype formula and asymmetry index (AI) were determined as 4m + 5sm and 3.64, respectively. Other karyotype parameters and asymmetries are given in Table .
Centaurea leptophylla (K. Koch) Tchich
In Flora of Turkey, no sectional position was assigned for Centaurea leptophylla due to some deficiencies in the specimen. For this reason, Centaurea leptophylla was examined in detail under a stereomicroscope to complete descriptive deficiencies concerning achene and pappus features. Based on these features, we suggest a taxonomic position, as well as characterizing its chromosomes in the present research.
Centaurea leptophylla can be recognized by its slender prostrate stems and fairly reduced simple appendages. According to our examination, the achene characteristics can be described as: achenes narrowly ovoid-oblong, 4.5–5 × 2.2–2.5 mm, superficially with sparsely pilose hairy and pappus double, deciduous, creamish-brown; inner up to 0.5 mm in one series, with scaly hairs, outer 8–10 mm in multiple series, with barbellate hairs.
The last molecular studies reported that the status of the section Pseudoseridia is highly controversial (Garcia-Jacas et al. Citation2006; Uysal et al. Citation2012) and not resolved yet. Because of this, we adopt the current position of this section in the Flora of Turkey (Wagenitz Citation1975). On the other hand, the precise taxonomic status of C. leptophylla was not determined by any paper until now. Therefore, according to the complementary description, we suggest that the species should be placed within the Pseudoseridia because of the similarity its appendage shape and achene features.
The chromosome number and morphology are first reported in this paper. Its chromosome number is 2n = 4x = 36 (Figure B). The karyotype formula and AI are determined as 4m + 5sm and 2.46, respectively. Other karyotype parameters and asymmetries are given in Table .
Section: Chartolepis
Centaurea demirizii Wagenitz
The species can be easily distinguished from others of the section by the creeping and delicate structure of its stems, as well as its aberrant pappus. The chromosome number and morphology are first reported in this paper. Its chromosome number is 2n = 16 (Figure C). The karyotype formula and AI are determined as 7m + 1sm and 2.99, respectively. Other karyotype parameters and asymmetries are given in Table .
Section: Cheirolepis
Centaurea saligna (K. Koch) Wagenitz
This species resides in the section Cheirolepis and is very distinctive species because of its conspicuous habit and characteristic large creamish-yellow appendages with hyaline texture and irregular cilia at the margins. The chromosome number and morphology are first reported in this paper. Its chromosome number is 2n = 4x = 36 (Figure D). The karyotype formula and AI were determined as 3m + 6sm and 1.71, respectively. Other karyotype parameters and asymmetries are given in Table .
Discussion
Four taxa belonging to the genus Centaurea were studied in terms of karyomorphology and the findings draw a detailed picture of the chromosome features of the species. Centaurea yaltirikii and C. demirizii had a diploid chromosome number; the remaining two species had tetraploid chromosome numbers. As a whole, the karyotypes of the analyzed species had a predominance of submetacentric chromosomes except from C. demirizii. Centaurea demirizii had mostly metacentric chromosomes. Centaurea leptophylla and C. yaltirikii share the same karyotype formula and they are fairly similar in terms of karyomorphologic features according to the different indices used. This karyomorphologic similarity could be associated with their taxonomical relationship and common chromosomal pattern in the evolutionary past. As emphasized in previous reports (Romaschenko et al. Citation2004), congruence between karyology and systematic position is very close in Centaurea and, therefore, karyomorphologic similarity among them can be evaluated as an insight in the determination of the taxonomical position of C. leptophylla. However, they had different chromosome numbers and ploidy levels. The chromosome number of Centaurea yaltirikii was counted as 2x = 18, but the chromosome number of C. leptophylla was counted as 4x = 36. Therefore, the first is a diploid and the second is a tetraploid species. The basic chromosome number is x = 9 for both species. Centaurea saligna is another tetraploid species having a basic chromosome number of nine (2n = 4x = 36). Polyploidy (rarely 3x and generally 4x, and 6x) is common in the genus Centaurea (Garcia-Jacas et al. Citation1998; Romaschenko et al. Citation2004; Uysal et al. Citation2009; Uysal and Köse Citation2009; Gömürgen et al. Citation2010). According to our results, a reduction in the chromosome size is observed in Centaurea leptophylla and C. saligna. According to Darlington (Citation1956), a reduction in the chromosome size is a consequence of polyploidy, as an adaptation to an increase in the number of chromosomes. The common basic chromosome number had been reported several times for the eastern species of different sections within Centaurea in previous studies (Bakhshi Khaniki Citation1996; Garcia-Jacas et al. Citation1997, Citation1998; Romaschenko et al. Citation2004; Uysal et al. Citation2009; Uysal and Köse Citation2009; Koçyiğit and Bona Citation2013). Basic chromosome numbers are well defined within Centaurea sections, and our results confirm the previous literature (Garcia-Jacas et al. Citation1997, Citation1998; Uysal et al. Citation2009).
All of the Centaurea taxa displayed similar morphology in the type of metacentric and submetacentric chromosomes. The shortest mean chromosome length (CL) (1.75 μm) was observed in Centaurea leptophylla and the longest (3.82 μm) was observed μin C. demirizii. This species also had the highest arm ratio (2.17), whereas Centaurea saligna had the lowest (1.55).
The analyzed species had small chromosomes according to the classification of Lima De Faria (Citation1980); their CL ranged from 1.66 μm to 3.82 μm, in a graded series within the complement. Centaurea demirizii, with a different basic chromosome number, can be evaluated as a very distinct species compared to the remaining three species because of its very large chromosomes. Hence, we determine that the rise in the CL of Centaurea is related with the reduction in the basic chromosome number.
It is widely assumed that an annual life cycle can affect the evolutionary dynamics of a particular genome. It has also been suggested that more and faster evolutionary divergence enables annual plants to penetrate less favorable and more unstable habitats (Ehrendorfer Citation1970). This evolutionary divergence can be represented by increased variability of chromosome number within a group of related plants (Nagl and Ehrendorfer Citation1974). Therefore, dysploidy is considered an active evolutionary mechanism in plants, especially in the Asteraceae (Garnatje et al. Citation2004). In accordance with this situation, subtribe Centaureinae also shows a complex descending dysploid chromosome series ranging from x = 16 to x = 7 (Garcia-Jacas et al. Citation1996), in which species adapted to more extreme habitats show lower chromosome numbers, correlated with other secondary adaptations that suggest a similar course of evolution (Guinochet and Foissac Citation1962; Garcia-Jacas et al. Citation1996; Susanna and Garcia-Jacas Citation2009; Uysal et al. Citation2009). Similarly, we hypothesize that the reduction in basic chromosome number of Centaurea demirizii may be related with the environmental conditions, as this plant is well adapted to arid areas and dry weather at the alpinic step of Irano-Turanian regions.
The total haploid complement length (TCL) in all of the analyzed species varied from 30.4 μm to 43.7 μm and polyploidy greatly contributes to the wide range in the TCL (Table ). For instance, the highest and lowest TCL values are 43.7 μm in tetraploid Centaurea saligna, and 30.4 μm in diploid C. demirizii, respectively. According to Martin et al. (Citation2009), C. virgata has diploid and tetraploid populations and we observed TCL values in polyploids almost double the values in diploids. The analyzed species showed a series of centromeric index (CI) values, ranging from 38 to 44. When the analyzed species were evaluated according to the CI, Centaurea demirizii was the most differentiated species. We determined that TCL and CI significantly distinguish the analyzed Centaurea species. Therefore, these indices may be useful especially in characterizing closely related species.
Satellites are useful markers in karyomorphological studies. The number, size, and distribution of satellites are good characteristics to distinguish related species or genera, and they were used by Lippi and Garbari (Citation2004) and Sosa et al. (Citation2011) among many others. We detected a satellite in the karyotype of the Centaurea demirizii species in only one chromosome pair and this satellite was positioned at the short arm of the fifth large chromosome (Figures C and C).
Figure 2. Idiograms of taxa belonging to the studied taxa. (A) C. yaltirikii; (B) C. leptophylla; (C) C. demirizii; (D) C. saligna.
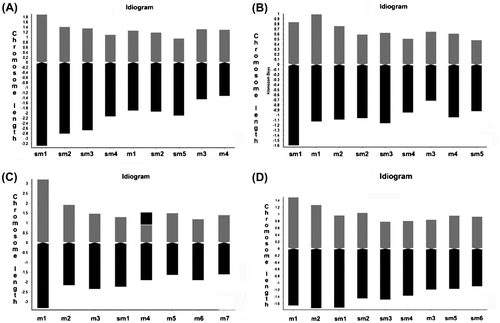
Figure 3. Karyograms of taxa belonging to the studied taxa. (A) C. yaltirikii; (B) C. leptophylla; (C) C. demirizii; (D) C. saligna.
Karyotype asymmetry is where a karyotype has a predominance of chromosomes with terminal/subterminal centromeres (intrachromosomal asymmetry) and highly heterogeneous chromosome sizes (interchromosomal asymmetry) (Peruzzi and Eroğlu 2013). The relative variation in CI (CVCI) is the best parameter that provides information about centromere heterogeneity (Zuo and Yuan Citation2011). The information resulting from our analyses indicated that the CVCI values included a moderate variation, ranging from 10.54 to 16.18. This means that our karyotypes are mostly symmetric in terms of variation in the position of the centromeres. Arnelas Seco and Devasa (2010) studied 21 taxa of the Jacea–Lepteranthus group of genus Centaurea L. and their CVCI values included a higher variation as compared to our results in terms of the heterogeneity of CI, ranging from 5.43 to 22.63. Centaurea saligna and C. demirizii had lower chromosomal heterogeneities with a CVCI value of around 10. The remaining species had moderately higher CVCI values. Therefore, Centaurea yaltirikii having the most asymmetric chromosomes had also the highest CI heterogeneity, with a 16.18 CVCI value. Similarly, it was reported that the Lactuca (Compositae) accessions had included a significant intraspecific variation in terms of chromosomal characteristics (Mousavi e al. Citation2013).
Concerning the measurement of interchromosomal asymmetry (A2), as explained in previous reports, the main point is to determine how different from each other are chromosome lengths of a complement, and the relative variation in chromosome length (CVCL) is perfectly suited for this (Paszko Citation2006). Our measurements indicates that the highest CVCL value belonged to the Centaurea demirizii species, at a value of 28.2; therefore, the species appears to be more differentiated in chromosome lengths as compared to the others. Centaurea saligna had the lowest CVCL value, at 17.16, and its chromosomes were highly similar in terms of length.
When considering karyotype asymmetry index (AI) values, there were several value intervals, ranging from 1.61 to 3.64. From these values, we can say that all of the species had clearly symmetric karyotypes, excluding Centaurea yaltirikii. The AI values displayed a large correlation with those of the CVCI. We confirmed that AI index, the CVCI and the CVCL have the potential to display even minor karyotypic variations as reported by Paszko (Citation2006).
Conclusions
The chromosome number and morphologies of four endemic Centaurea species were comprehensively reported for the first time in this paper. Our findings confirm previous reports that there is a predominance of x = 9 in the oriental sections of the Jacea group as opposed to western sections. According to the CVCL, CVCI, and A2 indices, a certain degree of heterogeneity and variable chromosome morphologies were observed in the studied Centaurea taxa.
We conclude that the analyzed Centaurea species have either a symmetric or asymmetric karyotype, characterized by the predominance of metacentric and submetacentric chromosomes. The chromosomes of the analyzed species were relatively small; in particular, they were smaller in the polyploid species. Although polyploidy contributes to increasing genome size, we assume that it may also cause small losses in some parts of the chromosomes, leading to a reduction of chromosome size, such as in the tetraploid species Centaurea leptophylla and C. saligna.
As a result of our study and the literature discussed herein, we conclude that there are significant differences between chromosomal characteristics in Centaurea and within Compositae and the reported notes about the intraspecific variation may be useful for taxonomy.
Acknowledgments
We thank the Scientific Investigation Project Coordinator of Selçuk University [project number: 2003/052] for their financial support.
References
- Arnelas Seco I, Devesa JA. 2010. Contribución al conocimiento cariológico del género Centaurea L. (Asteraceae) en la Península Ibérica. Grupo Jacea-Lepteranthus. Lagascalia. 30(1):407–445.
- Ayodele MS. 1999. Karyomorphological studies in some Nigerian species of Vernonia Schreb. (Asteraceae) with different growth forms. Feddes Repertorium. 110(7–8):541–553.
- Bakhshi Khaniki G. 1996. Karyological studies in six taxa of the genus Centaurea (Compositae) from Iran. Bot Chr (Patras). 12:55–65.
- Bovina IY, Polevova S. 1998. The palynomorphological study of some Centaurea species (Asteraceae). Botanicheskii Zhurnal. 83:42–51.
- Bowen CC. 1956. Freezing by liquid carbon dioxide in making slides permanent. Stain Technol. 31(2):87–90.
- Carr GD, King RM, Powell AM, Robinson H. 1999. Chromosome number in Compositeae XVIII. Am J Bot. 86(7):1003–1013.
- Charanarsri U, Kamemoto H, Tacashita M. 1973. Chromosome numbers in Oncidium and allied genera. Am Orchid Soc Bull. 42(6):518–524.
- Darlington CD. 1956. Chromosome botany. London: Allen & Unwin.
- Dydak M, Kolano B, Nowak T, Siwinska D, Maluszynska J. 2009. Cytogenetic studies of three European species of Centaurea L. (Asteraceae). Hereditas. 146(4):152–161.
- Ehrendorfer F. 1970. Evolutionary patterns and strategies in seed plants. Taxon. 19(2):185–195.
- Ekim T, Koyuncu M, Vural M, Duman H, Aytaç Z, Adıgüzel N. 2000. Red data book of Turkish plants (Pteridophyta and Spermatophyta). Ankara: TTKD-Van Yüzüncü Yıl University, Barışcan Ofset.
- Garcia-Jacas N, Susanna A. 1992. Karyological notes on Centaurea sect. Acrocentron. Plant Syst Evol. 179(1):1–18.
- Garcia-Jacas N, Susanna A, Garnatje T, Vilatersana R. 2001. Generic delimitation and phylogeny of the subtribe Centaureinae (Asteraceae): a combined nuclear and chloroplast DNA analysis. Ann Bot. 87(4):503–515.
- Garcia-Jacas N, Susanna A, Ilarslan R. 1996. Aneuploidy in Centaureinae (Compositae): is n = 7 the end of the series? Taxon. 45(1):39–42.
- Garcia-Jacas N, Susanna A, Ilarslan R, Ilarslan H. 1997. New chromosome counts in the subtribe Centaureinae (Asteraceae, Cardueae) from West Asia. Bot J Linn Soc. 125(4):343–349.
- Garcia-Jacas N, Susanna A, Mozaffarian V. 1998. New chromosome counts in the subtribe Centaureinae (Asteraceae, Cardueae) from West Asia III. Bot J Linn Soc. 128(4):413–422.
- Garcia-Jacas N, Susanna A, Mozaffarian V, Ilarslan R. 2000. The natural delimitation of Centaurea (Asteraceae: Cardueae): ITS sequences analysis of the Jacea group. Plant Syst Evol. 223(3):185–199.
- Garcia-Jacas N, Uysal T, Romashchenko K, Suárez-Santiago VN, Ertuğrul K, Susanna A. 2006. Centaurea revisited: A molecular survey of the Jacea group. Ann Bot. 98(3):741–753.
- Garnatje T, Vallès J, Vilatersana R, Garcia-Jacas N, Susanna A, Siljak-Yakovlev Y. 2004. Molecular cytogenetics of Xeranthemum L. and related genera (Asteraceae, Cardueae). Pl Biol. 6(2):140–146.
- Gömürgen AN, Erkara Potoglu I, Altınözlü H. 2010. Chromosome and pollen morphology of the rare endemic Centaurea lycopifolia Boiss. & Kotschy. Bangladesh J Bot. 39(2):223–228.
- Guinochet N, Foissac J. 1962. Sur les caryotipes de quelques espèces du genre Centaurea L. et leur signification taxonomique. Rév Cytol Biol Vég. 109(2):373–389.
- Gunjan K, Roy BK. 2010. Karyotype studies in dominant species of Aloe from Eastern India. Caryologia. 63(1):41–49.
- IUCN. 2001. IUCN red list categories and criteria, version 3.1. 51st Meeting of the IUCN Council, Switzerland.
- Koçyiğit M, Bona M. 2013. Chromosome numbers of five Turkish Centaurea L. (Asteraceae) species. Plant Biosyst. 147(4):1–9.
- Levan A, Fredga K, Sandberg AA. 1964. Nomenclature for centromeric position on chromosomes. Hereditas. 52(2):201–220.
- Lima De Faria A. 1980. Classification of genes, rearrangements and chromosomes according to the field. Hereditas. 93(1):1–46.
- Lippi MM, Garbari F. 2004. Leontodon villarsii (Willd.) Loisel. and L. rosani (Ten.) DC. (Asteraceae): nomenclatural, palynological, karyological, and micromorphological aspects. Plant Biosyst. 138(2):165–174.
- Martin E, Dinc M, Duran A. 2009. Karyomorphological study of eight Centaurea L. taxa (Asteraceae) from Turkey. Turk J Bot. 33(2):97–104.
- Meriç Ç, Arda H, Güler N, Dayan S. 2010. Chromosome number and nuclear DNA content of Centaurea kilaea (Asteraceae), an endemic species from Turkey. Phytol Balcan. 16(1):79–84.
- Mousavi SH, Hassandokht MR, Choukan R, Ghanbari A, Papini A. 2013. Genetic diversity of Iranian lettuce (Lactuca sativa. L) accessions revealed by cytological traits. Caryologia. 66(1):41–48.
- Mráz P, Bourchier R, Treier UA, Schaffner U, Müller-Scharer H. 2011. Polyploidy in phenotypic space and invasion context: a morphometric study of Centaurea stoebe s.l. Int J Plant Sci. 172(3):386–402.
- Nagl N, Ehrendorfer F. 1974. DNA content, heterochromatin, mitotic index and growth in perennial and annual Anthemidea (Asteraceae). Pl Syst Evol. 123(1):35–54.
- Paszko B. 2006. A critical review and a new proposal of karyotype asymmetry indices. Plant Syst Evol. 258(1):39–48.
- Peruzzi L, Eroglu HE. 2013. Karyotype asymmetry: again, how to measure and what to measure? Comp Cytogen. 7(1):1–9.
- Petit C, Freville H, Mignot A, Colas B, Riba M, Imbert E, Hurtrez-Bousses S, Virevaire M, Olivieri I. 2001. Gene flow and local adaptation in two endemic plant species. Biol Conserv. 100(1):21–34.
- Romaschenko K, Ertuğrul K, Susanna A, Garcia-Jacas N, Uysal T, Arslan E. 2004. New chromosome counts in the Centaurea jacea group (Asteraceae, Cardueae) and some related taxa. Bot. J. Linn. Soc. 145(3):345–352.
- Romero Zarco C. 1986. A new method for estimating karyotype asymmetry. Taxon. 35(3):526–530.
- Routsi E, Georgiadis T. 1999. Cytogeographical study of Centaurea L. sect Acrocentron (Cass.) DC. Asteraceae in Greece. Bot Helv. 109(2):139–151.
- Siljak-Yakovlev S, Solic ME, Catrice O, Brown SC, Papes D. 2005. Nuclear DNA content and chromosome number in some diploid and tetraploid Centaurea (Asteraceae: Cardueae) from the Dalmatia region. Plant Biol. 7(4):397–404.
- Sosa MM, Panseri AF, Fernández A. 2011. Karyotype analysis of the southernmost South American species of Stemodia (Scrophulariaceae). Plant Biosyst. 145(2):472–477.
- Susanna A, Garcia-Jacas N. 2007. Tribe Cardueae. In: Kadereit JW, Jeffrey C, editors. The families and genera of vascular plants, flowering plants. Eudicots. Asterales, Berlin: Springer. 8:123–147.
- Susanna A, Garcia-Jacas N. 2009. Cardueae (Carduoideae). In: Funk VA, Susanna A, Stuessy TF, Bayer RJ, editors. Systematics, evolution, and biogeography of Compositae. Vienna: IAPT. 293–313.
- Susanna A, Garcia-Jacas N, Soltis DE, Soltis PS. 1995. Phylogenetic relationships in tribe Cardueae (Asteraceae) based on ITS sequences. Am J Bot. 82(8):1056–1068.
- Tabur S, Civelek Ş, Öney S, Yilmaz Ergün ŞB, Kurşat M, Türkoğlu İ. 2012. Chromosome counts and karyomorphology of some species of Artemisia (Asteraceae) from Turkey. Turk J Bot. 36(3):235–246.
- Tonjan TR. 1980. Relation between chromosome number and some morphological feature of Centaureinae Less. representatives. Biologideskij Žurnal Armenii. 33(5):552–554.
- Uysal T. 2006. The morphological, caryological and molecular revision of the section Cheirolepis (Boiss.) O. Hoffm. of the genus Centaurea (Asteraceae) in Turkey. PhD Thesis Selçuk University Institute of Science, Konya.
- Uysal T. 2012. Centaurea L. In: Güner A, Aslan S, Ekim T, Vural M, Babaç MT, editors. Türkiye Bitkileri Listesi (Damarlı Bitkiler). İstanbul, Turkey: Nezahat Gökyiğit Botanik Bahçesi ve Flora Araştırmaları Derneği Yayını. 127–140 (in Turkish).
- Uysal T, Ertugrul K, Susanna A, Garcia-Jacas N. 2009. New chromosome counts in the genus Centaurea (Asteraceae) from Turkey. Bot J Linn Soc. 159(2):280–286.
- Uysal T, Köse B. 2009. A new Centaurea L. (Asteraceae) species from Turkey. Turkish J Bot. 33(1):41–46.
- Uysal T, Özel E, Ertuğrul K, Bozkurt M. 2012. Determination of genetic relationship among Cheirolepis (Centaurea/ Asteraceae) section and its relatives in Turkey. RJB. 2(3):104–109.
- Wagenitz G. 1955. Pollen Morphologie und Systematik in der Gattung Centaurea L. s. l. Flora. 142:213–279.
- Wagenitz G. 1975. Centaurea L. In: Davis PH, editor. Flora of Turkey and the East Aegean Islands. Edinburgh: Edinburgh Univ. Press. 5:465–585.
- Wagenitz G, Hellwig FH. 1996. Evolution of characters and phylogeny of the Centaureinae. In: Hind DJN, Beentje HG, editors. Compositae: systematics. Proceedings of the International Compositae Conference, Kew, 1994. Kew: Royal Botanic Gardens. 491–510.
- Weiss-Schneeweiss H, Stuessy TF, Siljak-Yakovlev S, Baeza CM, Parker J. 2003. Karyotype evolution in South American species of Hypochaeris (Asteraceae, Lactuceae). Plant Syst Evol. 241(3):171–184.
- Yıldız K, Minareci E, Cirpici A. 2009. A karyotypic study on Silene sect. Sclerocalycinae in Turkey. Nordic J Bot. 27(6):503–509.
- Zuo L, Yuan Q. 2011. The difference between the heterogeneity of the centromeric index and intrachromosomal asymmetry. Plant Syst Evol. 297(1):141–145.