Abstract
In the present study the potential toxic effect of prometryne herbicide was examined by investigating mitotic index, mitotic phase, chromosomal abnormalities, micronucleus frequency, 2C DNA content, and comet assay on the root tip cells of Allium cepa. The roots were exposed to 2, 3, 4 and 6 g l−1 concentrations within 12, 24 and 36 h. Mitotic index was markedly reduced by prometryne in each treatment group compared with the controls. The frequencies of mitotic phases have been changed. Prometryne significantly increased the anomaly cell percentage at all concentrations and application periods in comparison to their control. Mitotic abnormalities were recorded as disturbed prophase, c-mitosis, stickiness, laggards and chromatid bridges. The micronucleus was observed at interphase and its frequency was calculated in the test solutions used. Prometryne decreased the 2C DNA amount in onion root tips. Furthermore, the comet assay was applied to analyze the single stand breakages. Nearly all of the applied concentrations of prometryne enhanced DNA damage. In many cases negative correlation was found between 2C DNA amount and DNA damage; however, positive correlation was found between micronucleus formation and DNA damage.
Introduction
Pesticides are utilized in agricultural fields to prevent diseases caused by pests as well as during storage of agricultural products (Karaismailoglu et al. Citation2013). Pesticides and their derivatives (or products contaminated with pesticides) accumulate in living systems and induce hazards of mutagenicity, carcinogenicity or teratogenicity (Liman et al. Citation2011). However, the potential toxicity of most pesticides has not yet been studied widely. Therefore, evaluation of the toxic effects of pesticides is highly important and required for the determination of detrimental impacts on the living systems.
Herbicides are used in agriculture area for controlling weeds. Prometryne is a selective herbicide of the triazine family and used in Turkey to control some annual economic plants such as cotton, sunflower, celery, pigeon peas and dill agricultural areas at 2–3 g l−1 concentrations (EPA Citation1996; MARA Citation2009). This chemical blocks photosynthesis by interference with the Hill effect and corrupting electron flow and oxygen emission (Markus et al. Citation2008; Jin et al. Citation2012). Excessive amounts can cause high toxicity in plants which is indicated by symptoms such as tissue necrosis or modified metabolic pathways (Amado et al. Citation2006; Jiang and Yang Citation2009; Jin et al. Citation2012). Furthermore, several studies reported that use of prometryne has led to increased pollution levels in soil, water and air as a risk to the environment and human health (Fiveland Citation1977; Bardalaye and Wheeler Citation1985; Dikic et al. Citation2010). However, no research has been found presenting toxic or mutagenic effects of prometryne.
Plants are helpful for monitoring toxic impacts on living systems of the chemical agents (Singh et al. Citation2008) by observing chromosomal aberrations, nuclear DNA amount and micronuclei tests. The most common test plants are Allium cepa (Turkoglu Citation2009; Liman et al. Citation2011; Oyeyemi and Bakare Citation2013; Firbas and Amon Citation2014), Vicia faba (Khadra et al. Citation2012) and Helianthus annuus (Karaismailoglu et al. Citation2013). In particular, A. cepa is one of the most frequently used plants in toxicity tests because it is a highly sensitive and reliable test organism (Grant Citation1994).
The comet assay gives information about strand breaks in genomic DNA in cells, induced by toxic substances. This method has high sensitivity and simplicity, and it is often used to explain toxicity. Investigations about DNA damage including the comet test in plants are negligibly few, although the plants are the major recipients of substances including pesticides and heavy metals (Gichener et al. Citation2000; Turkoglu Citation2012).
The aim of this study was to examine the effects of prometryne herbicide on mitotic index, frequencies of the mitotic stages, chromosome abnormalities and 2C DNA amount in the root tips in A. cepa. Correlations among DNA breaks, 2C DNA amount and micronucleus percentage are present in the outcomes of this study.
Material and methods
Onions (2n = 16), 35–40 mm diameter, were purchased from a commercial market in Istanbul.
Prometryne [2,4-bis (isopropylamino)-6-methylthio-s-triazine; C10H19N5S; molecular weight: 241.37 g mol−1; CAS No: 7287-19-6] (EPA Citation1996) was obtained from a retail outlet and used as a stock solution for the toxicity tests. As a first step, the outer scales of the A. cepa bulbs were removed without damaging the primordia root and bulbs were let to grow in distilled water at 24°C in the dark. When root lengths of bulbs reached 1–2 cm length, they were treated with prometryne in concentrations of 2, 3, 4 and 6 g l−1for 12, 24 and 36 h. The treatment concentrations were selected based on the dosage of prometryne in agriculture, and its multiples (MARA Citation2009). Distilled water was used as control.
Root tips of the bulbs were fixed in ethanol:glacial acetic acid (3:1) and kept at 4°C overnight. Next they were washed with fixative free distilled water, were hydrolyzed in 1 N HCl at 60°C for 10 min and stained with Schiff’s reagent for 1.5 h in the dark. Five slides were chosen randomly from each application group for the cytological analysis and mitotic index (MI), micronucleus (MN) in interphase, and chromosome aberrations such as disturbed prophase, chromatid bridge, stickiness, c-mitosis and laggards were investigated in the dividing cells (Karaismailoglu et al. Citation2013; Karaismailoglu Citation2014a, Citation2014b).
The analysis of nuclear DNA content was performed following Turkoglu (Citation2009) with some modifications. For the determination of 2C DNA nuclear amount, root samples were hydrolyzed in 5 N HCl for 30 minutes at 25°C and stained with Feulgen for 1.5 h. Dyed-roots were squashed in 45% glacial acetic acid. Cytophotometric values of 2C telophase nuclei were measured. On average, 50 2C telophase nuclei were evaluated in two repetitions in each concentration of prometryne.
The comet assay (single cell gel electrophoresis) was performed following Gichner et al. (Citation2004); however small alterations were applied. A. cepa root samples exposed to prometryne (2, 3, 4 and 6 g l−1) were taken on a watch glass, and were chopped with a sharp blade to obtain the nuclei in a buffer (Tris). The slides were pretreated with 50 ml of 0.35% agarose gel in a saline phosphate buffer (pb) horizontally spreaded and dried. Nuclei solutions (20 ml) were added to 200 ml of agarose in pb, and then were spread over the slides. Nuclei were waited for h and slides were swilled in TAE buffer (40 mM tris-acetate buffer, 1 mM EDTA) in order to separate the salt. The slides were inserted into a horizontal gel electrophoresis tank including electrophoresis buffer and stained with 70 ml ethidium bromide (20 mg ml−1) for 8 min. In the next step 50 randomly selected nuclei for each slide were examined. Four slides were scored from each application. Each of the images was categorized with respect to the density of fluorescence of the comet tail and evaluated with one of the different genetic damage index categories [tip 0 (0), 1 (1–100 arbitrary units = AU), 2 (101–200 AU), 3 (201–300 AU), or 4 (301–400 AU)] (Kocyigit et al. Citation2005; Cavas Citation2011; Turkoglu Citation2012).
Data were evaluated by using analysis of variance (ANOVA) with SPSS (Citation2008) computer program and level of significance was fixed as p < 0.05. Dunnett multiple range test was used for the detection of statistical significance among the observed differences. The results of statistical analyses are presented in Tables , and .
Table 1. Effects of concentrations of prometryne and control on mitotic cell division of A. cepa.
Table 2. Type and frequency of chromosomal aberrations in the root tips of A. cepa exposed to concentrations of prometryne and control.
Table 3. The effects of prometryne herbicide on the micronucleus, nuclear DNA amount and DNA damage assays.
Results
The effect of different concentrations of prometryne herbicide on the MI and the frequency of mitotic phases are given in Table . In general, different concentrations of prometryne significantly reduced the MI of A. cepa root tip cells in comparison to the control (p < 0.05). Exceptionally, MI does not show significant difference in 2 g l−1 concentration of prometryne at all application times, when compared with the control group. There were strong differences found in MI of A. cepa root tips with other concentrations of prometryne in comparison to the control (p < 0.05) (Table ). As a consequence of the 6 g l−1 prometryne treatment, MI significantly fell at each exposure time (Table ). In addition to MI, when the mitotic phase frequencies were compared with the control in implementation times, there were also statistically significant outcomes (p < 0.05). Almost all of the applications significantly affected the mitotic phase frequencies (Table ). While the percentage of prophase increased, the frequencies of metaphase and anaphase-telophase declined in most of the analyzed cells. Furthermore, a significant rise in the frequencies of abnormality in phases with increasing prometryne concentrations was identified (Table ).
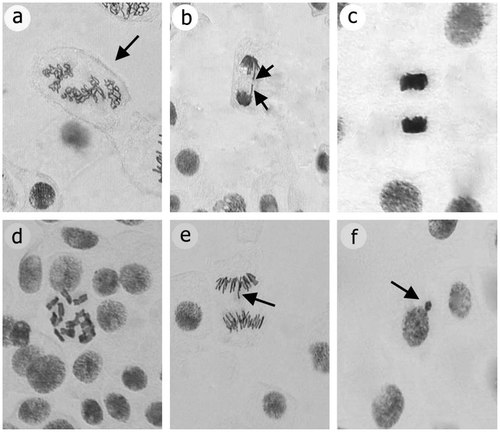
Chromosome abnormalities were investigated in mitotic phases. The types and percentages of chromosome abnormalities induced by applications are shown in Table and Figure . Treatments induced five types of common abnormalities: disturbed prophase, c-mitosis, stickiness, laggards and chromatid bridges.
Figure 1. Mitotic abnormalities caused by prometryne in root meristem cells of A. cepa: (a) disturbed prophase; (b) chromatid bridge; (c) stickiness; (d) c-mitosis; (e) laggards; (f) micronucleus.
The results of the micronucleus assay in A. cepa root tips exposed to control groups and concentrations of prometryne are presented in Table and Figure . The percentage of micronucleus induction found to be dependent on the treatment dose and found to be significantly different in all applications compared with the control (p < 0.05), except for 2 g l−1 application. It was markedly higher at 6 g l−1 than the other concentrations of prometryne in all the applications (Table ). Whereas 12, 24 and 36 h applications of 3 and 4 g l−1 concentrations of prometryne significantly enhanced MN frequency.
Nuclear DNA amounts of the treated groups and control are presented in Table . The amounts of DNA significantly decreased in A. cepa with prometryne compared with the control groups (p < 0.05).There was a greater decrease when the concentration was increased and the period of applications was elongated. The DNA amount ranged from 98.61 pg in the control to 30.14 pg with treatments of prometryne concentrations.
The results of the comet assay are presented in Table . DNA damage was significantly higher in almost all of the concentrations in comparison with the control (p < 0.05). The highest concentration induced the most considerable toxic effect compared with other applications (Table ). There was no difference detected between control and 2 g l−1 during the treatment period. We also aimed to explore the correlations between DNA amounts, DNA damage and micronucleus percentage. Negative correlation has been detected between 2C DNA amounts and DNA damage; however, there is positive correlation between DNA damage and micronucleus percentage.
Discussion
Haphazard or uncontrolled use of pesticides has frequently led to environmental contamination, resulting in adverse effects on living systems. Thus, assessment of the process pathways of prometryne and its effect on mitotic index, chromosomes and DNA, as applied in this investigation, provide helpful data about the effects of these commonly applied toxic substances (Turkoglu Citation2012).
Mitotic index may be utilized as a biological marker of cell increase, which measures the percentage of cells in different mitotic stages (Ping et al. Citation2012; Karaismailoglu et al. Citation2013; Karaismailoglu Citation2014a). The effects of various concentrations of prometryne on MI in A. cepa root tip cells are presented in Table . Mitotic influence mostly decreased significantly with increasing prometryne concentrations at each treatment time, compared with the control (p < 0.05) (Table ). However, at concentrations of 2 g l−1 prometryne, MI was not significantly different to the control at all application times (p < 0.05) (Table ). Furthermore, 6 g l−1 of prometryne was the most toxic dose, and had more mitodepressive impression than 2, 3 and 4 g l−1 concentrations at all treatment times. If the MI value decrease below 22% in comparison with the control, this situation causes lethal effects on living organisms. In addition, the decrements below 50% have sublethal effects and they are called the toxicity limit value (Panda and Sahu Citation1985; Yildiz and Arikan Citation2008; Karaismailoglu et al. Citation2013; Karaismailoglu Citation2014b). In this study, sublethal effect was found in 6 g l−1 concentration in comparison to the control in 12, 24 and 36 h applications, and sublethal effect values were determined as 38.34%, 42.13% and 45.94%, respectively.
These findings are consistent with results obtained by earlier investigations (Konuk et al. Citation2007; Liman et al. Citation2011; Ping et al. Citation2012; Karaismailoglu Citation2013, Citation2014b). In addition, decreases in MI could be the result of a slow progress of cells from S (synthesis) phase to M (mitosis) phase of the cell cycle (Patlolla et al. Citation2012) or may be due to the inhibition of DNA synthesis or prevention of the cell from entering mitosis (Sudhakar et al. Citation2001; Karaismailoglu et al. Citation2013; Karaismailoglu Citation2014b) following prometryne herbicide treatments.
The effects of the different concentrations of prometryne on phases of the cell cycle in the root cells of A. cepa are presented in Table . When the phase frequencies were compared with the control at the different treatment times, there were statistically significant outcomes (p < 0.05). As is illustrated in Table , the percentages of mitotic phases were significantly affected in virtually all treatments (p < 0.05). Generally, while the percentage of prophase increased, the percentages of metaphase and ana-telophase declined in analyzed cells. The effect on mitotic phases of the used prometryne concentrations could be attributed to the prevention of prophase and/or retention in the mitotic phases in response to mitotic stress (Scolnic and Halazonetis Citation2000; Liman et al. Citation2011; Karaismailoglu et al. Citation2013). Additionally, implemented concentrations of prometryne mostly induced a significant increase in the rates of abnormality in mitotic phases in A. cepa root tip cells (p < 0.05). Similar outcomes had been reported in previous studies (Kaymak and Goc Rasgele Citation2009; Inceer et al. Citation2009; Karaismailoglu Citation2013; Karaismailoglu et al. Citation2013).
Chromosome abnormalities can be used to investigate of the toxic effects of chemicals (Carita and Marin-Morales Citation2008). The effects of prometryne concentrations and the control treatments on mitotic abnormalities and percentages of total abnormality in the A. cepa root cells are summarized in Table . The observed chromosomal aberrations such as disturbed prophase, c-mitosis, stickiness, laggards and chromatid bridges are shown in Figure . According to Table and Figure , the most common type of chromosomal abnormality was stickiness, a chromatid tip abnormality (Badr Citation1983). According to Mercykutty and Stephen (Citation1980), stickiness could result from corruption of the structure of DNA (as depolymerization). Another common type of abnormality is c-mitosis. In this anomaly, prometryne prevents spindle creation, similar to the influence of colchicine (Badr Citation1983; Karaismailoglu et al. Citation2013). Chromatid bridges were one of the most common abnormalities, and could be with fragmentation and unification of chromatid (Shehab and Adam Citation1983). Moreover, a high percentage of disturbed prophase was also determined. This may occur due to chromatid erosion.
Stickiness, disturbed prophase and chromatid bridges are irreversible and toxic influence (Fiskesjö and Levan Citation1993), and might be linked with cellular death. In addition, laggards were shaped with failure of spindle fiber formation. This abnormality indicates a low toxic effect which may be reversible (Fiskesjö Citation1985).
Increased prometryne concentrations caused an increase of the percentage of total abnormalities. With application of the highest dose (6 g l−1) for each treatment time, toxicity was higher than expected. The test substance increased the abnormality rates of chromosomes in root tips and therefore indicates a toxic influence. These results are in agreement with previous investigations (Inceer et al. Citation2009; Turkoglu Citation2012; Ping et al. Citation2012; Karaismailoglu et al. Citation2013; Karaismailoglu Citation2014b).
Micronucleus tests have a key role in assessment of the toxicity effects of chemicals (Gebel et al. Citation1997; Karaismailoglu Citation2014a, Citation2014b). MN occurrence and its frequency in applied groups are given in Figure and Table . Generally, MN percentage markedly enhanced with increased prometryne dose when compared with the control, except for 2 g l−1 at all application times (p < 0.05). The MN frequency was markedly higher at the highest prometryne concentration (6 g l−1) than others. MN occurs with microtubules fractures and results in deteriorations at the ploidy levels (such as polyploidy or aneuploidy) because of causing fragments in chromosomes (Konuk et al. Citation2007).
The effects of prometryne on the nuclear DNA amounts in the A. cepa root tip cells are presented in Table . Table Increased prometryne concentrations mostly induced decreases in 2C DNA amounts in A. cepa, which may be due to the interference of prometryne in the double helix of DNA and the blocking of DNA synthesis (Shahin and El-Amoodi Citation1991). Similarly, a positive correlation has been found between mitotic activity and the amounts of DNA. This situation may be clarified by inhibiting of DNA synthesis in G1 or G2 in mitosis entry (Beau et al. Citation1976; Turkoglu Citation2012).
The comet assay, which is a rapid and sensitive test to assess the toxic hazard of pesticides, was used for detecting the toxicity of prometryne in A. cepa root cells (Table ) (Ribas et al. Citation1995). This assay allows the determination of DNA strand fragments in single cells. The comet test showed that applied doses of prometryne caused DNA damage or lesions in A. cepa root cells when compared with the controls. DNA damage occurrence with prometryne may be due to the increasing activity of free radicals, causing DNA strand breaks (Gichner Citation2003; Liman et al. Citation2011). Fairbairn et al. (Citation1995) reported that DNA lesions may occur due to the interaction of pesticides and DNA. The present study found that there are significant increases in the frequencies of both MN and DNA damage after prometryne applications on A. cepa root tips; however, 2C DNA amounts decrease with prometryne treatments (Table ). This finding show that nucleus losses may trigger micronucleated cells or DNA strain breaks, and there is antagonistic relation between nuclear DNA amounts and MN-DNA damages occurrences.
In conclusion, exposure to prometryne may offer a toxicological threat to chromosomes and DNA in Allium cepa. As this herbicide prevents product loss and increases yield in agricultural areas, it is not realistic to stop using it completely. However, our results suggest that if prometryne is used in concentrations below. 3 g l−1, which is the toxic limit of prometryne on A. cepa, the toxic influences on A. cepa would be reduced.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Amado LL, Rosa CDE, Leite AM, Moraes L, Pires WV, Pinho GLL, Martins CMG, Robaldo RB, Nery LEM, Monserrat JM, Bianchini A, Martínez PE, Gericitano LA. 2006. Biomarkers in croakers Micropogonias furnieri (Teleostei: Sciaenidae) from polluted and non-polluted areas from the Patos lagoon estuary (southern Brazil): evidences of genotoxic and immunological effects. Mar Pollut Bull. 52(2):199–206.
- Badr A. 1983. Mitodepressive and chromotoxic activities of two herbicides in A. cepa. Cytologia. 48(2):451–457.
- Bardalaye PC, Wheeler WB. 1985. Capillary gas chromatographic determination of prometryn and its degradation products in parsley. J Assoc Anal Chem. 68(4):750.
- Beau L, Schwarz OJ, Huges KW. 1976. Studies of the herbicide “paraquat” I. Effects of cell cycles and DNA synthesis in Vicia faba. Can J Genet Cytol. 18(1):93–99.
- Carita R, Marin-Morales MA. 2008. Induction of chromosome aberrations in the Allium cepa test system caused by the exposure of seeds to industrial effluents contaminated with azo dyes. Chemosphere. 72(5):722–725.
- Cavas T. 2011. In vivo genotoxicity evaluation of atrazine and atrazine–based herbicide on fish Carassius auratususing the micronucleus test and the comet assay. Food Chem Toxicol. 49(6):1431–1435.
- Dikic D, Sajli L, Benkovic V, Knezevic AN, Brozovic G, Lisicic D, Mojsovic A, Orsolic N. 2010. Brain toxicokinetics of prometryne in mice. Arh Hig Rada Toxicol. 61(1):19–27.
- [EPA] Environmental Protection Agency (US). 1996. Environmental protection and toxic substances, pesticides. 738-R-95-033. US Environmental Protection Agency. Washington (DC).
- Fairbairn DW, Olive PL, O’Neill KL. 1995. The comet assay: a comprehensive review. Mutat Res. 339(1):37–59.
- Firbas P, Amon T. 2014. Chromosome damage studies in the onion plants Allium cepa L. Caryologia. 67(1):25–35.
- Fiskesjö G. 1985. The Allium test as a standard in environmental monitoring. Hereditas. 112(1):99–112.
- Fiskesjö G, Levan A. 1993. Evaluation of the first ten MEIC chemicals in the Allium-test. Alta. 21(2):139–149.
- Fiveland TJ. 1977. Residues of linuron and prometryne in carrots and decomposition in soil in Southern and Northern Norway. Forsk Fors Landbruket. 28:345–363.
- Gebel T, Kevekordes S, Pav K, Edenharder R, Dunkelberg H. 1997. In vivo genotoxicity of selected herbicides in the mouse bone-marrow micronucleus test. Arch Toxicol. 71(3):193–197.
- Gichner T. 2003. DNA damage induced by indirect and direct acting mutagens in catalase-deficient transgenic tobacco cellular and acellular comet assay. Mutat Res. 535(2):187–193.
- Gichner T, Menke M, Stavreva DA, Schubert I. 2000. Maleic hydrazide induces genotoxic effects but no DNA damage detected by the Comet assay in tobacco and field beans. Mutagenesis. 15(5):385–389.
- Gichner T, Patková Z, Sza’ková J, Demnerová K. . 2004. Cadmium induces DNA damage in tobacco roots, but no DNA damage, somatic mutations or homologous recombinations in tobacco leaves. Mutat Res. 559(1–2):49–57.
- Grant WF. 1994. The present status of higher plant bioassays for the detection of environmental mutagens. Mutat Res. 310(2):175–185.
- Inceer H, Hayırlıoglu-Ayaz S, Ozcan M. 2009. Genotoxic effects of the insecticide cypermethrin on the root meristem cells of sunflowers (Helianthus annuus L.). Bull Environ Contam Toxicol. 83(5):652–656.
- Jiang L, Yang H. 2009. Prometryne-induced oxidative stress and impact on antioxidant enzymes in wheat. Ecotoxicol Environ Saf. 72(6):1687–1693.
- Jin ZP, Luo K, Zhang S, Zheng Q, Yang H. 2012. Bioaccumulation and catabolism of prometryne in green algae. Chemosphere. 87(3):278–284.
- Karaismailoglu MC. 2013. Deltamethrin ve quizalofop-p-etil pestisitlerinin Helianthus annuus L. (Ayçiçeği) kök ucu hücreleri üzerine mutajenik etkilerinin araştırılması [MSc Thesis]. Trabzon: Karadeniz Technical University.
- Karaismailoglu MC. 2014a. Investigation of the cytotoxic and genotoxic effects of Artemisia annua methanol extract with the Allium test. Ekoloji. 23(91):64–74.
- Karaismailoglu MC. 2014b. Evaluation of potential genotoxic effect of trifluralin in Helianthus annuus L. (Sunflower). Caryologia. 67(3):216–221.
- Karaismailoglu MC, Inceer H, Hayırlıoglu-Ayaz S. 2013. Effects of quizalofop-p-ethyl herbicide on the somatic chromosomes of Helianthus annuus (Sunflower). Ekoloji. 22(89):49–56.
- Kaymak F, Goc Rasgele P. 2009. Genotoxic effects of raxil on root tips and anthers of Allium cepa L. Caryologia. 62(1):1–9.
- Khadra A, Pinelli E, Lacroix MZ, Bousquet-Melou A, Hamdi H, Merlina G, Guiresse M, Hafidi M. 2012. Assessment of the genotoxicity of quinolone and fluoroquinolones contaminated soil with the Vicia faba micronucleus test. Ecotoxicol Environ Safe. 76(2):187–192.
- Kocyigit A, Keles H, Selek S, Guzel S, Celik H, Erel O. 2005. Increased DNA damage and oxidative stress in patients with cutaneous leishmaniasis. Mutat Res. 585(1–2):71–78.
- Konuk M, Liman R, Cigerci IH. 2007. Determination of genotoxic effect of Boron on Allium cepa root meristematic cells. Pak J Bot. 39(1):73–79.
- Liman R, Cigerci IH, Akyıl D, Eren Y, Konuk M. 2011. Determination of genotoxicity of fenaminosulf by Allium and comet tests. Pesticide Biochem Physiol. 99(1):61–64.
- [MARA] Ministry of Agricultural and Rural Affairs (Turkey). 2009. General directorate of protection and control; plant protection products. Ankara: Ministry of Agricultural and Rural Affairs of Republic of Turkey.
- Markus L, Gunnar S, Daniel B, Bob WK, Georg S, Walter T, Thomas K. 2008. Direct and indirect effects of pollutants on algae and algivorous ciliates in an aquatic indoor microcosm. Aquat Toxicol. 88(2):102–110.
- Mercykutty VC, Stephen J. 1980. Adriamycin induced genetic toxicity as demonstrated by Allium cepa test. Cytologia. 45(4):769–777.
- Oyeyemi IT, Bakare AA. 2013. Genotoxic and anti-genotoxic effect of aqueous extracts of Spondias mombin L., Nymphea lotus L. and Luffa cylindrica L. on Allium cepa root tip cells. Caryologia. 66(4):360–367.
- Panda BB, Sahu UK. 1985. Induction of abnormal spindle function and cytokinesis inhibition in mitotic cells of Allium cepa by the organophosphorus insecticide fensulfothion. Cytobios. 42(167/168):147–155.
- Patlolla AK, Berry A, May LB, Tehounwou PB. 2012. Genotoxicity of silver nanoparticles in Vicia faba: a pilot study on the environmental monitoring of nanoparticles. Int J Environ Res Public Health. 9(5):1649–1662.
- Ping KY, Darah I, Yusuf UK, Yeng C, Sasidharan S. 2012. Genotoxicity of Euphorbia hirta: an Allium cepa assay. Molecules. 17(7):7782–7791.
- Ribas G, Frenzilli G, Barale R, Marcos R. 1995. Herbicide-induced DNA damage in human lymphocytes evaluated by the single-cell gel electrophoresis (SCGE) assay. Mutat Res. 344(1–2):41–54.
- Scolnic D, Halazonetis T. 2000. Chfr defines a mitotic stress checkpoint that delays entry into metaphase. Nature. 406:430–435.
- Shahin SA, El-Amoodi KHH. 1991. Induction of numerical chromosomal aberrations during DNA synthesis using the fungicides nimrod and rubigan-4 in root tips of Vicia faba L. Mutat Res. 261(3):169–176.
- Shehab AS, Adam ZM. 1983. Cytological effects of medicinal plants in Qatar III. Mitotic effect of water extract of Anastatica hierochuntico L. on Allium cepa. Cytologia. 48:343–348.
- Singh P, Srivastava AK, Singh AK. 2008. Cell cycle stage specific application of cypermethrin and carbendazim to assess the genotoxicity in somatic cells of Hordeum vulgare L. Bull Environ Contam Toxicol. 81(3):258–261.
- Sudhakar R, Ninge Gowda KN, Venu G. 2001. Mitotic abnormalities induced by silk dyeing industry effluents in the cells of Allium cepa. Cytologia. 66(3):235–239.
- Turkoglu S. 2009. Genotoxic effects of mono-, di-, and trisodium phosphate on mitotic activity DNA content, and nuclear volume in Allium cepa L. Caryologia. 62(3):171–179.
- Turkoglu S. 2012. Determination of genotoxic effects of chlorfenvinphos and fenbuconazole in Allium cepa root cells by mitotic activity, chromosome aberration, DNA content, and comet assay. Pesticide Biochem Physiol. 103(3):224–230.
- SPSS Inc. Released 2008. SPSS Statistics for Windows, Version 17.0. Chicago: SPSS Inc.
- Yildiz M, Arikan ES. 2008. Genotoxicity of quizalofop-P-ethyl herbicide using the Allium cepa anaphase-telophase chromosome aberration assay. Caryologia. 61(1):45–52.
