Abstract
A cytological study was carried out on 16 taxa belonging to seven clades of Viburnum (Adoxaceae). Chromosome numbers of these 16 taxa were counted and karyotypes of 12 taxa were analyzed. Chromosome numbers of four species and karyotypes of nine taxa are new records. Three chromosome numbers 2n = 16, 18 and 32 of these 16 taxa were discovered, of which the corresponding basic chromosome number was x = 8 or 9. Most taxa showed relatively symmetrical karyotypes, while the well-known V. macrocephalum form. keteleeri, which has a long cultivation history and is culturally important in China, presented the most asymmetrical karyotype. Unweighted pair group method with arithmetic mean (UPGMA) cluster analysis of V. cylindricum and V. punctatum supports the recognized clades Coriacea and Punctata that were previously subsections of Megalotinus. The chromosomal pattern with few polyploids suggests that structural changes of karyotype at the diploid level may be the main trend in chromosomal evolution in Viburnum.
Introduction
Well known for its large inflorescence, diversity of leaf shapes and high ornamental value, plants of the genus Viburnum (Adoxaceae) are widely used in garden landscaping. Comprising approximately 175 species, Viburnum is a clade of shrubs or small trees distributed widely throughout the Northern Hemisphere, while its major diversity centers are in eastern Asia and Latin America (Rehder Citation1908; Killip and Smith Citation1931; Morton Citation1933; Kern Citation1951; Donoghue Citation1983; Hara Citation1983).
Morphological taxonomy originally divided Viburnum into 10 sections: Lentago, Megalotinus, Odontotinus, Opulus, Oreinotinus, Pseudotinus, Solenotinus, Tinus, Tomentosa and Viburnum (Oersted Citation1861; Hara Citation1983). Subsequently, Kern (Citation1951) divided the mostly Southeast Asian section Megalotinus into four subsections: Coriacea, Lutescentia, Punctata and Sambucina. According to phylogenetic studies, these four subsections are placed with confidence in different clades, and their disparate relationships are supported by morphological characters (Clement and Donoghue Citation2011). More recently, Clement et al. (Citation2014) proposed a formal classification system and phylogenetic definitions for 30 clades according to combined data of plastid genomes of 22 Viburnum accessions and a 10-gene dataset of 113 species.
Cytological studies by Sax and Kribs (Citation1930) initially reported a basic chromosome number of x = 9 in Viburnum. Simonet and Miedzyrzecki (Citation1932) subsequently reported the discovery of x = 8. Most species in Viburnum have a basic chromosome number of x = 9, whereas all the investigated species of Solenotinus are characterized by x = 8. In addition, x = 8 is also found in V. plicatum and V. plicatum var. tomentosum of Tomentosa, both of which have two forms: 2n = 16 or 18. Egolf (Citation1962) counted the somatic chromosome numbers of 153 taxa and found that diploids dominated in Viburnum while dysploids and polyploids existed in few species. However, karyotype analysis is only applied to 12 taxa in six clades (Huang et al. Citation1988, Citation1989; Wu Citation1997; Wang et al. Citation2001; Chen et al. Citation2003, Citation2008; Jin et al. Citation2007). For a better understanding of the cytological characters and karyotype patterns of Viburnum, the chromosome numbers of 16 taxa were counted and karyotypes of 12 taxa were analyzed.
Materials and methods
Plant material
Sixteen Viburnum taxa belonging to seven clades from the collections of Kunming Botanical Garden (KBG) were included in this study (Table ). V. hupehense and V. odoratissimum were cuttings introduced from Chengdu Botanical Garden (CBG). Seedlings of V. congestum and V. schensianum were from seeds collected from Kangding (Sichuan, China) and Yangcheng (Sichuan, China) respectively. Accession numbers in KBG of these 16 Viburnum taxa are detailed in Table .
Table 1. Somatic chromosome number (2n), ploidy level, previous chromosome counts, references, location and accession number in KBG of the investigated taxa.
Mitotic chromosome counts and karyotype analysis
Root tips were pretreated with saturated p-dichlorobenzene for 4–6 h at room temperature. After being fixed in absolute ethanol-acetic acid (3:1) for at least 30 min at 0°C, root tips were hydrolyzed in a 1:1 mixture of 1 N HCl and 45% acetic acid at 60°C for 1 min 30 s, then stained with 1% aceto-orcein for 24 h and squashed for cytological observation.
For each species, measurements were taken from at least 10 well-spread metaphase cells in no fewer than three different root tips. The designation of the centromere position as median (m), submedian (sm), subterminal (st) and terminal (t) followed Levan et al. (Citation1964) and the symmetry class (SC) of the karyotypes was made by following Stebbins (Citation1971). The morphology of the chromosomes was determined using the centromeric index (CI = short arm × 100/total length of the chromosome). The karyotype asymmetry was estimated using intrachromosomal (A1) and interchromosomal (A2) asymmetry indices suggested by Zarco (Citation1986). A cluster analysis of five karyomorphological features (average centromeric index (CI), average arm ratio (AR), ratio of the longest chromosome and the shortest chromosome length (Lt/St), asymmetry indices (A1 and A2)) for 21 Viburnum taxa was carried out to examine karyotype similarity. The program STATISTICA v.10.0 was used to calculate the average Euclidean distance and to generate an unweighted pair group method with arithmetic mean (UPGMA) dendrogram.
Results
Chromosome counts and basic chromosome number
Chromosome numbers of 16 Viburnum taxa were counted, and 2n = 16, 18 and 32 were found (Table ). The somatic chromosome numbers of four species (V. congestum, V. foetidum var. ceanothoides, V. koreanum and V. punctatum) are reported for the first time. Based on basic chromosome number, these 16 taxa could be divided into two groups: those with a basic chromosome number of x = 8 (V. odoratissimum, V. plicatum and V. plicatum var. tomentosum), and those with a basic chromosome number of x = 9 (the other 13 taxa). Furthermore, a tetraploid (V. odoratissimum, 2n = 4x = 32) was found in this study.
Karyotype analysis
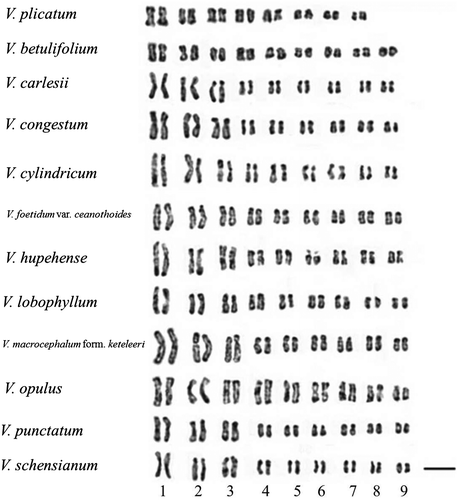
Karyotypes of 12 Viburnum taxa were analyzed in the present study, and nine of them (V. betulifolium, V. carlesii, V. congestum, V. foetidum var. ceanothoides, V. hupehense, V. lobophyllum, V. opulus, V. punctatum and V. schensianum) are new reports. Karyomorphological details of these 12 Viburnum taxa are presented in Figures and . These data were combined with the karyotype data from nine taxa previously reported by other researchers, shown in Table . Parameters for the 21 taxa include: somatic chromosome number (2n), karyotype formula, average chromosome length, chromosome length range, total karyotype length (TKL), average centromeric index (CI), average arm ratio (AR), ratio of the longest chromosome and the shortest chromosome length (Lt/St), asymmetry index (A1 and A2) and symmetry class (SC).
Figure 1. Mitosis metaphase chromosomes of 12 Viburnum taxa. (a) V. plicatum, 2n = 16; (b) V. betulifolium, 2n = 18; (c) V. carlesii, 2n = 18; (d) V. congestum, 2n = 18; (e) V. cylindricum, 2n = 18; (f) V. foetidum var. ceanothoides, 2n = 18; (g) V. hupehense, 2n = 18; (h) V. lobophyllum, 2n = 18; (i) V. macrocephalum form. keteleeri, 2n = 18; (j) V. opulus, 2n = 18; (k) V. punctatum, 2n = 18; (l) V. schensianum, 2n = 18. Scale bars = 5 μm.
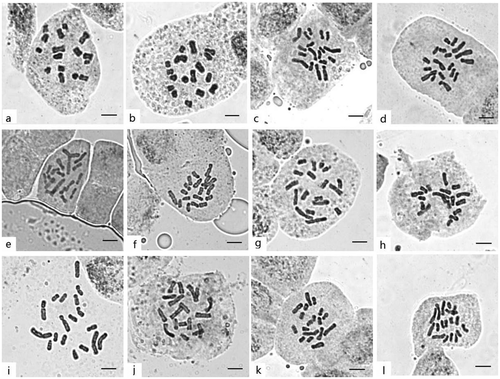
Table 2. Karyomorphological features of Viburnum taxa.
Of the 21 taxa listed in Table , except for V. erosum and V. macrocephalum form. keteleeri, which have telocentric and subtelocentric chromosomes respectively, all taxa show relatively symmetrical karyotypes, consisting of metacentric and submetacentric chromosomes with minor variations among different taxa. The average chromosome length varied from 2.59 μm (V. plicatum in Tomentosa) to 4.47 μm (V. opulus in Opulus). V. plicatum (2n = 16) in Tomentosa had the shortest total karyotype length of 41.50 μm, while in other 20 taxa with 2n = 18, the total karyotype length ranged from 50.04 μm (V. betulifolium in Succotinus) to 80.45 μm (V. opulus in Opulus). Besides, V. plicatum of Tomentosa showed a more symmetrical karyotype with an intrachromosomal asymmetry index (A1) of 0.12 and an average centromeric index (CI) of 46.79. Of all the investigated taxa, V. macrocephalum form. keteleeri of Euviburnum presented the most asymmetrical karyotype with an A1 index of 0.41 and CI of 36.66. The interchromosomal asymmetry index (A2) ranged from 0.15 (V. sargentii in Opulus) to 0.38 (V. punctatum in Punctata and V. hupehense in Succotinus). An asymmetry indices disperation diagram representing the karyotype relations between A1 and A2 of 21 taxa is presented in Figure a.
Figure 3. Karyotype relationships of the 21 studied taxa from Viburnum. (a) Asymmetry indices dispersion diagram representing the karyotype relations in 21 Viburnum taxa. (b) The UPGMA dendrogram of Viburnum taxa, constructed on the basis of karyological characters.
Notes: For species numbers, see Table . ■ Coriacea; ▴ Succotinus; ● Opulus; + Pseudotinus; □ Punctata; △ Tomentosa; ○ Euviburnum.
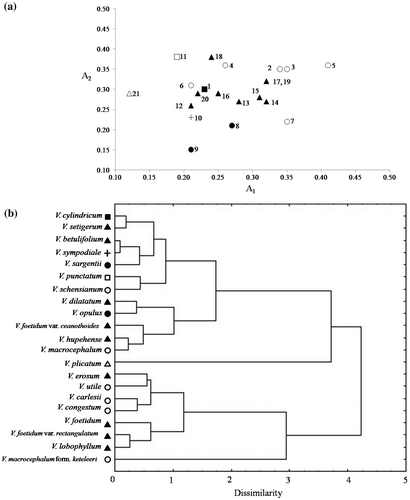
Three types of symmetry class were found: 2A, 1B and 2B. The 2A type included V. sargentii and V. utile; 1B type included four taxa (V. cylindricum, V. betulifolium, V. plicatum and V. schensianum); and the remaining 15 taxa belonged to the 2B type. The UPGMA dendrogram, based on five karyomorphological features (CI, AR, Lt/St, A1 and A2) for 21 Viburnum taxa, is presented in Figure b. Two major clusters were formed, comprising 13 and eight Viburnum taxa respectively.
Discussion
Chromosome numbers
Chromosome numbers of the studied taxa were consistent with previous counts or one of the previous chromosome numbers, as the reports of chromosome numbers of five taxa (V. carlesii, V. lobophyllum, V. odoratissimum, V. plicatum and V. plicatum var. tomentosum) vary between different studies (Table ), in that dysploid (2n = 20, 22), diploid (2n = 16, 18), triploid (2n = 27), tetraploid (2n = 32) and pentaploid (2n = 40) are found in existence. Egolf (Citation1962) had ascribed the occurrence of dysploidy in V. carlesii to self-pollination or cross-pollination between a triploid and a diploid which would produce progeny with additional chromosomes. In this study, V. carlesii, V. lobophyllum, V. plicatum and V. plicatum var. tomentosum were all diploid with 2n = 16 or 18 while V. odoratissimum was a tetraploid with 2n = 32. These data are consistent with the study of Egolf (Citation1962) in that diploid forms dominate in Viburnum while dysploid and polyploid forms are less common. The stability of chromosome numbers in Viburnum lowers the barrier for breeding artificial cultivars by hybridization, and the phylogenetic analysis also suggests the occurrence of natural hybridization in the process of speciation, in that V. prunifolium may be a hybrid of V. rufidulum and V. lentago (Winkworth and Donoghue Citation2005).
In this study, the basic chromosome number of V. odoratissimum, V. plicatum and V. plicatum var. tomentosum was found to be x = 8, while that of other species was x = 9. In agreement with Egolf’s (Citation1962) studies, it is clear that x = 9 is the primary chromosome base number and x = 8 is in fewer species. Phylogenetic studies using chloroplast gene regions, ITS and duplicated nuclear gene GBSSI support the position of V. clemensiae as a sister to all other Viburnum species (Donoghue et al. Citation2004; Winkworth and Donoghue Citation2004, Citation2005; Clement and Donoghue Citation2011; Moore and Donoghue Citation2007, Citation2009). The basic chromosome number of V. clemensiae may be critical in the determination of the ancestral state of the basic chromosome number of Viburnum. Further cytology studies on V. clemensiae should be conducted to address this problem and to reveal the ancestral state of chromosomes in Viburnum.
Karyotype analysis
In the nine taxa with karyotype data reported previously, the number of metacentric and submetacentric chromosomes of V. macrocephalum varies in different studies (Wu Citation1997; Huang et al. Citation1989). In this study, karyotype data of Huang et al. (Citation1989) was quoted (Table ), because no detailed data of individual homologous chromosome pairs are available in Wu’s (Citation1997) study. The karyotype analysis of these 21 taxa showed high consistency and symmetry of chromosome characters in Viburnum, in that most chromosomes were metacentric and submetacentric and 2B was the most common and highest class of asymmetry. The consistency of cytological characters could contribute to successful hybridization, explaining the occurrence of large numbers of excellent horticultural hybrids in Viburnum.
Of the three taxa with previously studied karyotypes, karyotype formula and symmetry class of V. macrocephalum form. keteleeri were consistent with the analysis of Jin et al. (Citation2007), whereas those of V. cylindricum and V. plicatum differed from previous reports (Wu Citation1997; Wang et al. Citation2001). In this study, V. cylindricum and V. plicatum were both 1B type and their genomes were composed of metacentric chromosomes. In reports of Wang et al. (Citation2001) and Wu (Citation1997), they are 2B type comprising some submetacentric chromosomes. For V. cylindricum, karyotype differences may be caused by geographical variations within species as reported in the genus Achillea (Aksu et al. Citation2013). In Wu’s (Citation1997) report of V. plicatum, leaf-buds were used for karyotype study rather than root tips in this study. Different plant materials used could lead to karyotype differences in V. plicatum. A similar situation was reported in the chromosome number counts of Viburnum in the studies of Egolf (Citation1962) and Janaki Ammal (Citation1953).
V. macrocephalum form. keteleeri of Euviburnum presented the most asymmetrical karyotype in this study. Its subtelocentric chromosomes and relatively high average arm ratio (1.85) could have occurred via a shift of the centromere position from median to subterminal or terminal chromosomes, or through accumulation of differences in relative size between individual chromosomes, as reported by Liu et al. (Citation2006) in Leucadendron (Proteaceae). Hou et al. (Citation1997) proposed that artificial breeding of tobacco may affect its karyotype characters and result in a development from symmetry to asymmetry, and V. macrocephalum form. keteleeri is a well-known ornamental plant with a long cultivation and cultural history in China, which may be the cause of its asymmetrical karyotype.
Cladistic analysis of morphological characters indicates that Megalotinus subsections Coriacea (represented by V. cylindricum) and Punctata (represented by V. punctatum) are not united (Donoghue Citation1983). Clement and Donoghue (Citation2011) also proposed the dissolution of section Megalotinus which was supported by phylogenetic analysis and morphological characters including branching pattern, inflorescence types and trichomes. In this research, V. cylindricum and V. punctatum did not cluster together in the UPGMA cluster analysis (Figure b), supporting the fact they are not each other’s closest relatives and the recognized clades Coriacea and Punctata.
Karyotype asymmetry varies to a relatively large extent (Figure a) in Succotinus and Euviburnum, and the UPGMA cluster analysis (Figure b) also shows separations of these two clades, indicating the chromosomal diversity of these clades. Chromosomal structure may change rather rapidly in the evolution of these two clades during environmental change, which is supported by the hypothesis that frequent niche shifts may contribute to the diversification of Succotinus (Spriggs et al. Citation2015). The chromosomal pattern with few polyploids observed suggests that structural changes of karyotype at the diploid level may be the main trend in chromosomal evolution in Viburnum.
This study reported new records of chromosome numbers and karyotypes for some Viburnum taxa. In addition, an extensive karyotype analysis was made for Viburnum, which showed the relatively consistent karyotype characteristics throughout the genus and indicated systematic relationships among taxa and clades from the cytological perspective. This preliminary report will contribute to further studies to address the taxonomy and evolutionary questions in Viburnum.
Funding
This study was supported by the Independent Research Program of the Chinese Academy of Sciences [Grant Number KSCX2-EW-J-24].
Acknowledgments
The authors are grateful to Dr Jane Marczewski (editor of newsletter of the Systematics Association) for her help with the English. We thank the anonymous reviewers for suggestions that improved the paper.
Additional information
Funding
References
- Aksu N, Inceer H, Hayirlioǧlu-Ayaz S. 2013. Karyotype analysis of six Achillea L. (Asteraceae, Anthemideae) taxa from Turkey. Caryologia. 66(2):103–108.
- Bedi YS, Bir SS, Gill BS. 1982. Cytological studies in certain woody members of family Caprifoliaceae. J Tree Sci. 1:27–34.
- Chen RY, Song WQ, Li XL, Li MX, Liang GL, Chen CB. 2003. Chromosome atlas of major economic plants genome in China (III). Beijing: Science Press; p. 101–102.
- Chen RY, Song WQ, Li XL, Li MX, Liang GL, Chen CB. 2008. Chromosome atlas of major economic plants genome in China (V). Beijing: Science Press; p. 130.
- Clement WL, Arakaki M, Sweeney P, Edwards EJ, Donoghue MJ. 2014. A chloroplast genome tree for Viburnum (Adoxaceae): implications for character evolution and phylogenetic classification. Am J Bot. 101:1029–1049.
- Clement WL, Donoghue MJ. 2011. Dissolution of Viburnum section Megalotinus (Adoxaceae) of Southeast Asia and its implications for morphological evolution and biogeography. Int J Plant Sci. 172:559–573.
- Donoghue MJ. 1983. A preliminary analysis of phylogenetic relationships in Viburnum (Caprifoliaceae s.l.). Syst Bot. 8:45–58.
- Donoghue MJ, Baldwin BG, Li J, Winkworth RC. 2004. Viburnum phylogeny based on chloroplast trnk intron and nuclear ribosomal ITS DNA sequences. Syst Bot. 29:188–198.
- Egolf DR. 1956. Cytological and interspecific hybridization studies in the genus Viburnum [PhD thesis]. New York (NY): Cornell University, 131 pp.
- Egolf DR. 1962. A cytological study of the genus Viburnum. Journal of the Arnold Arboretum. 43:132–172.
- Hara H. 1983. A revision of the Caprifoliaceae of Japan with reference to allied plants in other districts and the Adoxaceae. Tokyo: Academia Scientific Books.
- Hou XG, Qu ZQ, Zhang ZP. 1997. Karyotype studies of cultivated tobacco. J Wuhan Bot Res. 15(3):208–214.
- Huang SF, Wang YQ, Qiu JX. 1988. A karyotypical study of the 4 species of genus Viburnum. Forest Res. 1(3):320–324.
- Huang SF, Zhao ZF, Qi QY. 1989. Karyotype analysis for Viburnum macrocephalum and V. sympodiale. J Nanjing Forest Univ. 13(4):16–20.
- Janaki Ammal EK. 1953. Chromosomes and the species problem in the genus Viburnum. Curr Sci Bangalore. 22:4–6.
- Jin B, Fang RC, Wang Q, Zhou WZ. 2007. Karyotype analysis of Viburnum macrocephalum f. keteleeri. J Yangzhou Univ (Agric Life Sci Ed). 28(1):92–94.
- Kern JH. 1951. The genus Viburnum (Caprifoliaceae) in Malaysia. Reinwardtia. 1:107–170.
- Killip EP, Smith AC. 1931. The South American species of Viburnum. Bull Torrey Bot Club. 57:245–258.
- Levan A, Fedga K, Sandberg AA. 1964. Nomenclature for centromeric position on chromosomes. Hereditas. 52:201–220.
- Liu H, Yan GJ, Shan FC, Sedgley R. 2006. Karyotype in Leucadendron (Proteaceae): evidence of the primitiveness of the genus. Bot J Linn Soc. 151:387–394.
- Moore BR, Donoghue MJ. 2007. Correlates of diversification in the plant clade Dipsacales: Geographic movement and evolutionary innovations. Am Nat. 170:S28–S55.
- Moore BR, Donoghue MJ. 2009. A Bayesian approach for evaluating the impact of historical events on rates of diversification. Proc Natl Acad Sci USA. 106:4307–4312.
- Morton CV. 1933. The Mexican and Central American species of Viburnum. Contrib US Natl Herb. 26:339–366.
- Oersted AS. 1861. Til belysning af slaegten Viburnum. Videnskabelige Meddelelser fra Dansk Naturhistorisk Forening i Kjobenhavn. 13:267–305.
- Poucques ML. 1949. Recherches caryologiques sur les Rubiales. Revue Generale de Botanique. 56:5–27, 74-138, 172-188.
- Rehder A. 1908. The viburnums of eastern Asia. In: Sargent CS, editor. Trees and Shrubs. Vol. II. Part II. Boston: Houghton Mifflin. p. 105–116.
- Sax K, Kribs DA. 1930. Chromosomes and phylogeny in Caprifoliaceae. J Arnold Arb. 11:147–153.
- Simonet M, Miedzyrzecki C. 1932. Etude caryologique de quelques especes arborescentes ou sarmenteuses dornement. Comptes rendus des séances de la societe de biologie et de ses filiales. 111:969–973.
- Spriggs EL, Clement WL, Sweeney PW, Madrinan S, Edwards EJ, Donoghue MJ. 2015. Temperate radiations and dying embers of a tropical past: the diversification of Viburnum. New Phytol. 207(2):340–354.
- Stebbins GL. 1971. Chromosome evolution in higher plants. London: Edward Arnold; p. 1–215.
- Sugiura T. 1936. Studies on the chromosome numbers in higher plants with special reference to cytokinesis. I. Cytologia. 7:544–595.
- Thomas JL. 1961. The cytology of some cultivated species of Viburnum. J Arnold Arb. 42:157–164.
- Wang BY, Yin JT, Wang YH. 2001. Studies on the karyotypes of two species in Viburnum. Caprifoliaceae. J Yunnan Univ. 23:79–82.
- Winkworth RC, Donoghue MJ. 2004. Viburnum phylogeny: evidence from the duplicated nuclear gene GBSSI. Mol Phylogenet Evol. 33:109–126.
- Winkworth RC, Donoghue MJ. 2005. Viburnum phylogeny based on combined molecular data: Implications for taxonomy and biogeography. Am J Bot. 92:653–666.
- Wu YM. 1997. Research on the karyotypes of two species of Viburnum L. J Nanjing Forest Univ. 21(2):89–91.
- Zarco CR. 1986. A new method for estimating karyotype asymmetry. Taxon. 35:526–530.