Abstract
Cytology, morphology and ecology were determined from 21 natural populations of Aconitum heterophyllum in Kashmir and Ladakh Himalayas. Data revealed a high body of variability among various morphological characters including plant height, foliage, floral and tuber attributes at intra-population levels. Plant height varied from 98 ± 4.6cm to 36.1 ± 4.5cm. The tuber crude drug production values ranged from 1.22 to 1.7 g/plant. It was generally lower at lower altitudes and higher at higher altitudes. The number of flowers varied from nine to 11; however, it was directly linked with altitude. A similar trend was observed for number of seeds/fruit. Despite this variability, the species was restricted to specific ecological niches with critically low population density facing onslaught of over-exploitation. The present study contributes to insight some aspects of the cytogenetic diversity related to the distribution range of the studied populations with respect to different ecological factors. In addition, the chromosome study depicted that all the populations were diploid (2n = 16); however, the meiotic course varied from normal to abnormal with 11 populations showing abnormalities. These populations exhibited reduction of pollen fertilities up to 20–40% as well as formation of heterogeneous sized pollen grains.
Introduction
Aconitum heterophyllum Wall. (Ranunculaceae) is commonly known as atis or patris. The species is distributed between 2400 and 4500 m asl in temperate and alpine regions of the Himalayas. Due to its medicinal importance and high market value, indiscriminate harvesting of tubers of the species from the wild has led to categorization of the species as critically endangered (International Union for Conservation of Nature (IUCN) Citation1993). It is a high value (Rs. 4000/kg) medicinal species with an estimated annual demand of over 400 MT (Ved and Goraya Citation2008).
The tubers of the species are used for therapeutic purposes, e.g. anti-arthritic or analgesic. The tubers contain the alkaloids aconitine, mesaconitine, hypaconitine, atisine, heteratisine, telatisine, and atidine (Murti and Khorana Citation1968; Mori et al. Citation1989), and have been reported to show significant antibacterial, antipyretic, and enzyme inhibition activity (Nisar et al. Citation2009). They are also used to treat poisoning from scorpion and snake bites, fevers of contagious diseases as well as inflammation in the intestines (Tsarong Citation1994). The main ingredients for industrial use are atisine and aconitine.
A number of studies of this species have been performed including chemical characterization (Jabeen et al. Citation2011), cultivation (Nautiyal et al. Citation2006; Srivastava et al. Citation2011), molecular profiling, conservation and sustainable utilization (Nautiyal and Nautiyal Citation2004; Seethapathy et al. Citation2013), reproductive biology (Siddique, Wafai, et al. Citation1998), ecology (Bhat et al. Citation2014) and cytology (Siddique, Beigh, et al. Citation1998; Rani et al. Citation2011).
Although the species has a wide range of traditional uses and demonstrated potential medicinal properties, studies describing morphological, cytological and ecological aspects in detail on population basis are lacking. In addition, quantitative information on a species plays a fundamental role in formulating a conservation strategy and in understanding the ecology of the species (Uniyal et al. Citation2002). Hence, the present study was carried out in Kashmir and Ladakh Himalayas with the following objectives: (i) to investigate intra-population genetic diversity; (ii) to assess intra-population morphological variability; and (iii) to evaluate the ecological features of the species.
Materials and methods
Field surveys and exploration
Extensive field surveys were carried out in different localities of Kashmir and Ladakh Himalayas covering a wide range of habitats. The plants for cytomorphological population studies were collected during 2013–2014. The young seedlings of A. heterophyllum, underground rhizomes, floral parts and seeds were collected in different seasons. The different surveyed areas/localities are shown in Figure and Table .
Figure 1. Map showing the surveyed localities of Aconitum heterophyllum from Kashmir and Ladakh Himalayas.
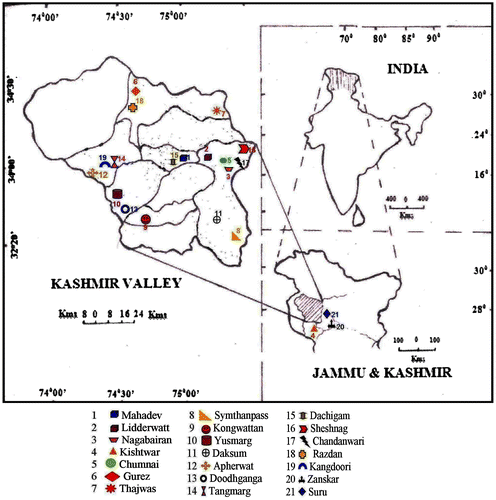
Table 1. Information on populations with locality (geographical coordinates and altitude), nature of meiotic course and habitat of the investigated populations of Aconitum heterophyllum from Kashmir and Ladakh Himalayas.
Morphology and phenotypic variability
With the help of different morphological parameters based on field observations, varying phenotypic characters were recorded to determine the actual differences. Various populations were analyzed for plant structure, number of shoots per plant, rhizome dimensions, plant height, leaf number and dimensions, flower structure and dimensions as well as seed size and number. Both qualitative and quantitative parameters were studied on the basis of different morphological features. In all minimum 15 individual plants were selected for each population on a particular site.
Cytological study
For meiotic studies, flower buds were collected in the field from plants growing under natural conditions from different localities of selected areas of Kashmir and Ladakh Himalayas. These flower buds were collected from 15 randomly selected plants of each population and fixed in Carnoy’s fixative (6:3:1 ethanol/chloroform/acetic acid v/v/v) for 24 h. Flower buds were washed and preserved in 70% ethanol at 4°C until used. Smears of appropriate-sized flower buds were made, using standard acetocarmine technique (Belling Citation1921). About 20–50 fresh slides in each case were prepared from different anthers/flowers for different individuals of a particular population and then were analyzed in each case. To confirm the chromosome number in case of normal meiosis, around 50 pollen mother cells (PMCs) were observed at different stages of meiosis, preferably at diakinesis/metaphase I/anaphase I, II. In case of abnormal meiosis, however, more than 300 PMCs were considered to ascertain the type and frequency of various abnormalities per plant. Pollen fertility was estimated by mounting mature pollen grains in glycerol-acetocarmine (1:1) mixture (Marks Citation1954). Nearly 400–500 pollen grains were analyzed in each case for evaluating pollen fertility and pollen size. Well-filled pollen grains with stained nuclei were taken as apparently fertile, while shriveled and unstained pollen grains were counted as sterile. Photomicrographs of pollen mother cells and pollen grains were made from freshly prepared slides using Optika Digital Imaging System (OPTIKA SRL, Italy).
Ecological study
Four plots (20 × 20 m) were identified in each location. Ten quadrats (50 × 50 cm) were laid randomly in each plot following Kershaw (Citation1973). Quadrat data were used to determine per cent frequency, abundance, density and total basal cover of each species present in community by using the methods of Misra (Citation1968). Abundance to frequency ratio for different species was determined for eliciting the distribution pattern in terms of A/F ratio as regular (A/F < 0.025), random (A/F = 0.025–0.05) and contagious (A/F > 0.05). Importance value index (IVI) of each species was calculated for the determination of dominance and ecological success of a species (Curtis and McIntosh Citation1950). The degree of presence of a species was determined following Braun-Blanquet (Citation1951) and the frequency values were used to determine degree of constancy. Species having frequency of 1–20 was considered as rare, 21–40 seldom present, 41–60 often present, 61–80 mostly present and between 81–100 constantly present.
Results
Morphological variation
For intra-population investigations 11 quantitative morphological characters of 15 individuals of each population were studied (Table ). The data pertaining to different morphological parameters (plant height, tuber length, tuber thickness, dry weight of tubers, number of leaves/plants, leaf dimensions, number of branches/plants, length of floral axis, number of flowers/plants, number of seeds/fruits, and seed weight, were recorded from the studied populations) showed wide significant variations among the 21 populations (Table ). Populations SK-KH11 and SK-LH20 exhibited the highest and lowest values, respectively, for plant height (98 ± 4.6 cm) and 36.1 ± 4.5 cm). Plants from populations SK-KH18, SK-LH21, SK-KH1, SK-KH2 and SK-KH7 had longer and thicker tubers than other populations. Populations SK-KH9, SK-KH10 and SK-KH14 had lower tuber length and thickness as well as the lowest dry weight (g)/plant. The two populations from Ladakh (SK-LH20 and SK-LH21) both had tuber dry weight values of 1.70 ± 0.1 g/plant, which was the highest value among all the populations, followed by SK-KH19, SK-KH18 and SK-KH6. Population SK-KH11 had the highest number of leaves per plant while the lowest was recorded in SK-KH7. In the SK-KH18 population the maximum number (10.8 ± 2.6) of branches per plant was noted while the minimum value (9.8 ± 2.2) was recorded in the SK-KH13 population. The highest leaf diameter was measured in the SK-KH4 population (8.24 ± 1.1 × 5.5 ± 1.9cm); it was lowest (6.93 ± 1.7 × 3.92 ± 0.8cm) in the SK-LH21population. The maximum total number of flowers/plant (10.8 ± 2.6) was in the SK-KH18 population and the minimum (9.8 ± 2.2) in the SK-KH13 population. The highest values for number of seeds/fruit and seed weight (mg/10 seeds) were recorded in the SK-KH16 population and the lowest in the SK-KH10 population. The greatest length of the floral axis was observed in the SK-KH11 population and the lowest in the SK-LH20 population.
Table 2. Intra-population morphological variations in A. heterophyllum from Kashmir and Ladakh Himalayas.
Overall assessment revealed that the populations inhabiting higher altitudes generally had stunted growth, fewer leaves and more flowers. The flowers of such populations were much brighter than the populations collected at lower altitudes. Further, the numbers of seeds were higher in most higher altitude populations. A similar trend was shown in the dry weight of tubers.
Cytological study
All the 21 populations of A. heterophyllum had a chromosome number of 2n = 16 at different stages of meiosis (Figures a–c). The meiotic course and microsporogenesis in 10 populations were normal with high pollen fertilities, whereas in the other 11 populations these were abnormal with low pollen fertilities (Table ). The different abnormalities observed included cytomixis, fragmented chromatin, chromatin stickiness, unoriented bivalents, chromosomal bridges and laggards, or multipolarity seen at different stages of meiosis, which ultimately result in abnormal microsporogenesis.
Figure 2. (a–c) PMCs at metaphase-I (2n = 16); (d–f) PMCs at different stages of meiosis depicting cytomixis (arrows showing transfer of chromatin in d and e); (e) hyperploid (I) and empty (II) PMCs; (f) PMCs with fragmented chromatin (double arrowed PMCs); (g) PMC at metaphase-I showing chromatin stickiness; (h) PMC at metaphase-I with unoriented bivalents (arrowed); (i) PMC at anaphase-I showing chromosomal laggards (arrowed); (j) PMC at anaphase-I showing chromosomal bridge (arrowed); (k) PMC at telophase II exhibiting five poles; (l) diad with micronuclei (arrowed); (m) a triad; (n) tetrad with micronuclei (arrowed); (o) fertile (double arrowed) and sterile (single arrowed) pollen grains; (p) smaller (arrowed) and large sized pollen grains. Scale= 10 μm.
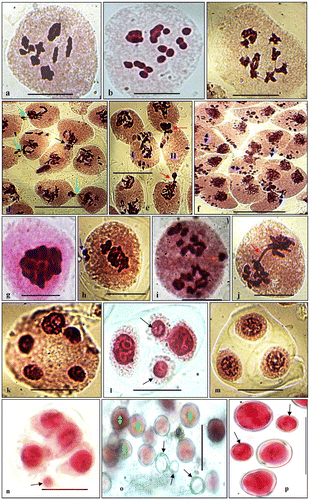
Table 3. Data on abnormal meiotic course in different populations of A. heterophyllum from Kashmir and Ladakh Himalayas.
Cytomixis was the main abnormality of the meiotic system in nine populations (Table ). Transfer of chromatin between PMCs was seen from the early stages of meiosis up to pollen formation and involved a large number of PMCs per group (Figures d–f). Cytomixis in these populations resulted in the formation of PMCs with variable chromosome numbers in the form of hypo- and hyperploids PMCs (Figure f). Further, the frequency of cytomixis was noticed to be variable (Table ). Fragmented chromatins were observed in the PMCs during early stages of the meiosis (Figure d). Chromatin stickiness was the second most frequent meiotic anomaly from early prophase I to telophase II (T-II), involving partial or often complete clumping of bivalents/chromosomes (Figure g). The unoriented bivalents at metaphase I (Figure h) were observed with high frequency in the SK-KH3 population (Table ). This population often showed chromosomal laggards and bridges at anaphases and telophases (Figures i, j) as well as few multipolar PMCs at T-II (Figure k). As a consequence of chromosomal laggards, micronuclei were produced during the tetrad stage formation (Figures 2l–n). These meiotic abnormalities led to the induction of abnormal polarity of the spindle and production of nuclei of variable numbers and sizes during microsporogenesis in the form of monads, dyads, triads or polyads along with or without micronuclei (Figures l–n; Table ).
Table 4. Data on abnormal microsporogenesis in different populations of A. heterophyllum from Kashmir and Ladakh Himalayas.
As a result of these meiotic abnormalities, fertile pollen grains of variable sizes were formed together with some sterile pollen grains in these abnormal populations (Figures o) along with reduced pollen fertilities (Table ). However, in some of the populations (SK-KH2, SK-KH8, SK-KH18), a few giant sized pollen grains (possibly 2n unreduced pollen grains; Figure 2p) were observed. The frequency of such pollen grains in these populations was about 2.65% and were almost double sized to the normal (reduced) ones (Figure p).
Ecological study
The details of different ecological parameters of the 21 populations of A. heterophyllum at varying altitudes are presented in Table . The species grows well on shady moist alpine slopes. Population SK-KH2 and SK-KH18 showed the highest frequency, density and abundance at altitudes of 3400 m and 3500 m respectively. The lowest frequency was recorded in SK-LH20 population, with minimum density (0.5 individuals m−2) in both SK-LH20 and SK-LH21 populations as well as lowest abundance for the SK-LH21 population. Both these populations belong to Ladakh Himalayas. Total basal cover was highest in SK-KH2 and decreased simultaneously in SK-LH20 and SK-LH21in Ladakh. These populations also depicted variation in Importance Value Index (IVI) (Table ). The SK-KH18 population had maximal IVI while it was minimum in the SK-LH20 population in Ladakh. Degree of constancy (presence of a population/species in a given community) was measured as “often” at 12 sites, “seldom” at seven sites and “mostly” at two sites (see Table ). In distribution pattern, the abundance/frequency ratios (A/F) obtained showed that 90.47% of populations were randomly distributed whereas two populations, SK-KH9 and SK-LH20, revealed regular and contagious distribution respectively (Table ).
Table 5. Analysis of intra-population ecological parameters of A. heterophyllum from Kashmir and Ladakh Himalayas.
Discussion
The present study reveals that in response to their highly specific ecological environments, the populations of A. heterophyllum have developed a spectacular diversity in their morphological characters. A high degree of quantitative intra-population variation with respect to different morphological traits was found (Table ) and confirmed the effect of different environmental characters on plant phenotype. On the basis of the analysis of the intra-populational differences of the pooled data, plasticity in phenotypic characters is naturally abundant. The intra-population variations may be related to the populations’ habitats. According to Schlichting (Citation1986), the expression of morphological trait variability can show differentiation of plant populations in a particular habitat. Talebi et al. (Citation2014) showed that different populations of same species that grow under different ecological conditions alter their morphological features for adaption with their habitat condition. However, in some cases, the arrangements of populations in morphological and ecological plots were similar. The plasticity in morphological characters enables individuals of populations to establish in different habitat, and such variations provide a base for evolution of new taxa. The variations in the length and width of leaves observed here may be sensitive to the varying environmental conditions, in line with previous observations made by Lynn and Waldren (Citation2001). According to Kuniyal et al. (Citation2003), the production of more flowers has been considered as an adaptive feature in high altitude plants subjected to a variety of stress conditions; this also applies to high-altitude populations studied here. Pigliucci et al. (Citation1997) and Kuniyal et al. (Citation2002) suggested that available soil nutrients also play an important role in determining morphological variations in plants. The phenotypic variability as observed in the present study helps the species to adapt in various eco-edaphic conditions. The observed variations between the studied populations may also result from variations in the genetic structure of populations. The geographic range of species could be a good predictor of genetic diversity of natural populations, where narrowly distributed or endemic species attain lower genetic diversity levels than their widespread congeners (Gitzendanner and Soltis Citation2000; Cole Citation2003; Mateu-Andrés Citation2004; Orellana et al. Citation2009). Premoli et al. (Citation2001) is of the view that widespread species, often consisting of historically larger and more continuous populations, maintain higher polymorphism than endemics and are less affected by drift, which tends to erode genetic variation in more geographically restricted species (Boroń et al. Citation2011).
Aconitum L. contains 300 species globally (cf. Rani et al. Citation2011). Of these, 181 species/197 cytotypes have been determined, with chromosome numbers of 2n = 16–64, including 42.54% polyploids species (Rani et al. Citation2011). Of 27 taxonomically known species from India, chromosome numbers are known in 12; 50% have a polyploid nature; and x = 8 is be the base number for the genus (Rani et al. Citation2011). The present reports of 2n = 16 in all the populations are thus in agreement with previous reports of the species.
The occurrence of meiotic anomalies in some populations indicates the existence of intraspecific genetic diversities. Such genetic differences have been seen previously in different plant species (Fadaei et al. Citation2010; Jeelani et al. Citation2012; Kumar et al. Citation2013; Rani et al. Citation2013). Cytomixis and chromatin stickiness result from genetic factors (Fadaei et al. Citation2010) or environmental factors (Nirmala and Rao Citation1996) as well as genetic–environmental interaction (Baptista-Giacomelli et al. Citation2000). Cytomixis or occurrence of multipolar cells and meiotic irregularities in anaphase segregation of chromosomes seems to be responsible for the formation of large-sized pollen grains and low pollen fertility in these meiotically abnormal populations, as has been reported earlier in several angiosperms (Rani et al. Citation2011). Hypo- and hyperploid PMC formation is attributed to cytomixis (Fadaei et al. Citation2010; Jeelani et al. Citation2012; Kumar et al. Citation2013; Rani et al. Citation2013), which, especially when accompanied by other meiotic abnormalities, leads to anomalous microsporogenesis resulting in the formation of variable-sized pollen grains (Nirmala and Rao Citation1996). The larger ones may be unreduced 2n pollen grains, as has been reported in several plant species (Vorsa and Bingham Citation1979; Jeelani et al. Citation2012; Rani et al. Citation2013). The formation of unreduced gametes is of evolutionary significance as it can lead to the production of plants with higher ploidy levels through polyploidization (Villeux Citation1985). The other presently observed meiotic abnormalities include chromosomal laggards and chromatin bridges seen at anaphase/telophase and aberrant spindle activity in the PMCs. The presence of extra chromatin material in the recipient meiocytes due to chromatin transfer also contributed to the formation of micronuclei as seen earlier by Bhat et al. (Citation2006).
According to Uniyal et al. (Citation2002), studies on quantitative assessment play a vital role in the ecology of the species and in determining the performance of populations under different sets of conditions. It also provides information about the specialized ecological requirements of a taxon (Kaul and Handa Citation2001). Thus information collected quantitatively at present reveals that populations of A. heterophyllum are unevenly distributed with low population density and restricted distribution. The IVI is efficient for determination of the status of distribution and availability across varying environmental and biotic conditions (Negi et al. Citation1992). Nautiyal et al. (Citation1997) and Pandey et al. (Citation2000) reported that low seed viability, barriers of reproductive phases by juvenile phases, fronts and early snow fall coupled with biotic interference prevent seed maturation and reduced plant population in most alpine vegetation. Thus such plants emerge through underground penetrating tubers, and this has been recorded in the present study. However, in Kashmir Himalayas tubers of A. heterophyllum are exploited for their medicinal use, which is a causing severe threat to the existence of their populations. The present analysis makes it clear that populations of A. heterophyllum are inconsistent as no subpopulations/pockets estimated to contain more than 250 mature individuals (on the basis of density) and all the individuals of species are in specific pockets and mostly occur often. Therefore, the species is endangered in Kashmir Himalayas as also evaluated by IUCN (Citation1993), which has conservation implications.
In the populations investigated here, the parameters related to species richness had lower values in two populations of Ladakh Himalayas which are found at higher altitudes, compared to other species from Kashmir Himalayas. Hortal et al. (Citation2013) also suggested that species richness can decrease with altitude but not with habitat diversity.
Conclusion
In summary, the Himalayan species showing the highest genetic diversity usually inhabit extreme habitats. The values of genetic diversity are more closely related to the ecological conditions than to the size of the area of distribution. Considering the higher frequency of occurrence in some populations is indicative that species have potential for better performance in these sites (habitats) in the region and can be used for mass propagation/cultivation. The quantitative information on a species plays a vital role in formulating a conservation plan and in understanding the ecology of the species; this is helpful in determining the status of species and can be applied in conservation strategies. The occurrence of limited variation of chromosome numbers but enormous diversity in meiotic behavior at intraspecific level in the species demands extensive exploration on a population level, to score different cytotypes, morphotypes and ecotypes, and to determine the best chemotype for future medicinal use.
Acknowledgements
The authors are grateful to the University Grants Commission, New Delhi for the award of Dr D.S. Kothari Post-Doctoral Fellowship to Dr Syed Mudassir Jeelani and Department of Science and Technology, New Delhi, for the Young Scientist Fellowship to Dr Savita Rani. Thanks are also due to the Head, Division of Floriculture, Medicinal & Aromatic Plants (FMAP), Shere-e-Kashmir University of Agricultural Sciences and Technology of Kashmir, Shalimar, Srinagar, (Jammu & Kashmir), India, for arranging the necessary laboratory facilities.
References
- Baptista-Giacomelli FR, Palgliarini MS, Almeida JL. 2000. Meiotic behavior in several Brazilian oat cultivars (Avena sativa L.). Cytologia. 65(4):371–378.
- Belling J. 1921. On counting chromosomes in pollen mother cells. Am Nat. 55(641):573–574.
- Bhat D, Joshi GC, Kumar R, Tewari LM. 2014. Phytosociological features and threat categorization of A. heterophyllum Wall. ex Royle and A. ferox Wall. ex Ser. in Kumaun Himalaya. J Ecol Nat Env. 6(3):111–118.
- Bhat TA, Parveen S, Khan AH. 2006. MMS-Induced cytomixis in pollen mother cells of broad bean (Vicia faba L.). Turk J Bot. 30(2006):273–279.
- Boroń P, Zalewska-Gałosz J, Sutkowska A, Zemanek B, Mitka J. 2011. ISSR analysis points to relict character of Aconitum bucovinense Zapał. (Ranunculaceae) at the range margin. Acta Soc Bot Pol. 80(4):315–326.
- Braun-Blanquet J. 1951. Pflanzensoziologie. Wien: Springer- Verlag.
- Cole CT. 2003. Genetic variation in rare and common plants. Ann Rev Ecol Syst. 34:213–237.
- Curtis JT, McIntosh RM. 1950. The interrelation of certain analytic and synthetic phytosociological characters. Ecology. 31(3):434–455.
- Fadaei F, Sheidai M, Asadi M. 2010. Cytological study the genus Arenaria L. (Caryophyllaceae). Caryologia. 63(2):149–156.
- Gitzendanner MA, Soltis PS. 2000. Patterns of genetic variation in rare and widespread plant congeners. Am J Bot. 87(6):783–792.
- Hortal J, Carrascal LM, Triantis KA, Thébault E, Meiri S, Sfenthourakis S. 2013. Species richness can decrease with altitude but not with habitat diversity. Proc Natl Acad Sci USA. 110(24):E2149–E2150.
- International Union for Conservation of Nature. 1993. Draft IUCN Red List Categories. Gland, Switzerland: IUCN.
- Jabeen N, Rehman S-U, Bhat KA, Khuroo MA, Shawl AS. 2011. Quantitative determination of aconitine in Aconitum chasmanthum and Aconitum heterophyllum from Kashmir Himalayas using HPLC. J Pharm Res. 4(8):2471–2473.
- Jeelani SM, Kumari S, Gupta RC. 2012. Meiotic studies in some selected angiosperms from the Kashmir Himalayas. J Syst Evol. 50(3):244–257.
- Kaul MK, Handa SS. 2001. Medicinal plants of crossroads of Western Himalaya. In: Samantl SS, Dhar U, Palni LMS, editors. Himalayan medicinal plants: potential and prospects, Nainital, India: Gyanoday Prakashan. p. 73–87.
- Kershaw KA. 1973. Quantitative and dynamic plant ecology. London: Edward Arnold; p. 308.
- Murti BSR, Khorana ML. 1968. Identification of aconites and estimation of alkaloids. Indian J Pharm. 30(9):206–208.
- Kumar S, Jeelani SM, Rani S, Gupta RC, Kumari S. 2013. Cytology of five species of subfamily Papaveroideae from the Western Himalayas. Protoplasma. 250(1):307–316.
- Kuniyal CP, Bhadula SK, Prasad P. 2002. Morphological and biochemical variations among the natural populations of Aconitum atrox (Bruhl) Muk. (Ranunculaceae). J Plant Biol. 29(1):91–96.
- Kuniyal CP, Rajsekaran C, Prasad P, Bhadula SK. 2003. Propagation of a threatened medicinal herb Aconitum atrox (Bruhl) Muk. through tuber segments. Plant Genet Resour Newsl. 135:59–62.
- Lynn DE, Waldren A. 2001. Morphological variations in populations of Ranunculus repens from lime stone lake (Turlough) in the west of Ireland. Ann Bot. 87(1):9–17.
- Marks GE. 1954. An acetocarmine glycerol jelly for use in pollen fertility counts. Stain Technol. 29(1):277.
- Mateu-Andrés I. 2004. Low levels of allozyme variability in the threatened species Antirrhinum subbaeticum and A. pertegasii (Scrophulariaceae): implications for conservation of the species. Ann Bot. 94(6):797–804.
- Misra R. 1968. Ecology work-book. New Delhi, India: Oxford and IBH Publication.
- Mori T, Ohsawa T, Murayama M. 1989. Studies on Aconitum species. VIII. Components of “Kako-Bushi-Matsu”. Heterocycles. 29(5):873–884.
- Nautiyal MC, Nautiyal BP. 2004. Agrotechniques for high altitude medicinal and aromatic plants. Dehradun, India: Mahendra Pal Singh Bishen Pal Singh.
- Nautiyal BP, Pandey N, Bhatt AB. 1997. Analysis of vegetation pattern in an alpine zone in North West Himalaya: a case study of Garhwal Himalaya with reference to diversity and distribution patterns. Int J Ecol Env Sci. 23(1):49–65.
- Nautiyal BP, Vinay P, Chauhan RS, Purohit H, Nautiyal MC. 2006. Cultivation of Aconitum species. J Trop Med Plants. 6(2):193–200.
- Negi GCS, Rikhari HC, Singh SP. 1992. Phenological features in relation to growth forms and biomass accumulation in an alpine meadow of the Central Himalaya. Vegetatio. 101(2):161–170.
- Nirmala A, Rao PN. 1996. Genesis of chromosomal numerical mosaicism in higher plants. Nucleus. 39:151–175.
- Nisar M, Ahmad M, Wadood N, Lodhi MA, Shaheen F, Choudhary MI. 2009. New diterpenoid alkaloids from Aconitum heterophyllum Wall.: Selective butyrylcholinestrase inhibitors. J Enzyme Inhibition Med Chem. 24(1):47–51.
- Orellana MR, Blanché C, Simon J, Bosch M. 2009. Genetic diversity within and among disjunct populations of the Mediterranean island endemic Delphinium pictum and D. requienii (Ranunculaceae). Folia Geobot. 44(1):47–63.
- Pandey N, Nautiyal BP, Bhatt AB. 2000. Studies on vegetation analysis, plant form and biological spectrum of an alpine zone of north-west Himalaya. Trop Ecol. 40(2):163–166.
- Pigliucci M, Dhorio P, Schlichting CD. 1997. Phenotypic plasticity of growth trajectories in two species of lobelia in response to nutrient availability. J Ecol. 85(3):265–276.
- Premoli AC, Souto CP, Allnutt TR, Newton AC. 2001. Effects of population disjunction on isozyme variation in the widespread Pilgerodendron uviferum. Heredity. 8(3):337–343.
- Rani S, Kumar S, Jeelani SM, Kumari S, Gupta RC. 2011. Meiotic studies in some members of Ranunculaceae from Western Himalaya (India). Caryologia. 64(4):405–418.
- Rani S, Kumar S, Jeelani SM, Kumari S, Gupta RC. 2013. Impaired male meiosis, morphology and distribution pattern of diploid and tetraploid cytotypes of Bupleurum lanceolatum Wall. (Apiaceae) from the Western Himalayas. Pl Syst Evol. 299(9):1801–1807.
- Schlichting CD. 1986. The evolution of phenotypic plasticity in plants. Ann Rev Ecol Syst. 17:667–693.
- Seethapathy GS, Balasubramani SP, Venkatasubramanian P. 2013. nrDNA ITS sequence based SCAR marker to authenticate Aconitum heterophyllum and Cyperus rotundus in Ayurvedic raw drug source and prepared herbal products. Food Chem. 145:1015–1020.
- Siddique MAA, Beigh SY, Wafai BA. 1998. Patris (Aconitum heterophyllum) Ranunculaceae – an important endangered N.W. Himalayan drug plant – problems and prospects. Proc Natl Acad Sci India. 12:23–25.
- Siddique MAA, Wafai BA, Mehmooda. 1998. Breeding system analysis of an important medicinal plant (Aconitum heterophyllum Wall ex Royle) of Kashmir Himalaya. I. Cytogenetics, resource allocation and propagation. In: Govil JN, editor. Glimpses in plant research current concepts of multidiscipline approach to the medicinal plants (Part I). New Delhi: Today and Tomorrow Printers and Publishers; p. 179–189.
- Srivastava N, Sharma V, Dobriyal AK, Kamal B, Gupta S, Vikash SJ. 2011. Influence of presowing treatments on in vitro seed germination of Ativisha (Aconitum heterophyllum Wall.) of Uttarakhand. Biotech. 10(2):215–219.
- Talebi SM, Salahi Esfahani G, Azizi N. 2014. Inter and intrapopulation variations in Stachys inflata Benth. Based on phenotype plasticity (an ecological and phytogeographical review). Int Res J Biol Sci. 3(2):9–20.
- Tsarong TJ. 1994. Tibetan medicinal plants. New Delhi: Tibetan Medical Publications.
- Uniyal SK, Awasthi A, Rawat GS. 2002. Current status and distribution of commercially exploited medicinal and aromatic plants in upper Gori Valley, Kumaon Himalaya. Uttaranchal. Curr Sci. 82(10):1246–1252.
- Ved DK, Goraya GS. 2008. Demand and supply of medicinal plants in India. New Delhi and Bangalore, India: National Medicinal Plants Board and Foundation for Revitalization of Local Health Traditions.
- Villeux R. 1985. Diploid and polyploidy gametes in crop plants: mechanisms of formation and utilization in plant breeding. In: Janick J, editor. Plant breed rev 3. Westport, CT: AVI Publishing; p. 442.
- Vorsa N, Bingham ET. 1979. Cytology of 2n pollen formation in diploid alfa, Medicago sativa. Can J Genet Cytol. 21(4):525–530.
