Abstract
The critically endangered Nardostachys grandiflora DC is a highly renowned medicinal and aromatic plant of the Western Himalayan region, traditionally used in treating heart palpitations, convulsions, epilepsy and insomnia, as well as being a source of aromatic oil used as incense, flavoring agent and in quality perfumes. This species is on the verge of extinction due to over-exploitation and habitat destruction and needs immediate preventive measures. Despite being essential for domestication, there have been few studies of the natural variation, breeding and genetic system of this species possibly due to its remote location and sparse populations. The present study has revealed that N. grandiflora, which is restricted to specific ecological niches in high alpine regions 3000–5000 m asl, is under stress due to its infrequent flowering nature, which limits generation of new variation, although some intra-population variability with regard to lamina margin (dentate and wavy) and type of stigma (capitate and trilobed) were observed. This limited variability prevents the species from colonizing new niches, as it mostly multiplies through rhizome ramets. Several interesting features have also been revealed, e.g. presence of bicolpate, tricolpate, tetracolpate and pentacolpate pollen grains as well as isobilateral, decussate and tetrahedral types of tetrad within the same or in different flowers. This study also establishes a new diploid chromosome number (2n = 78), the plant’s genomic allohexaploidy, up to 58% cross pollination; protoandry, its infrequent flowering nature, a high pollen ovule ratio of 6135 ± 327.86 per flower and poor seed set.
Introduction
Nardostachys grandiflora DC (syn N. jatamansi, N. chinensis) locally known as jatamansi and nard, a monotypic species considered as the most primitive genus in Valerianaceae (Weberling Citation1975; Government of Nepal Citation1993), is a perennial rhizomatous herb growing in sub alpine/alpine areas of the northwestern Himalayan region of Pakistan, India, Nepal, China and Tibet, mostly growing in steep, moist, rocky, undisturbed grassy slopes at 3000–5000 m asl (Chauhan and Nautiyal Citation2005). The species has been assessed as ‘Endangered’ in Arunachal Pradesh, Sikkim and Himachal Pradesh, India, ‘Critically Endangered’ in Uttrakhand and Kashmir Himalayas, India (Kaul Citation2001) and ‘Vulnerable’ in Nepal during the 2001 CAMP (Conservation Assessment and Management Plan) workshop (Bhattarai et al. Citation2002). It is listed in CITES (Convention on International Trade in Endangered Species of Wild Flora and Fauna) appendix II and the Red Data Book of Indian Plants (Nayar and Sastry Citation1988; Mulliken Citation2000).
Recorded use of N. grandiflora rhizomes in relieving pain dates back to the sixteenth-century “Compendium of Materia Medica” in China (Fu Citation1993). The fibrous rhizomes, used as incense, yield an important essential oil known as spikenard oil that is effective in treating leprous wounds (CSIR Citation1966; Thakur and Hussain Citation1989; Amataya and Sthapit Citation1994; Mulliken Citation2000; Mulliken and Crofton Citation2008) and incense sticks made from its rhizomes are sold in Middle East markets (Burbage Citation1981). In India, in traditional Unani and Ayurvedic systems of medicine, it is valued for its antispasmodic properties which are helpful in treating abnormal heart palpitations and constipation, as well as to regulate urination, menstruation and digestion (Jain Citation1994). Indian classical medical texts, such as Charak samhita and Shushruta samhita, have prescribed smoking pellets (small balls used for smoking) made from the rhizome for treating coughs, hiccups and asthma. The rhizomes are used as a nervine tonic, stimulant, external painkiller, antiepileptic, antiseptic and also in treating hysteria, convulsions, heart palpitations, high blood pressure, fever, anxiety, insomnia, asthma, acidity and,bronchial problems (Chauhan Citation1984; Government of Nepal Citation1993). Rhizomes are used by amchis (traditional medicine practitioners trained in Tibetan medicine) for treating complaints including epilepsy, wounds, coughs, colds and high blood pressure (Ghimire et al. Citation2005). In Pakistan, it is used to treat hysteria, epilepsy, neurosis, insomnia, constipation, scorpion stings, Bell’s palsy, Parkinson’s disease, indigestion and age related deafness (Kazmi and Siddiqui Citation1953; Zaman and Khan Citation1970; International Trade Centre Citation1982; Khan and Zaidi Citation1989). It has also been used as a single ingredient to treat hypertension, as an aphrodisiac and to aid memory (Mulliken Citation2000; Mulliken and Crofton Citation2008). The most recent review of this species has been provided by Kamini and Raina (Citation2013).
The majority of N. grandiflora supplies come from Nepal (82 ± 5%), India (13 ± 5%) and Bhutan (5 ± 4%) and are exported to France, Spain, Germany, USA and others, mostly as unprocessed rhizomes, as well as essential oil and marc in smaller amounts (Mulliken Citation2000; Olsen Citation2005; Mulliken and Crofton Citation2008). India imports about 1000 t of rhizomes annually from Nepal (Olsen Citation2005; Mulliken and Crofton Citation2008) and nard oil fetches about 12,000–30,000 Indian rupees (≈US$240–600) per liter in international markets (Jhunjhunwalla Citation2010). N. grandiflora in trade is sometimes confused with Valeriana jatamansi (Mulliken Citation2000); also rhizomes of Valeriana jatamansi, Selinum candollei and Selinum vaginatum can be mixed in with it leading to adulteration (Singh et al. Citation2011).
Over-exploitation and habitat destruction have necessitated urgent steps to conserve and domesticate this species; for this suitable agro-practices are needed as well as better strains for optimal productivity. As is the case with most high-value temperate endangered medicinal species, genetic improvement has received almost no attention. Scientific studies have been limited to aspects of its taxonomy, distribution, ecophysiology, phytochemistry and ethnobotanical uses (CSIR Citation1966; Samant et al. Citation1998; Prakash Citation1999; Airi et al. Citation2000), but studies of its reproductive potential, genetic and breeding system, which are essential for its conservation and improvement, are lacking.
Materials and methods
Phenological studies were carried out during 2011–2012 on randomly selected plants sourced from Tirthan Wildlife Sanctuary, Kullu, Himachal Pradesh, India (altitude 2400–3000 m asl; 31° 41′55.75″ N, 77°31′46.90″ E).
The vegetative and floral studies were conducted as per standard literature (Lawrence Citation1951; Weberling Citation1989; Kuafman et al. Citation1989). Fresh leaves were preserved in a formalin:acetic acid:alcohol solution (5:5:90) at room temperature, dechlorophylled by boiling in chloral hydrate solution (50 g dissolved in 20 ml water) to determine the palisade ratio, vein-islet/vein termination number and stomatal index as per Fahn (Citation1967).
Splitting of anther wall showing pollen grains was taken as sign of dehiscence, whereas a shining surface of stigma tip was considered as receptive stage (observed under hand magnifying lens). Pollen–ovule ratio was calculated as per Cruden (Citation1977) and pollen viability was calculated on the basis of 1% acetocarmine staining test. Fruit and seeds were studied at maturity stage.
Young floral buds fixed in Carnoy’s fixative (absolute alcohol, glacial acetic acid and chloroform in the ratio of 1:1:1) for 24 h, washed and then stored in 70% absolute alcohol at low temperature before use for meiotic studies. Freshly prepared 1% acetocarmine stain was used for chromosomal staining by usual squash method.
Pollination studies were analyzed using a t-test (15 replications with minimum of 15 flowers per replication). For open pollination unopened healthy floral buds were tagged and left as such, whereas for assessing autogamy, healthy floral buds, about to open, were enclosed in butter paper bags. Increase in ovary size and its transformation into fruit was taken as the basis of fruit set. The number of flowers that finally transformed into fruit out of the total number of flowers tagged for a particular treatment/replication was used for calculating the fruit set percentage. As the fruit in this species is dry achene, the fruits that appeared firm on pressing between the fingers were considered as having developed seed and seed set was calculated on the basis of total number of flowers tagged. Germination test was performed at 23 ± 2°C in a growth chamber and radicle protuberances were taken as sign of germination.
Statistical analysis was conducted as per Gomez and Gomez (Citation1984). Ocular and stage micrometers (ERMA, Tokyo, Japan) were used for micro-measurements and microscopic examination was made using an Olympus trinocular research microscope (model CH20iBIMF, New Delhi, India).
Results
Phenology
Plants (Figure a) of N. grandiflora are erect perennial rhizomatous herbs with an aerial stem (attaining a height of 20.55 ± 2.28 cm), consisting of a rosette of radical leaves at the base and a seasonal pubescent, quadrangular, hollow flowering shoot bearing cauline leaves topped by floral heads. Flowering and fruiting occurs during June to September, with underground perennation occurring from October onwards; sprouting occurs the following year after the snow melts. The perennating rootstock consists of stout, cylindrical rhizomes (Figure b) covered with reddish brown remains of dry petioles of previous radical leaves and adventitious roots.
Radical and cauline leaves are mostly estipulate, petiolated (radical) or sessile (cauline), simple, lanceolate (radical) or ovate (cauline), glabrous, longitudinally veined and lamina entire with mostly wavy smooth margin having acute tip and attenuate base. However, a single plant having lamina with dentate margin was also observed in the natural population. Histological features including the palisade ratio, vein islet and termination number/mm2 and stomatal type/index (Figure d) are determined here for the first time in this species and are given in Table .
Figure 2. N. grandiflora (a) transverse section of ovary; (b) capitate stigma; (c) trilobed stigma; (d) stomata.
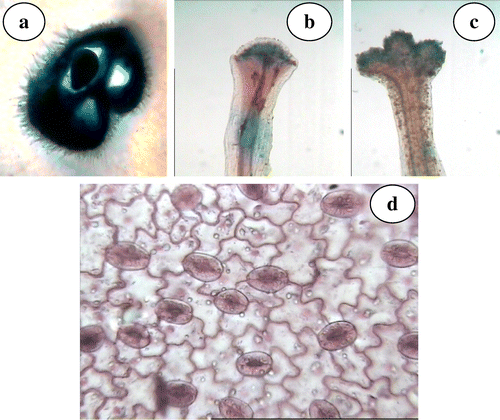
Table 1. Some leaf (radical) constants of N. grandiflora.
There is a single flowering shoot per plant (Figure c), which bears two to five condensed dichasial cyme heads, with the main largest terminal head having eight to 25 flowers and lateral heads having three to nine flowers, where each cyme head is subtended with a pair of ovate, greenish involucre bracts. Bud opening is asynchronous and not more than two to eight flowers are open at any time in the main terminal cyme with only one to two having dehiscing anthers.
Flowers are bracteate, sessile, complete, actinomorphic (or irregular due to the absence of the fifth stamen and with only single ovule in one of the three locules of ovary), bisexual and epigynous. Floral bracts are pubescent, oblong and pale green with size decreasing from basal to terminal flowers. A gamosepalous calyx, at the rim of the thalamus cup, is constituted of five, distinct, valvate, dentate and pubescent lobes which are purple towards the margins and green at the middle. Calyx size increases after fertilization and remains persistent till fruit dispersal. The corolla is tubular at base with five free valvate lobes above, campanulate, purple with pinkish tinge and whitish hairs inside, becoming pale white later.
Four well-developed free, epipetalous, haplostamenous and oppositipetalous stamens represent the androecium. One extra rudimentary anther is also present in some flowers. Anthers are bithecous, exerted, dorsifixed, kidney shaped and violet colored, dehiscing by longitudinal slits. Filaments are purple above and with a white hairy base.
The epigynous gynoecium is represented by three fused carpels. Ovary is trilocular (Figure a) bearing single anatropous ovule showing apical placentation. Linear style bears capitate (Figure b) as well as trilobed (Figure c) papillate stigmas in different flowers, and is purple colored above and whitish towards the base. Stigmatic tip is papillate and violet colored. Fruit is dry pubescent indehiscent achene with persistent calyx bearing a single seed.
Qualitative, quantitative and phenological parameters of this species are summarized in Tables and .
Table 2. Qualitative and quantitative features of N. grandiflora.
Table 3. Pheno-phases of N. grandiflora.
Breeding system studies
Anther, stigma maturity and pollen ovule ratio
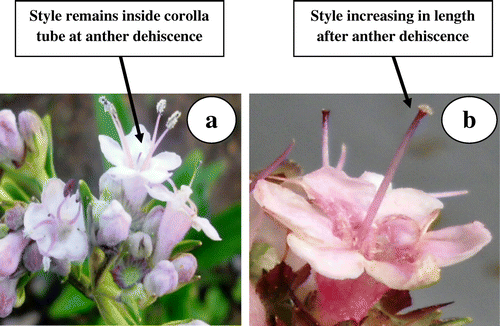
Anther dehiscence is initiated (Figure a) 20–25 days after floral bud appearance and dehiscing anthers are exerted out of corolla tube. The stigma tip is initially positioned below the level of anthers, and later gets pushed out of corolla tube due to increased stylar growth after complete anther dehiscence. At this stage the stigma tip becomes visibly glossy, indicating receptivity (Figure b).
The number of pollen grains ranges from 5320 to 7840 (average 6135 ± 327.86) per flower. With only a single ovule per flower, pollen ovule ratio works out to be 6135 ± 327.86 per flower.
Male meiosis
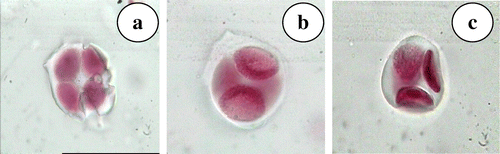
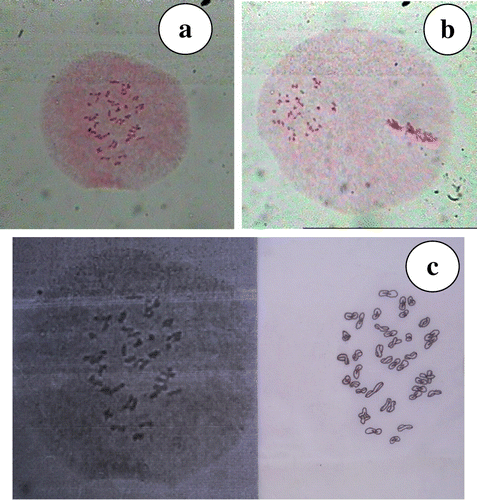
A uniform number of 39 bivalents at diplotene and metaphase-I (Figure a, c) followed by 39–39 segregation of chromosomes at anaphase-I (Figure b) stage was observed in all the pollen mother cells studied. The bivalents were characterized by both interstitial as well as terminal chiasmata and no disjunctional abnormalities were observed. However, three types of tetrads, i.e. isobilateral (Figure a), decussate (Figure b) and tetrahedral (Figure c) were observed in the same as well as different anthers. The normal meiosis is reflected in high pollen stainability of 92.12%.
Figure 4. N. grandiflora (a) metaphase-I; (b) anaphase-I; (c) diagramatic representation of metaphase-I.
Spherical to oval shaped pollen grains were mostly tricolpate (Figure a). However, bicolpate (Figure b), tetracolpate (Figure c) and pentacolpate (Figure d) pollen grains were also observed within the same anther (Figure e), with some anthers having only tricolpate pollen grains. Pollen stainability was found to marginally increase with increase in pollen aperture number, with the lowest (86.38 ± 0.83%) observed in bicolpate and the highest (99.1 ± 0.8%) in pentacolpate pollen grains (Table ).
Figure 6. N. grandiflora (a) tricolpate pollen grain; (b) bicolpate pollen grain; (c) tetracolpate pollen grain; (d) pentacolpate pollen grain; (e) all pollen morphs in one anther.
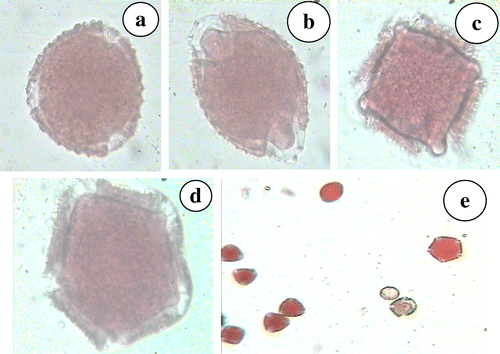
Table 4. Dynamics of different type of pollen grains in the same flower.
Pollination studies
The potential of open and autogamous pollination was evaluated to determine their effect on fruit set and seed germination. Insect pollinators, including different species of bees, flies, butterflies, and ants were observed visiting the flowers. Maximum fruit and seed set of (18.94% and 8.08%) were obtained in open pollination which was statistically higher than self pollination (4.52% and 1.85%). However, seeds from both these sources exhibited about 71% germination (Table ).
Table 5. Effect of different pollination methods on fruit set, seed set and seed germination in N. grandiflora.
Discussion
The floral characters of plant species are indicators of the type of breeding system, having a bearing on conservation and domestication; such studies are lacking in N. grandiflora. Most of the morphological and floral characters studied agreed with earlier records (Clarke Citation1885; Amatya and Sthapit Citation1994; Dutta and Jain Citation2000). However, some plants with a dentate margined radical leaf lamina, in contrast to the common wavy margin, were present in wild populations, which is a first record for this species. Leaf variants (narrow and broad) have also been reported in another endangered temperate Himalayan perennial herb Picrorhiza kurroa (Nautiyal and Nautiyal Citation2004). Similar variations have also been reported in Gaultheria fragrantissima (Vijayakumar and Paulsamy Citation2010).
Some leaf features, including palisade ratio, vein islet number, vein termination number and stomatal type/index/size have been studied for the first time during the present study (Table ). Anomocytic stomata were observed, as in taxa of Ranunculaceae, Malvaceae, Scrophulariaceae, Cucurbitaceae and Geraniaceae (Fahn Citation1967).
The presence of capitate as well as trilobed stigmas in different flowers, indicating the existence of different floral types in this species, is also reported here for the first time. Similar features have been reported in Valeriana jatamansi (a gynodioecious species of family Valerianaceae) wherein female flowers posses trifid stigmas and bisexual flowers posses unifid stigmas (Raina Citation2006; Nawchoo et al. Citation2012).
These hitherto unknown leaf and floral features have enriched our knowledge about this species and have the potential of providing leads in any further genetic improvement programs.
Phenology
Phenological episodes, e.g. sprouting (May onwards after snow melting), flowering (July to August), seed setting (August to October) followed by perennation were observed as per earlier reports (Ghimire et al. Citation2005; Chauhan et al. Citation2008). However, the asynchronous flowering pattern in this species is significant as it is generally encountered in species that require pollinator services, e.g. insects, for effecting allogamous pollination; asynchronous flowering overcomes the risk of pollinator limitations. Another important phenological feature observed in this species was its infrequent flowering behavior. Out of an approximate population size of about 3500 plants growing at the study site, only 50–60 plants bore flowers in a season and this trend was repeatedly observed for two seasons (2011 and 2012). This limits the possibility of good seed production and genetic variation.
Anther dehiscence and stigma receptivity
The temporal and spatial separation between male and female phase to a large extent determines the breeding system of a species. Anther dehiscence prior to the stigma becoming receptive indicates the protoandrous nature of this species. Stigmas become receptive only after complete anther dehiscence, at which stage they protrude out of the corolla tube presenting themselves to pollinators. In Gentiana kurroo, another temperate critically endangered species, stigmatic lobes remain adpressed to each other till complete anther dehiscence, unfolding later and becoming receptive (Raina et al. Citation2003). Protoandry has been reported in many other temperate Himalayan medicinal plants, e.g. Aconitum heterophyllum, A. balfourii, Inula racemosa, Podophyllum hexandrum, Digitalis purpurea, Digitalis lanata, Digitalis grandiflora, and Rheum emodi (Behera Citation2002; Wafai et al. Citation2005; Wani et al. Citation2006, Citation2009; Nazir et al. Citation2008; Nautiyal et al. Citation2009a, Citation2009b). However, Chauhan et al. (Citation2008) reported N. grandiflora as protogynous.
Temporal separation of the maturity of two sexes within a flower can minimize fitness losses from self-pollination within flowers, reduce interference between male female functions and increase female fitness (Lloyd and Webb Citation1986). In N. grandiflora dichogamy appears to play a key role in ensuring cross fertilization, which is crucial for generating variation in an otherwise infrequent flowering and mostly vegetatively multiplying species.
Pollination studies and breeding behavior
A maximum fruit set of 18.94% occurred in open pollinated flowers, but only 4.52% fruit set was observed in passive autogamy. The same trend was noticed in seed set, with 8.08% and 1.85% seed set in open pollination and passive autogamy respectively, indicating preference of its flowers for cross pollination. Based on fruit/seed set percentage, it is estimated that flowers of N. grandiflora exhibit lower chances of self pollination (23.86–42%), compared to higher chances of cross pollination (58–76%). According to a general hypothesis in alpine botany, selfing rates should increase with increasing altitudes (Schroter Citation1926; Bliss Citation1962; Garcia and Totland Citation2009; Korner and Paulsen Citation2009), as pollinator abundance and activity have been shown to become limiting factors for successful pollination due to hostile climatic conditions at higher altitudes (Arroyo et al. Citation1982, Citation2006; Bingham and Orthner Citation1998; Medan et al. Citation2002). However, Eritricium nanum has higher cross pollination rates at high altitudes, and higher selfing rates at lower altitudes (Wirth et al. Citation2010); also, in Chaetanthera renifolia (Asteraceae) of the Andean region, long-lived stigmas allow extended cross pollination (Diaz et al. Citation2011), leading to higher cross pollination rates at high altitudes. The infrequent flowering habit observed in this species limits the chances of generating new genetic combinations, as the species mostly multiplies vegetatively. Hence the meager number of flowers produced in a growing season, as well as offsetting pollinator limitation, is more adapted to cross pollination than self pollination.
Our findings support the conclusion of Chauhan et al. (Citation2008) that this species exhibits both cross as well as self pollination, but disagree with Chauhan et al. (Citation2008), who found that mostly self pollination occursCitation. Wirth et al. (Citation2010) could not demonstrate a higher selfing rate in the alpine species Eritrichium nanum (Boraginaceae) and called for a critical re-examination of the hypothesis of higher selfing in alpine species.
Other direct evidence of the mostly cross pollinating behavior of N. grandiflora established during the present study includes attractive floral attributes, flowers aggregated into a head, dichogamy (protandrous) and receptive stigma protruding out of corolla tube. Insect pollinators such as species of bees, flies, butterflies and ants, were actively observed visiting the flowers.
Meiotic behavior
The present observation of n = 39 and 2n= 78 chromosomes indicates a new chromosome number for this species; two earlier reports give x = 13 in Nardostachys (Engel Citation1976) and 2n = 26 in N. grandiflora (Goldblatt Citation1984). As per Stebbins (Citation1971), base numbers (x) above 12 are secondary in origin (denoted as X2) and x=13 in N. grandiflora (Engel Citation1976) should be denoted as X2 =13. Family Valerianaceae exhibits a dysploid series of five basic chromosome numbers: x = 15 in American Valerianella; x = 13 in Nardostachys; x = 11 in Patrinia and Valeriana; x = 8 in Cantranthus, Fedia, Valeriana and Valerianella; and x = 7 in Valeriana and Valerianella (Engel Citation1976). With X2 = 13, the cytotype studied here appears to be a hexaploid and due to regular meiosis is also a genomic allohexaploid. The present studies as well as earlier reports in this species reveals the existence of at least two cytotypes (i) 2n = 26 (Goldblatt Citation1984); and (ii) 2n = 78 (present study). This wide variation in chromosome numbers points to the possibility of the existence of other intermediate cytotypes that have evaded detection so far. If these are located, a new source of genetic variability would be opened up in this species.
Meiotic events observed in this species followed a normal pattern which was reflected in high pollen viability of 92.12% and compares well with earlier reports of 81.89% (Chauhan et al. Citation2008). However, the course of meiosis from telophase-II to pollen formation is eventful. Three type of tetrads – isobilateral, decussate and tetrahedral – were observed within the same flower. Different types of tetrad emerge as a result of successive or simultaneous cytokinesis during microsporogenesis and generally dicots are considered to follow simultaneous cytokinesis post telophase-II stage (Furness and Rudall Citation2004), but in the present cytotype it appears that some of the pollen mother cells undergo successive cytokinesis as well. Furness and Rudall (Citation2004) also found that different tetrad shapes influence pollen aperture numbers, which may be the reason for the occurrence of bi, tri, tetra and pentacolpate pollen grains observed in this species. Of the different type of pollen grains observed, the tricolpates were far in excess (>73.8%) of other types. This preeminence of tricolpate pollen grains has been explained by Beiatrice et al. (Citation2010) on the basis of tetrad type and cytokinesis pattern in angiosperms. In Clypeola aspera (Brassicaceae) 3- and 4-colpate pollen have been observed in the same individual (Keshavarzi et al. Citation2012). Beiatrice et al. (Citation2010) demonstrated the role of cytokinesis in pollen aperture pattern ontogeny in Epilobium roseum (Onagraceae) and Paranomous reflexus (Proteaceae).
Increased pollen aperture number offers a potential advantage because it increases the number of prospective germination sites, thus facilitating contact between at least one aperture and the stigmatic surface (Furness and Rudall Citation2004). It appears that N. grandiflora also has this advantage. Also, pollen grains with more apertures (4-and 5-colpate) were more stainable in comparison to 2- and 3-colpate ones, indicating better fitness potential of such pollen grains. The pollen ovule ratio is also a good indicator of cross ability pattern of any species (Cruden Citation1977). The pollen ovule ratio (6135 ± 327.86) per flower compares well with the earlier report of 5222.4± 922.63 (Chauhan et al. Citation2008) and indicates obligate outcrossing (Cruden Citation1977). This also supports our findings that N. grandiflora favors cross pollination.
Acknowledgements
The study was conducted under All India Co-ordinated Research Project (AICRP) on Medicinal, Aromatic Plants and Betelvine (MAPB) of Indian Council for Agriculture Research (ICAR), New Delhi, and financial support from ICAR is acknowledged.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Airi S, Rawal RS, Dhar U, Purohit AN. 2000. Assessment of availability and habitat preference of Jatamansi: critically endangered medicinal plant of west Himalaya. Curr Sci. 79(10):1467–1470.
- Amatya G, Sthapit V. 1994. Note on Nardostachys jatamansi. J Herb Spices Med Plants. 2(2):39–47.
- Arroyo MTK, Munoz MS, Henriquez C, Bottraud TI, Perez F. 2006. Erratic pollination, high selfing levels and their correlates and consequences in an altitudinally widespread above tree-line species in the high Andes of Chile. Acta Oecol. 30(2):248–257.
- Arroyo MTK, Primack R, Armesto J. 1982. Community studies in pollination ecology in the high temperate Andes of central Chile. I. Pollination mechanisms and altitudinal variation. Am J Bot. 69(1):82–97.
- Behera MC. 2002. Studies on reproductive biology and anatomy of Gentiana kurroo Royle [Master’s thesis]. [Solan, Himachal Pradesh (India)]: Dr. Yashwant Singh Parmar University of Horticulture and Forestry, Department of Forest Products.
- Beiatrice A, Sophie N, Leanne D, Adrienne R. 2010. The influence of tetrad shape and intersporal callose wall formation on pollen aperture pattern ontogeny in two eudicot species. Ann Bot. 106(4):557–564.
- Bhattarai N, Kharki M, Tandon V. 2002. Report of the conservation assessment and management plan workshop in Nepal. Med Plant Cons. 8:28–30.
- Bingham RA, Orthner AR. 1998. Efficient pollination of alpine plants. Nature. 391(6664):238–239.
- Bliss LC. 1962. Adaptations of arctic and alpine plants to environmental conditions. Arctic. 15(2):117–144.
- Burbage MB. 1981. Report on a visit to Nepal: the medicinal plant trade in the Khardep area – study of the development potential. London: Tropical Products Institute.
- Chauhan NS. 1984. Medicinal wealth of Pabbar valley in Himachal Pradesh [Master’s thesis]. [Palampur, Himachal Pradesh (India)]: Himachal Pradesh Krishi Vishav Vidyalaya.
- Chauhan RS, Kaul MK, Kumar A, Nautiyal MC. 2008. Pollination behavior of Nardostachys jatamansi DC.: endangered medicinal and aromatic herb. Sci Hortic. 117(1):78–81.
- Chauhan RS, Nautiyal MC. 2005. Commercial viability of cultivation of an endangered medicinal herb Nardostachys grandiflora DC. at three different agroclimatic zones. Curr Sci. 89(9):1481–1488.
- Clarke CB. 1885. The flora of British India. Vol. 3. Hooker JD, editor. London: L. Reene and Co.
- Cruden RW. 1977. Pollen ovule ratio: a conservative indicator of breeding system in flowering plants. Evolution. 31(1):32–46.
- CSIR. 1966. Wealth of India: dictionary of raw material and industrial products. vol 7. New Delhi: Public and Information Directorate, Council for Scientific and Industrial Research (CSIR).
- Diaz CT, Gonzalez SG, Stotz GC, Morales PT, Paredes B, Millaqueo MP, Gianoli E. 2011. Extremely long-lived stigmas allow extended cross-pollination opportunities in high Andean plant. PLoS ONE. 6(5):e19497. doi:10.1371/journal.pone.0019497.
- Dutta R, Jain P. 2000. CITES listed medicinal plants of India: identification manual. Delhi: Trade Record Analysis of Flora and Fauna in Commerce (TRAFFIC) India and WWF India.
- Engel K. 1976. Beitrage zur Systematik der Valerianaceae a unter besonderes Berucksichtigung systematischer Ergebnisse [Contributions to the systematics of Valerianaceae with special consideration of systematic results] [PhD Dissertation]. [Germany]: University of Giessen. p. 196.
- Fahn A. 1967. Plant anatomy. Oxford: Pergamon Press; p. 543.
- Fu W. 1993. History of traditional Chinese medicines. Siachuan (China): Bashu Publication.
- Furness CA, Rudall PJ. 2004. Pollen aperture evolution: crucial factor for eudicot success? Trends Plant Sci. 9(3):154–158.
- Garcia CR, Totland O. 2009. Pollen limitation in the alpine: a meta-analysis. Arct Antarct Alp Res. 41(1):103–111.
- Ghimire SK, Mckey D, Aumeeruddy TY. 2005. Conservation of Himalayan medicinal plants: harvesting patterns and ecology of two threatened species, Nardostachys grandiflora DC. and Neopicrorhiza scrophulariiflora (Penel) Hong. Biol Conser. 124(4):463–475.
- Goldblatt P. 1984. Index to plant chromosome numbers, 1979–1981. Monogr Syst Bot MO Bot Gard. 8:1–427.
- Gomez KA, Gomez AA. 1984. Statistical procedures for agricultural research. 2nd ed. New York (NY): John Wiley.
- Government of Nepal. 1993. Medicinal Plants of Nepal. Vol. 3. 4th ed. Bulletin of the Department of Medicinal Plants. Kathmandu (Nepal): Government of Nepal, Ministry of Forests and Soil Conservation, Department of Medicinal Plants.
- International Trade Centre. 1982. Markets for Selected Medicinal Plants and their Derivatives. United Nations Conference on Trade and Development/General Agreement on Tariff and Trade. Geneva, Switzerland.
- Jain SK. 1994. Medicinal plants. 5th ed. New Delhi (India): National Book Trust.
- Jhunjhunwalla A. 2010. Local market report: natural essential oil of Indian origin. Indian Perfum. 54(2):13.
- Kamini, Raina R. 2013. Review of Nardostachys grandiflora: an important endangered medicinal and aromatic plant of western Himalaya. Forest Prod J. 63(1/2):67–71.
- Kaul MK. 2001. Technical report on establishment of gene bank of medicinal plants of N.W. Himalaya. Jammu (India): Department of Biotechnology, Government of India, and Regional Research Laboratory (RRL).
- Kazmi MA, Siddiqui IA. 1953. Medicinal plants of Astore and upper Guraiz valleys. Pak J Forest. 3(4):186–212.
- Keshavarzi M, Abassian S, Sheidai M. 2012. Pollen morphology of the genus Clypeola (Brassicaceae) in Iran. Phytol Balc. 18(1):17–24.
- Khan ANH, Zaidi SH. 1989. Propagation and regeneration technology of pharmacopeial medicinal plants of the temperate regions of Pakistan. Bulletin No. 8. Peshawar (Pakistan): Biological Sciences Research Division, Pakistan Forest Institute.
- Korner C, Paulsen J. 2009. Exploring and explaining mountain biodiversity. In: Spehn EM, Korner C, editors. Data mining for global trends in mountain biodiversity, 1–10. Florida (USA): CRC Press.
- Kuafman PB, Carison TF, Dayanadan P, Evans ML, Fisher JB, Parks O, Wells JR. 1989. Plants, their biology and importance. New York, NY: Hopper and Raw Publishers.
- Lawrence GHM. 1951. Taxonomy of vascular plants. New York, NY: McMillan.
- Lloyd DG, Webb CJ. 1986. The avoidance of interference between the presentation of pollen and stigmas in angiosperms. New Zealand J Bot. 24(1):135–162.
- Medan D, Montaldo NH, Devoto M, Mantese A, Vasellati V, Bartoloni NH. 2002. Plant–pollinator relationships at two altitudes in the Andes of Mendoza. Argentina. Arct Antarct Alp Res. 34(3):233–241.
- Mulliken TA. 2000. Implementing CITES for Himalayan medicinal plants Nardostachys grandiflora and Picrorhiza kurroa. Traffic Bull. 18(3):63–72.
- Mulliken TA, Crofton P. 2008. Review of the status, harvest, trade and management of seven Asian CITES-listed medicinal and aromatic plant species. Bonn (Germany): Federal Agency for Nature Conservation.
- Nautiyal MC, Nautiyal BP. 2004. Agrotechniques for high altitude medicinal and aromatic plants. Dehradun (India): High Altitude Plant Physiology Research Centre, Srinagar, Uttaranchal, India; p. 99–133.
- Nautiyal BP, Nautiyal MC, Khanduri VP, Rawat N. 2009a. Floral biology of Aconitum heterophyllum Wall.: critically endangered alpine medicinal plant of Himalaya. India. Turk J Bot. 33(1):13–20.
- Nautiyal BP, Nautiyal MC, Rawat N, Nautiyal AR. 2009b. Reproductive biology and breeding system of Aconitum balfourii (Benth) Muk: a high altitude endangered medicinal plant of Garhwal Himalaya. Res J Med Plants. 3(2):61–68.
- Nawchoo IA, Rather AM, Ganie AH, Jan TR. 2012. Need for unprecedented impetus for monitoring and conservation of Valeriana jatamansi – a valuable medicinal plant of Kashmir Himalaya. Agri Sci Res J. 2(7):369–373.
- Nayar MP, Sastry ARK. 1988. RED data book of Indian plants. vol 2. Calcutta (India): Botanical Survey of India.
- Nazir R, Zafar R, Wafai BA. 2008. Reproductive ecology of medicinally important Kashmir Himalayan species of Digitalis L. Plant Species Biol. 23(2):59–70.
- Olsen CS. 2005. Trade and conservation of Himalayan medicinal plants: Nardostachys grandiflora DC. and Neopicrorhiza scrophulariiflora (Pennel) Hong. Biol Cons. 125(4):505–514.
- Prakash V. 1999. Indian Valerianaceae. Jodhpur (India): Scientific Publisher; p. 22.
- Raina NS. 2006. Studies on the progeny performance of floral morphotypes in Valeriana jatamansi Jones [PhD thesis]. [Solan, Himachal Pradesh (India)]: Dr. Yashwant Singh Parmar University of Horticulture and Forestry, Department of Forest Products.
- Raina R, Behera MC, Rana RC, Sharma YP. 2003. Reproductive biology of Gentiana kurroo Royle. Curr Sci. 85(5):667–670.
- Samant SS, Dhar U, Plani LMS. 1998. Medicinal plants of Indian Himalaya: diversity distribution and potential values. Nainital (India): Gyanodaya Prakashan.
- Schroter C. 1926. Das Pflanzenleben der Alpen [The plant life in the Alps]. Zurich (Switzerland): Albert Raustein.
- Singh V, Dubey P, Srivastav S, Rawat AKS. 2011. Botanical standardization of the jatamansi, their substitutes and adulterants species. Indian J Trad Knowl. 10(4):599–603.
- Stebbins GL. 1971. Chromosome evolution in higher plants. London: Arnold.
- Thakur PRS, Hussain A. 1989. Major medicinal plants of India. Lukhnow (India): Central Institute for Medicinal and Aromatic Plants, India; p. 28–30.
- Vijayakumar KK, Paulsamy S. 2010. Crude and ecological densities of certain variants of the medicinal shrub, Gaultheria fragrantissima Wallich in Shola forests of Nilgiris, the Western. Ghats. J Life Sci. 2(1):21–25.
- Wafai BA, Siddiqui MAA, Nawachoo IA, Dar NA, Bhat MA, Mohi-Ud-Din GG. 2005. Studies on reproductive biology of some endangered medicinal herbs as a prelude to their ex situ conservation. Ind Bot Soc. 84(4):88–106.
- Wani PA, Ganaie KA, Nawchoo IA, Wafai BA. 2006. Phenological episodes and reproductive strategies of Inula recemosa (astersaceae): critically endangered medicinal herb of N W Himalaya. Int J Bot. 2(4):388–394.
- Wani PA, Nawchoo IA, Wafai BA. 2009. The role of phenotypic plasticity, phenology, breeding behaviour and pollination systems in conservation of Rheum emodi Wall. ex Meisn. (Polygonaceae): a threatened medicinal herb of North West Himalaya. Int J Plant Reprod Biol. 1(2):179–189.
- Weberling F. 1975. On the systematics of Nardostachys (Valerianaceae). Taxon. 24(4):443–452.
- Weberling F. 1989. Morphology of flower and inflorescence. New York, NY: Cambridge University Press.
- Wirth LR, Graf R, Gugerli F, Landergott U, Holderegger R. 2010. Lower selfing rate at higher altitudes in the alpine plant Eritrichium nanum (Boraginaceae). Am J Bot. 97(5):899–901.
- Zaman MB, Khan MS. 1970. Hundred drug plants of West Pakistan. Peshawar (Pakistan): Pakistan Forest Institute.