Abstract
A karyological study of eight populations of Plantago albicans L. (Plantaginaceae) collected from the central high-plain steppe in the province of Djelfa in Algeria was undertaken for the first time. Chromosome numbers were determined as 2n = 2x = 10 for populations growing at medium altitudes (600–900 m) and 2n = 4x = 20 for those growing at higher altitudes (1000–1300 m) of arid bioclimates. Intermediate chromosome numbers 2n = 6, 8, 9, 12, 14, 15, 17 and 18 were found in individuals of the studied populations and were probably aneuploid forms. The analysis of the karyotype asymmetry indices showed that the diploid cytotype (2n = 2x = 10) is characterized by ancestral chromosomes that are symmetrical, large and homogeneous, while the tetraploid cytotype (2n = 4x = 20) is more evolved with asymmetric, small and heterogeneous chromosomes. Based on our findings, it can be suggested that environmental factors and geographical location might have an effect on the genetic structure and evolution of P. albicans populations in the central steppe of Algeria.
Introduction
The genus Plantago (Plantaginaceae) consists of roughly 200 annual and perennial herbs and sub shrubs with a worldwide distribution (Rønsted et al. Citation2002). Some species are cultivated for their various medicinal and economic uses (Pramanick and Sen Raychaudhuri Citation1997), others are high feed-value pastoral species (Le Houérou and Ionesco Citation1973).
Plantago albicans L. is a rosette hemicryptophyte native to the Mediterranean area, Asiatic Turkey and Iran (Greuter et al. Citation1989; Tutel al. Citation2005; Pedrol Citation2009) recognizable by its silky hairy aspect and lanceolate leaves with wavy margins (Coste Abbé Citation1937; Quézel and Santa Citation1963). The flowers are typically wind-pollinated without attractive morphological features (Soekarjo Citation1992). Its rhizomatous basis bears suckers that ensure plant survival in dry years (Puech et al. Citation1998) and enables active vegetative multiplication during the growing season (Pontanier et al. Citation2003).
Plantago albicans is widely distributed along a large altitudinal gradient of 0–1300 m (Veiga-Barbosa and Pérez-García Citation2014). It grows in wastelands, slopes and stony pastures, on dry and sun-exposed soils (Pedrol Citation2009) in Spain, but is less frequent and even rare in Italy where it is restricted to dry and sandy habitats (Pignatti Citation1982). In North Africa it colonizes open semi-arid to arid areas in very heterogeneous environments (Puech et al. Citation1998) and may equally well occur on deep, sandy soil or on surfaces of leveled silt with sharply dipping strata (Vernet Citation1958). It belongs to the Macrochloa tenacissima (alfa-grass) communities on stony glacis in Eastern Tafoughalt in Morroco (Acherkouk et al. Citation2011). In the coastal desert of Egypt, P. albicans was reported to occur on semi-stabilized eolian deposits (El-Nahrawy Citation2011).
In Algeria, P. albicans is among 20 Plantago species reported to grow in various geographical habitats and bioclimatic conditions (Quézel and Santa Citation1963). It is found throughout the country, but is less common in the coastal Tell, growing at less than 400 mm of rainfall per year (Le Houérou Citation1995) on more-or-less fixed coarse sands, within matorral and M. tenaccissima and Artemisia heba halba communities (Pouget Citation1980; Djebaili Citation1984) and can reach the Saharan Northern border at the district of Biskra (Ozenda Citation2004).
Karyological studies on the genus Plantago revealed a high variation in ploidy levels. Petit and Thompson (Citation1999) consider polyploidy as a major process in plant evolution. According to Lumaret et al. (Citation1997) natural polyploidy is often related to a longer life span, vegetative reproduction and higher competitive ability with increased frequency with latitude in the northern hemisphere and high-altitude regions of the world (Grant Citation1981; Brochmann et al. Citation2004). An increase in ploidal level often has been associated with speciation and the origin of novel adaptations as stated by Levin (Citation2002).
From previous reports, Van Dijk and Delden (Citation1990) concluded that more than two thirds of Plantago species were polyploids. This fact was confirmed by Vamosi et al. (Citation2007) who stated that polyploidy is more common than the original diploid state in the genus. According to Groves and Hair (Citation1971) polyploidy level in the genus Plantago reaches up to 16-ploids found in Lake Sylvester, NW Nelson in New Zealand at 1317 m.
While abundant literature exists on cytotaxonomic aspects within the genus Plantago worldwide, no karyological records on the genus have been reported for Algeria. The main objective of the present study is, therefore, to detail somatic chromosome characteristics of Plantago albicans, including their morphology and number within eight populations from the central steppe of Algeria collected in various eco-geographical conditions. Our findings may provide useful information to improve management and conservation of genetic resources of this species in Algeria.
Materials and methods
The material used in the present study was collected during a survey conducted in May–June 2013 in the district of Djelfa (Algerian central steppe) along a north–south transect (Figure ). Sampling was done in homogeneous stations where the main dominant species were observed (Table ). From each P. albicans population at least 30 plants were randomly sampled. Voucher specimens are deposited at the National Institute of Agronomic Research INRAA, Algiers, Algeria.
Figure 1. Distribution of populations of Plantago albicans L. in the province of Djelfa (central steppe of Algeria).
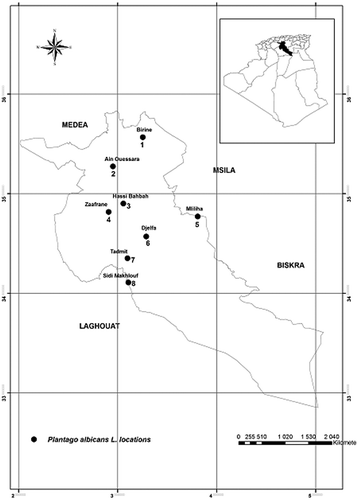
Table 1. Bioclimatic and floristic characteristics of the sampling stations.
Botanical determination of the sampled plant material was done on whole individuals with vegetative and reproductive parts using morphological traits as described in the flora of Algeria (Quézel and Santa Citation1963) as follows:
stemless herb, stem prostrate;
3–5 veined leaves in general caulescent, very hispid;
5–7 mm wide ears usually dense and uninterrupted;
sepals equal, oval, widely scarious;
petals broadly ovate, glabrous; and
fruit in the form of capsule with two oblong seeds.
Furthermore, samples were compared with specimens of P. albicans deposited at the Herbarium of the Ecole Nationale Supérieure Agronomique (ENSA, ex. INA), Algiers, Algeria.
Chromosome counts were carried out on metaphase plates observed in actively growing root meristems, using the Feulgen technique (Jahier et al. Citation1992). Root tips (10–15 mm length) were obtained from germinated seeds, derived from a random plant sample, and placed in sterile Petri dishes at room temperature for 48 h. They were pre-treated in α-bromonaphthalene (2 h) and fixed in 3:1 absolute ethanol:acetic acid at room temperature (24 h). Root tips were then rinsed in distilled water and hydrolyzed at 60°C for 10 min with 1 N HCl, stained in Schiff's reagent for 1–2 h in darkness and finally squashed in 45% acetic acid. Chromosome measurements were based on at least three metaphase plates. Observations were made using a Zeiss-Axiostar photomicroscope (Zeiss, Göttingen, Germany) and photographs of the best preparations were made with the same microscope.
The karyograms were drawn from mitotic metaphases. Length measurements of the short arm (S), the long arm (L) and the total length (C) of each pair of chromosomes from the best metaphase plates were made using cytogenetics software (MicroMeasure software package Version 3.3, Department of Biology, Colorado State University, Fort Collins, USA). The average of total lengths of all chromosomes (Χ), the ratio of long arm and short arm (R) and centromeric index (CI) were calculated.
To assess the existence of published chromosome numbers in P. albicans, several online databases and florae were consulted (Index to Plant Chromosome Numbers (IPCN), Chromosome Counts Database (CCDB); International Association for Plant Taxonomy / International Organization of Plant Biosystematists (IAPT/IOPB) Chromosome Data, CHROBASE (chromosome numbers for the Italian flora), Flora Iberica, and Flora Europea).
Results
Variation of chromosome numbers within populations
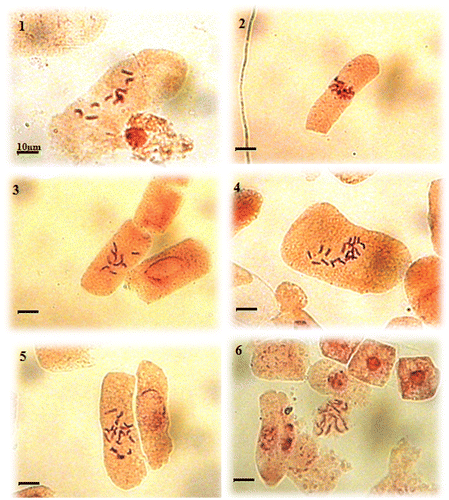
Chromosome counts performed on all metaphase plates (Table ) of all populations showed the existence of two chromosome numbers 2n = 10 and 2n = 20 (Figure ) with high frequencies: 17–62% and 25–82%, respectively. The other numbers (2n = 6, 8, 9, 12, 14, 15, 17 and 18) found in some cells can be assumed to be observation errors or probably aneuploidy phenomena.
Table 2. Cell number, absolute frequencies (AF) and relative frequencies (RF) of the different chromosome numbers found in populations of Plantago albicans L. in the Algerian central steppe.
Figure 2. Microphotographs of somatic metaphases in Plantago albicans L. 2n = 10 (1 to 3) and 2n = 20 (4 to 6).
On the other hand, geographical distribution and frequencies of both chromosome numbers 2n = 10 and 2n = 20 were different. The chromosome number 2n = 10 was less widespread. It was found in six populations with high frequencies in the lower altitudes (600–900 m). Conversely, the chromosome number 2n = 20 had a wider distribution; it was found in all populations and was more frequent at higher altitudes (1000–1300 m).
Karyotype study and karyogram setting
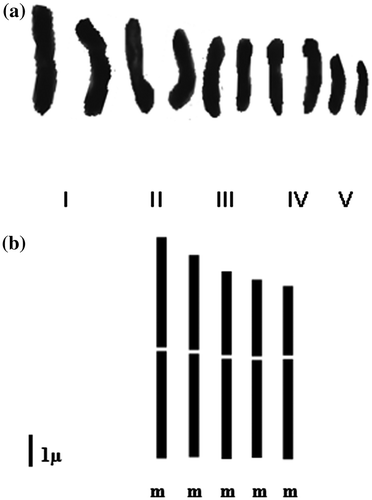
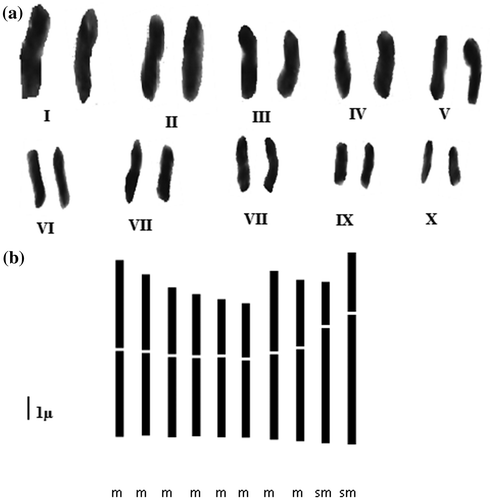
Morphometric parameters performed on all chromosomes of the diploid (2n = 2x = 10) and tetraploid (2n = 4x = 20) cytotypes allowed identification of the homologous chromosomes presented in Tables and .
Table 3. Karyomorphological parameters of the diploid cytotype (2n = 2x = 10) of Plantago albicans L. in the Algerian central steppe.
Table 4. Karyomorphological parameters of the tetraploid cytotype (2n = 4x = 20) of Plantago albicans L. in the Algerian central steppe.
Within the diploid cytotype, chromosome length varied from 3.50 to 7.07 μm with an average total length of 5.15 μm. The values of the asymmetry index IC indicate that all chromosomes are metacentric (Figure ). Within the tetraploid cytotype, chromosome length ranged between 2.21 and 5.95 μm with an average total length of 5.23 μm. The values of the asymmetry index IC revealed eight pairs of metacentric (m) and two pairs of sub-metacentric (sm) chromosomes in the karyotype (Figure ). The formulae of the two cytotypes are 5m + 0sm + 0st for the diploids and 8m + 2sm + 0st for the tetraploids.
Study of asymmetric karyotypes
Values of the two asymmetry indices applied to the diploid and tetraploid cytotypes of Plantago albicans are shown in Table .
Table 5. Values of asymmetry indices of the diploid and tetraploid cytotypes of Plantago albicans L. from the Algerian central steppe.
While the intra-chromosomal asymmetry index A1 (Romero Zarco Citation1986) of diploids was lower than that of tetraploids, the A2 inter-chromosomal index was higher. Similarly, the asymmetry index of Paszko (Citation2006) shows a low value of AI in the diploid cytotype compared to that of the tetraploid cytotype.
Discussion
The majority of samples of the studied P. albicans populations have a chromosome number of 2n = 2x = 10 or 2n = 4x = 20. McCullagh (Citation1934) considered Plantago to be a monophyletic genus with 6 as the original base number from which derived the other basic numbers (x = 5 and x = 4). It can therefore be deduced that our P. albicans populations would be diploids (2n = 2x = 10) and tetraploids (2n = 4x = 20) with the basic number x = 5. These counts are new records for P. albicans from Algeria.
Our chromosome counts are consistent with those found by several authors (Puech Citation1987, Citation1988, Citation1992; Puech et al. Citation1998) on samples of P. albicans originating from North Africa and Spain. For the same species, Mohsenzadeh et al. (Citation2008) reported two chromosome numbers (2n = 8 and 2n = 10) in a population from Iran.
We have not encountered any hexaploid individuals (2n = 6x = 30). These were reported in rainy regions in Tunisia and Morocco (Puech Citation1988), and in dry sandy areas of Italy (Peruzzi and Cesca Citation2002) with the karyotypic formula: 2n = 6x = 30 = 6M + 6stsat + 6sm + 6M + 6sm. Badr and El-Kholy (Citation1987) and Badr et al. (Citation1987) also found hexaploids in populations from the northern coastal region of Egypt. Known chromosome counts of P. albicans are summarized in Table .
Table 6. Chromosome counts reported for Plantago albicans L.Table Footnotea
The general analysis of the available counts reveals that the three ploidy levels (2x, 4x and 6x) are present in the Mediterranean distribution area of P. albicans with the prevalence of hexaploids (2n = 6x = 30, reported in Italy, Spain, Greece, Tunisia, Egypt and Morocco) that seem to be more septentrional. Conversely, tetraploids (2n = 4x = 20) have been found in steppe and arid areas of Spain, Tunisia and Algeria in the present study. Finally, diploids (2n = 2x = 10) have been reported so far only in the Maghreb countries (Morocco and Tunisia) and Algeria in the present study.
Our karyological data showed that individuals of populations sampled at middle elevations (600–900 m) are characterized by a diploid cytotype (2n = 2x = 10) with symmetrical large size and homogeneous chromosomes. Meanwhile, individuals of populations sampled at higher altitudes (1000–1300 m) have a tetraploid cytotype (2n = 4x = 20) evolved, with asymmetrical, small and heterogeneous chromosomes. This fact suggests that eco-geographical conditions might have an effect on changes in chromosome number and genomic structure of P. albicans.
Moreover, tetraploids in the present study belong predominantly to populations growing within the alfa-grass (Macrochloa tenacissima) steppes or their facies of degradation that preferentially occupy high glacis, matorrals and sparse forests at high altitudes. The Algerian steppe is found at altitudes above 600 m (except the Hodna basin steppe: 400 m), which implies an altitudinal gradient that brings more rainfall and lower temperatures compared to lowlands (Le Houérou et al. Citation1977; Pouget Citation1980); this could probably explain the presence of tetraploids at higher elevations as polyploids were reported to have a wider ecological range of tolerances (Schulz-Schaeffer Citation1980). A similar tendency appeared in a study where one hexaploid cytotype (2n = 36) of Plantago depressa was found at 1500 m, while two tetraploid cytotypes (2n = 24) were collected 200 m lower (up to 1300 m) (Bala and Gupta Citation2011).
Other studies have attempted to correlate changes in P. albicans chromosome numbers with eco-geographical conditions. Hence, reports of Puech (Citation1987, Citation1988, Citation1992) stated that the populations from the Tunisian uplands are hexaploids, like those of the wetter regions of the north, whereas those of lowlands in the south are tetraploids and then diploids.
Adaptability implies also a wider geographical distribution, as shown by the analysis of the distribution of P. media cytotypes in Europe where the ancestral diploid was found to be more restricted than that of the derived tetraploid (Van Dijk et al. Citation1992).
Karyotypic evolution in angiosperms involves both aneuploidy and polyploidy (Levin and Wilson Citation1976). Although the process of aneuploidy is reported to be exceedingly rare and involving a very large number of generations (Levin Citation2002), the phenomenon is known within the genus Plantago. Aneuploidy was even reported to be common in the genus by Gorenflot (Citation1985) who reported counts of 8, 9, 10, 11, 12 and 13 to occur in P. coronopus. In the same species, the same and other numbers were also found in other studies (Böcher et al. Citation1953, Citation1955; Fernandes and Frana Citation1972).
About a third of the Plantago species growing in Algeria have been reported to have aneuploidy in their chromosome counts; e.g. in Plantago lanceolata (2n = 12) the following numbers were found: 2n = 14 (Munshi et al. Citation1994), 2n = 13 (Fernandes and Frana Citation1972; Sareen et al. Citation1994; Munshi et al. Citation1995) and 2n = 6 (Baquar and Abid Askari Citation1970). In P. major counts of 2n = 22 and 2n = 6 were reported (Laane Citation1969; Sharma and Koul Citation1995). In P. maritima, counts of 2n = 18 and 2n = 6 were found (Skalinska et al. Citation1961; Laane Citation1969; Gorenflot and Marcotte Citation1970). Stack and Brown (Citation1969) found 2n = 4 in P. subulata, whereas Dhar and Koul (Citation1994, Citation1995) reported counts of 2n = 13 and 2n = 14 in P. lagopus. In P. albicans counts of 2n = 12 and 2n = 31 were reported (McCullagh Citation1934; Puech Citation1987). In light of all the above-mentioned records, deviant counts of P. albicans found in the present study could be assumed as aneuploids that appear mostly as reduced numbers of polyploids. This corroborates the conclusions of Jones (Citation1970) who stated that reduction in number is much more frequent than increase. Aneuploid reduction in chromosome number, and polyploidy are, according to Stebbins (Citation1966), characteristic of many species groups which are outcrossing and occupy pioneer habitats. On the other hand, aneuploidy was reported to possibly contribute to phenotypic variability, and hence adaptability, of neopolyploid populations and to enable transitions between euploid chromosome numbers (Ramsey and Schemske Citation2002).
In the present study, polyploidy in P. albicans seems to be related to environmental factors such as elevation. Changes in environmental conditions and hybridization were reported to confer adaptive capacities enabling polyploids to colonize newly available areas (Stebbins Citation1950, Citation1984), a fact that can promote adaptive evolutionary changes (Otto and Whitton Citation2000). Furthermore, the North African steppe has specific features and its transitional position between two contrasting areas (the desert to the south and the Mediterranean to the north) offers the opportunity to study evolutionary trends of not only P. albicans but also other native species.
To fully determine the cytotaxonomic status of P. albicans in Algeria, more advanced molecular-based genetic characterization is required for a better preservation of the biodiversity of steppe rangelands in Algeria including this species.
Acknowledgements
Thanks are due to Thiziri Khedim and Ouarezki Meriem from Mouloud Mammeri Unversity (Tizi Ouzou District) for technical assistance.
Additional information
Funding
References
- Acherkouk M, Maatougui A, El Houmaiz MA. 2011. Communautés végétales et faciès pastoraux dans la zone de Taourirt-Tafoughalt du Maroc oriental: Ecologie et inventaire floristique. Acta Bot Malacit. 36:125–136.
- Angiosperm Phylogeny Group. 2009. An update of the Angiosperm Phyllogeny Group classification for the orders and families of flowering plants: APG III. Bot J Linn Soc. 161:105–121.
- Badr A. 1980. Chromosome counts of six Egyptian plants. UAR J Bot. 23:127–129.
- Badr A, El-Kholy MA. 1987. Chromosomal studies in the Egyptian flora. 2. Karyotype studies in the genus Plantago L. Cytologia. 52(4):725–731.
- Badr A, Labani R, Elkington TT. 1987. Nuclear DNA variation in relation to cytological features of some species in the genus Plantago L. Cytologia. 52(4):733–737.
- Bala S, Gupta RC. 2011. Chromosomal diversity in some species of Plantago (Plantaginaceae). Int J Bot. 7(1):82–89.
- Baquar SR, Abid Askari SH.1970. Chromosome numbers in some flowering plants of West Pakistan. Genetica Iberica. 22:1–11.
- Bartolo G, Brullo S, Pavone P. 1978. Numeri cromosomici per la flora italiana: 484–493. Inform Bot Ital. 10(2):267–277.
- Bartolo G, Brullo S, Pavone P, Terrasi MC. 1980. Contributo alla cariologia del genere Plantago in Sicilia e Calabria meridionale. G Bot Ital. 114(3–4):100.
- Böcher TW, Larsen K, Rahn K. 1953. Experimental and cytological studies on plant species I. Kohlrauschia prolifera and Plantago coronopus. Hereditas. 39(1–2):289–304.
- Böcher W, Larsen K, Rahn K. 1955. Experimental and cytological studies on plant species III. Plantago coronopus and allied species. Hereditas. 41(3–4):423–453.
- Brochmann C, Brysting AK, Alsos IG, Borgen L, Grundt HH, Scheen AC, Elven R. 2004. Polyploidy in arctic plants. Biol J Linn Soc. 82(4):521–536.
- Brullo S, Pavone P, Terrasi MC. 1985. Considerazioni cariologiche sul genere Plantago in Sicilia. Candollea. 40(1):217–230.
- Coste Abbé H. 1937. Flore descriptive et illustrée de la France, de la Corse et des contrées limitrophes. Paris. Librairie des Sciences et des Arts. 3:141–143.
- Dhar MK, Koul AK. 1994. Genetic diversity among Plantagos XXVI. Ring chromosome aneuploids of Plantago lagopus L. Proc Indian Natl Sci Acad. B60(2):143–149.
- Dhar MK, Koul AK. 1995. A new trisomic in Plantago lagopus L. Chromosome Inf Serv. 58:31–33.
- Djebaili S. 1984. Steppe algérienne, phytosociologie et écologie. Alger: Office des Publications Universitaires. 177 p.
- El-Nahrawy MA. 2011. Egypt: Country pasture/forage resources profile. Rome: Food and Agriculture Organization of the United Nations.
- Fahmy TY. 1955. La cytologie du Plantago albicans en Tunisie. Recl. trav. lab. bot. géol. zool. Fac sci Univ Montpel., Sér Bot. 7(1):115–126.
- Fernandez-Casas J. 1977. Recuentos cromosmicos en plantas vasculares españolas. Saussurea. 8:33–55.
- Fernandes A, Frana F. 1972. Contribution la connaissance cytotaxinomique des spermatophyta du Portugal. VI. Plantaginaceae. Bol. Soc. Brot. 46(2a sèrie):465–501.
- Gonzalez F, Silvestre S. 1980. Numeros cromosmicos para la flora española, 141–149. Lagascalia. 9(2):261–266.
- Gorenflot R. 1985. Niveaux et diversité des variations intra-individuelles. Bull Soc Bot France. Actualités Botaniques. 132(2):7–17.
- Gorenflot R, Marcotte JL.1970. Polyploidisation naturelle dans le complexe du Plantago maritima L. C. r. hebd. séances Acad. sci. 270:1911–1914.
- Grant V. 1981. Plant speciation. 2nd ed. New York, NY: Columbia University Press.
- Greuter WR, Burdet HM, Long G. 1989. Med-Checklist. Genève: Conservatoire et Jardin Botaniques.
- Groves BE, Hair JB. 1971. Contributions to a chromosome Atlas of the New Zealand Flora. 15 miscellaneous families, New Zeal J Bot. 9(4):569–575.
- Humphries CJ, Murray BG, Bocquet G, Vasudevan K. 1978. Chromosome numbers of phanerogams from Morocco and Algeria. Botaniska Notiser. 131(4):391–406.
- Jahier J, Chevre AM, Delourme R, Eber F, Tangay AM. 1992. Techniques de cytogénétique végétale. Paris: Editions de l’INRA; p. 184.
- Jones K. 1970. Chromosome changes in plant evolution. Taxon. 19(2):172–179.
- Laane MM. 1969. Meiosis and structural hybridity in some Norwegian plant species. Blyttia. 27(3):141–173.
- Le Houérou HN. 1995. Bioclimatologie et biogéographie des steppes arides du Nord de l’Afrique. Diversité biologique, développement durable et désertisation. Options Méd Série B. 10:1–396.
- Le Houérou HN, Ionesco T. 1973. L’appétibilité des espèces de la Tunisie steppique. Rome: Division Production et Protection des Plantes, FAO; p. 68.
- Le Houérou HN, Claudin J, Pouget M. 1977. Etude bioclimatique des steppes algériennes (Avec une carte bioclimatique à 1/1.000.000ème). Bull Soc Hist Nat Afr Nord. 3(3):33–74.
- Levin DA. 2002. The role of chromosomal change in plant evolution. New York (NY): Oxford University Press. 226 p.
- Levin DA, Wilson AC. 1976. Rates of evolution in seed plants: net increase in diversity of chromosome numbers and species numbers through time. Proc Natl Acad Sci USA. 73(6):2086–2090.
- Lorenzo-Andreu A. 1951. Cromosomas de plantas de la estepa de Aragon, III., A. Anales Estac. Exp. Aula Dei. 2(2):195–203.
- Lumaret R, Guillerm JL, Maillet J, Verlaque R. 1997. Plant species diversity and polyploidy in islands of natural vegetation isolated in extensive cultivated lands. Biodivers Conserv. 6(4):591–613.
- McCullagh D. 1934. Chromosomes and chromosome morphology in Plantaginaceae I. Genetica. 16(1–2):1–44.
- Mohsenzadeh S, Nazeri V, Mirtadzadini SM. 2008. Chromosome numbers of fifteen species of Plantago L. (Plantaginaceae) From Iran. Iran J Bot. 14(1):47–53.
- Munshi A, Langer A, Koul AK.1994. A novel aneuploid in Plantago lanceolata L. Nucleus (Calcutta). 37(1, 2):23–24.
- Munshi A, Sareen S, Langer A, Koul AK.1995. Cytogenetic studies on primary trisomics of Plantago lanceolata L. Proc Indian Natl Sci Acad B. 61(4):339–346.
- Otto S, Whitton J. 2000. Polyploid incidence and evolution. Annu Rev Genet. 34:401–437.
- Ozenda P. 2004. Flore et végétation du Sahara. Paris: Centre National de Recherche Scientifique Editions; p. 566.
- Paszko B. 2006. A critical review and a new proposal of karyotypes asymmetry indices. Plant Syst Evol. 258(1–2):39–48.
- Pedrol J. 2009. Plantago L. In: Benedi C, Rico E, Güemes J, Herrero A, editors. Flora Iberica. vol XIII. CSIC: Real Jardín Botánico, Madrid; p. 4–38.
- Peruzzi L, Cesca G. 2002. Chromosome numbers of flowering plants from Calabria, S Italy. Willdenowia. 32(2):33–44.
- Petit C, Thompson JD. 1999. Species diversity and ecological range in relation to ploidy level in the flora of the Pyrenees. Evol Ecol. 13(1):45–66.
- Pignatti S. 1982. Flora d'Italia. Bologna: Edagricole.
- Pontanier R, Diouf M, Zaafouri MS. 2003. Ecologie et régime hydrique de deux formations à Acacia raddiana au nord et au sud du Sahara (Tunisie, Sénégal) In: Grouzis M, Le Floc’h E, editors. Un arbre au désert: Acacia raddiana. Paris: Institut de Recherche pour le Développement (IRD). p. 79–101.
- Pouget M. 1980. Les relations sol-végétation dans les steppes Sud algéroises [Thèse Doctorat d’Etat]. Paris: ORSTOM; p. 555.
- Pramanik S, Sen Raychaudhuri S. 1997. DNA content, chromosome composition, and isozyme patterns in Plantago L. Bot Rev. 63(2):124–139.
- Puech S. 1987. Fertilité, aptitudes germinatives et caryotype dans deux populations de Plantago albicans L. (Plantaginaceae) en Tunisie. Bull Soc Bot France. Lettres Bot. 134(2):145–154.
- Puech S. 1988. Graines, aptitudes germinatives et caryotype de populations de Plantago albicans L. (Plantaginaceae) de Tunisie. Bull Soc Bot France. Lettres Bot. 135(4/5):353–359.
- Puech S. 1992. Productions inflorescentielles, aptitudes germinatives et garnitures chromosomiques de populations de Plantago albicans L. (Plantaginaceae) du Maroc, Bull Soc Bot France. Lettres Bot. 139(2):155–160.
- Puech S, Rascol JP, Michel V, Andary C. 1998. Cytogenetics and adaptation to increasingly arid environments: the example of Plantago albicans L. (Plantaginaceae). Biochem Syst Ecol. 26(3):267–283.
- Quézel P, Santa S. 1963. Nouvelle flore de l’Algérie et des régions désertiques méridionales. CNRS. 2:861–866.
- Ramsey J, Schemske DW. 2002. Neopolyploidy in flowering plants. Annu Rev Ecol Syst. 33:589–639.
- Romero Zarco C. 1986. A new method for estimating karyotype asymmetry. Taxon. 35(3):526–530.
- Rønsted N, Chase MW, Albach DC, Bello MA. 2002. Phylogenetic relationship within Plantago (Plantaginaceae): evidence from nuclear ribosomal ITS and plastid trnl-F sequence data. Bot J Linn Soc. 139(4):323–338.
- Runemark H. 1967. Studies in the Aegean flora X. Cytologic and morphologic notes on Plantago. Botaniska Notiser. 120(1):9–16.
- Sareen S, Koul AK, Langer A. 1994. A novel trisomic in Plantago lanceolata L. New Bot. 21(1–4):29–35.
- Schulz-Schaeffer J. 1980. Cytogenetics. Plants, animals, humans. New York (NY): Springer-Verlag. 446 p.
- Sharma N, Koul AK. 1995. Reproductive strategies in weeds Plantago major, P. lanceolata and their cultivated ally P. ovata. Proc Indian Natl Sci Acad B. 61(6):471–478.
- Skalinska M, Piotrowicz M, Sokolowska-Kulczycka A, Kalita J, Jaworska H, Czapik R, Czapska D, Gacek E, Banach-Pogan E, Wcislo H. 1961. Further additions to chromosome numbers of Polish Angiosperms. Acta Soc Bot Pol. 30(3):463–489.
- Soekarjo R. 1992. General morphology in Plantago. In: Kuiper PJC, Bos M, editors. Plantago: a multidisciplinary study. Berlin: Springer-Verlag; p. 6–12.
- Stack SM, Brown WV.1969. Somatic and premeiotic pairing of homologues in Plantago ovata. Bull Torrey Bot Club. 96(2):143–149.
- Stebbins GL. 1950. Variation and evolution in plants. New York (NY): Columbia University Press.
- Stebbins GL. 1966. Chromosomal variation and evolution. Science. 152(3728):1463–1469.
- Stebbins GL. 1984. Polyploidy and the distribution of the arctic-alpine flora: new evidence and a new approach. Bot Helv. 94:1–13.
- Tutel B, Kandemir I, Kus S, Kence A. 2005. Classification of Turkish Plantago L. species using numerical taxonomy. Turk J Bot. 29(1):51–61.
- Vamosi JC, Goring SJ, Kennedy BF, Mayberry RJ, Moray CM, Neame LA, Tunbridge ND, Elle E. 2007. Pollination, floral display, and the ecological correlates of polyploidy. Funct Ecosyst Commun. 1(1):1–9.
- Van Dijk P, Van Delden W. 1990. Evidence for autotetraploidy in Plantago media and comparisons between natural and artificial cytotypes concerning cell size and fertility. Heredity. 65(3):349–357.
- Van Dijk P, Hartog MV, Van Delden W. 1992. Single cytotype areas in autopolyploid Plantago media L. Biol J Linn Soc. 46(4):315–331.
- Veiga-Barbosa L, Pérez-García F. 2014. Germination of mucilaginous seeds of Plantago albicans (Plantaginaceae): effects of temperature, light, pre-sowing treatments, osmotic stress and salinity. Aust J Bot. 62(2):141–149.
- Vernet A. 1958. Climates and vegetation. Arid Zone Research, Climatology, Review of Research, 75–101, Paris: United Nations Educational Scientific and Cultural Organization.
- Vogt R, Oberprieler C. 2009. Chromosome numbers of North African Phanerogams. IX. In: Marhold K, editor. IAPT/IOPB chromosome data 8. Taxon. 58:1282–1283. E3–E9.