Abstract
This study was performed to explore the genotoxic and apoptotic effects of Cissus quadrangularis L. ethanolic extract in A431 cells. Cissus quadrangularis L. (CQ) is widely used in Ayurvedic medicines. Estimation of cytotoxicity and apoptotic effects of CQ extract in A431 cells was assessed over 24 h. An 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay showed the CQ extract induced cytotoxicity in a dose-dependent manner. However, CQ extract (100 μg ml−1) induced significant production of intracellular reactive oxygen species (ROS) in A431 cells. With acridine orange (AO) staining analysis, a significant induction of chromosomal condensation was observed at higher dose of CQ extract. DNA fragmentation analysis showed that CQ extract caused DNA damage in A431 cells in a concentration-dependent manner. Our results suggest that CQ extract can induce cell death in the A431 cell line. The study points towards the capability of the CQ extracts to produce stress and in consequence apoptosis and genome instability.
Introduction
Cissus quadrangularis L. has been widely used as medicine in India and is popularly known as Hadjod. Cissus quadrangularis (CQ) extracts have been used as antiviral and antibacterial agents and as free radical scavengers (Chidambara et al. Citation2003; Panthong et al. Citation2007; Balasubramanian et al. Citation2010). Ethyl ethanoate extracts of Cissus quadrangularis were found to suppress NF-kB activation and stimulation of heme oxygenase-1 (Srisook et al. Citation2011). Acetonic extracts of Cissus quadrangularis exerted anti-inflammatory activity against COX and 5-LOX enzymes. Different Cissus quadrangularis extracts were used to treat bone fracture healing and osteoporosis (Muthusami et al. Citation2011). Skin is the first protection against infection, heat and injury for the body and contains fat, water and vitamins. Molassiotis et al. (Citation2006) reported that skin cancers occurred due to excessive exposure to ultraviolet radiation. Prajapati et al. (Citation2003) reported that extracts of CQ stem produces irritation on skin due to the presence of many methyl tritiacontonic acid and calcium oxalate. Extracts of CQ have been used in preventing metabolic disorders and as a scavengers and antioxidants in vitro (Mallika and Shyamala Citation2005; Oben et al. Citation2006). A mixture of stem extracts of CQ and sesame oil was used in treatment of skin disease and coughs (Rastogi and Mehrotra Citation1995). Jainu et al. (Citation2006) reported that CQ extracts (0.5 g kg−1) increased glycoproteins and secretion of mucin. Ethonolic extracts of CQ (0.5 g kg−1) control loss of bone in mice after feeding for 11 weeks (Banu et al. Citation2012). Studies of the genotoxic characteristics of man-made and naturally occurring substances are important if they have wide use as herbal remedies, therapeutic products, ingredients of food or other environmental chemicals.
The current analysis was performed to screen the remedial importance of Cissus quadrangularis extracts. Therefore, this study was prepared to evaluate cellular toxicity and apoptotic effect of Cissus quadrangularis extract in A431 cells.
2. Materials and methods
2.1. Chemicals
Propidium iodide, 2,7-dichlorofluorescin diacetate (DCFH-DA), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO, USA). DMEM/F-12 medium, fetal bovine serum and penicillin-streptomycin were obtained from Invitrogen Co. (Carlsbad, CA, USA).
2.2. Preparation of Cissus quadrangularis extract
Cissus quadrangularis L. (Vitaceae) was collected from India and its stem part was dried for four to five months in the dark, and then ground and extracted consecutively by using Soxhlet apparatus with water and ethyl alcohol. For extraction 200 g of Cissus quadrangularis powder was used. We achieved ethanolic extracts of 1.10 g and water extracts of 1.0 g.
Dry extracts were dissolved in DMSO and liquefied in phosphate buffer saline and filtered at 3000 × g for 15 min. We used supernatant to examine the preventive effect on A431 cell growth.
2.3. A431 cell culture and treatment with CQ extract
A431 cells were procured from NCCS, Pune, India. A431 cells were developed as a single layer in DMEM added with antibiotics (1%) and fetal calf serum (10%) at 37°C in an incubator (5% carbon dioxide). A431 cells were treated with different concentrations (25, 50, 100 and 250 μg ml−1) CQ extracts for 24 h. Untreated A431 cells were used as control.
2.4. Cell morphology
The morphology of cells after exposure of CQ extract (0, 10, 25, 50,100 and 250 μg ml-1) for 24 h was seen under inverted phase contrast microscopy (Nikon ECLIPSE Ti-S, Tokyo, Japan).
2.5. MTT assay
Cytotoxicity of different concentrations (0, 25, 50, 100 and 250 μg ml-1) of CQ extract was evaluated by MTT assay for 24 h according Mossman et al. (Citation1983) method.
2.6. Reactive oxygen species
Intracellular reactive oxygen species (ROS) production was observed by using 2,7-dichlorodihydrofluorescein diacetate a. fluorescent quest for ROS measurement. It is oxidized into a fluorescent compound 2’, 7’ –dichlorofluorescein (DCF) with ROS (Keston and Brandt, Citation1965). Intensity of fluorescent was measured with spectrophotometer fluorescent (Varian, Victoria, Australia.) at 500 nm (excitation wavelength) and 529 nm (emission wavelength).
A set of cells (5 × 104 per well) was analyzed for intracellular ROS using fluorescence microscope (Nikon ECLIPSE Ti-S).
2.7. Apoptosis by DAPI staining
The apoptotic effect of CQ extract on A431 cells was analyzed by using fluorescent nuclear dye DAPI (Lewandowska et al. Citation2013).
2.8. DNA fragmentation assay
CQ extract (25, 50, and 100 μg ml−1) treated and untreated A431 cells (2 × 106) were washed twice with PBS (1×) without calcium and magnesium. Lysis of cells was performed in lysis buffer containing EDTA (10 mM l−1), 0.5% Triton X-100 and TRIS (10 mM l−1, pH 8). Proteinase K (1 mg ml−1) was added and cells were incubated for 1 h at 37°°C followed by a 10 min incubation at 70°C. Then RNAase (200 μg ml−1) was added and cells were incubated for another 1 h at 37°C. Samples were transferred to 2% agarose gel and run with 40 V at 3 h. DNA fragments were visualized by UV illuminator.
2.9. Statistical analysis
Results of triplicate observations were presented as average ± SE and tested by one-way analysis of variance (ANOVA). P-values less than 0.05 were considered statistically significant.
3. Results
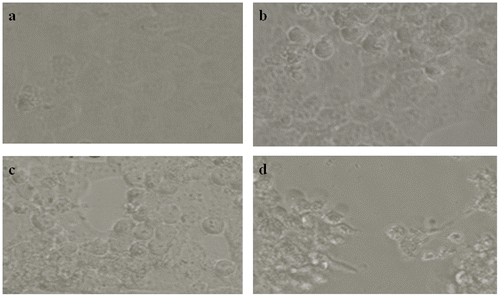
3.1. Morphological changes
There are no modifications in A431 cells at 10 μg ml−1 but at 25, 50,100 and 250 μg ml−1 CQ extract treated A431 cells transformed into a circular shape and disconnected from the surface (Figure b–d).
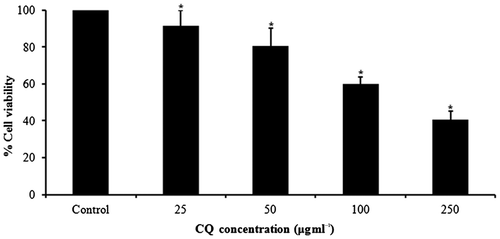
3.2. CQ extract induced cytotoxicity in A431cells
MTT data indicated dose-dependent toxicity in A431 cells due to CQ extract (Figure ). The cell toxicity was 8.46% at 25 μg ml−1, 19.37% at 50 μg ml−1, 40% at 100 μg ml−1 and 59.26% at 250 μg ml−1 of CQ extract over 24 h (Figure ).
3.3. CQ extract induced ROS generation and oxidative stress
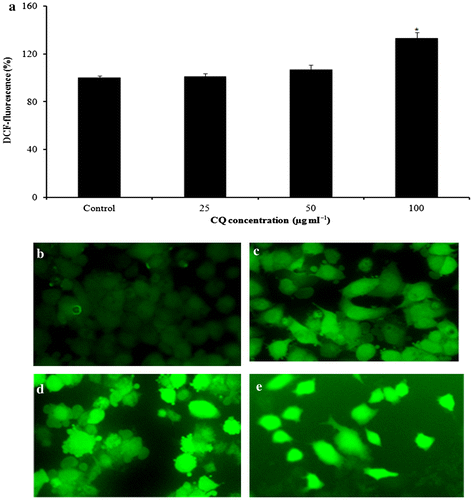
The capacity of CQ extract to produce oxidative stress was decided by quantifying the ROS levels in A431 cells. Data revealed that CQ extract encouraged the intracellular ROS production in a dose-dependent basis (Figure ).
Figure 3. CQ extract induced ROS generation in A431 cells. (a) Percentage of ROS production due to CQ extract in A431 cells. (b–e) Photomicrographs captured with a fluorescence microscope after CQ extract exposure for 24 h: (b) untreated; (c) at 25 μg ml−1; (d) at 50 μg ml−1; (e) at 100 μg ml−1.*p < 0.05 versus control.
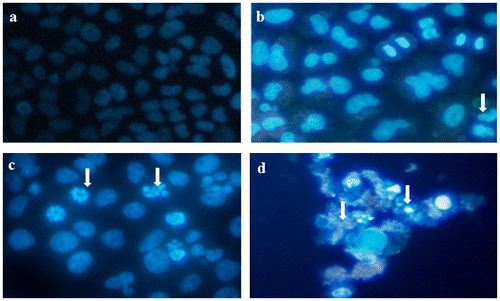
3.4. Induction of chromosomal condensation by CQ extract
Chromosome shrinkage was observed by DAPI staining. A431 cells treated with CQ extract (25, 50 and 100 μg ml−1) for 24 h induced chromatin shrinkage in dose-dependent basis (Figure ).
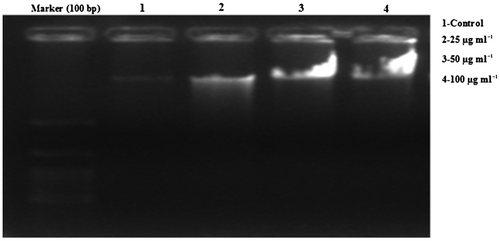
3.5. DNA ladder assay
The most important parameter for apoptosis is DNA fragmentation. Figure shows that exposure of A431 cells to CQ extract resulted in the development of fragments that may be detectable through electrophoretic measurement as a character of ladder form.
4. Discussion
Edible plant material contains many micro-constituents and it is active in organisms. CQ extract has shown cytotoxic activity against A431 skin cancerous cells and can be effectively used in treatment of skin cancer. This work reported the preventive character of CQ extract in A431 cells. The present data indicate that CQ extract induces cytotoxicity and apoptotic activity against A431 cells. A 250 μg ml−1 CQ extract prevents growth of A431 cells.
In the present study apoptotic activity induced by CQ extract was confirmed by acridine orange (AO) markers and disintegration of DNA are established markers of cell death. Cytotoxicity of CQ extract was measured by the 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide test, and a dose-related toxicity of CQ extract was observed.
Oxidative stress is a discrepancy between the appearance of reactive oxygen species and a living system’s capability to detoxify the reactive species or to heal the damage. Sotgia et al. (Citation2011) reported that cells induce toxic effects by the production of free radicals and super oxides, which degrades cell components such as lipid, protein and DNA. Diers et al. (Citation2012) documented that mitochondria act as metabolic centers in cells of eukaryotic organisms and source of reactive oxygen species. During apoptosis, mitochondria undergo specific damage that results in loss of their function. CQ extract induce the ROS (free radicals) production which leads to activation of the apoptotic pathway and consequently changing the cellular morphology and finally DNA fragmentation and cell death.
Intracellular reactive oxygen species were evaluated in CQ extract treated A431 cells by DCFH-DA. Reactive oxygen species are a group of different free radicals such as OH•, and O2-, and can degrade cell components (Rana Citation2008). ROS produce free radicals due to contact with cell organelles such as degradation of mitochondria. On the other hand reactive oxygen species produced through stimulation enzyme NADPH oxidase that is liable for super oxide generation phagocytic cells. ROS can oxidize and decrease cell molecules as a effect cell damage. We found that CQ extract significantly generated toxic effect in A431 cells. DNA damage may either induce carcinogenesis or cell death, thus disrupting normal cell function. We observed the apoptotic potential of CQ extract in A431 cells by DNA fragmentation assay, which is capable of identifying fragmented DNA.
In conclusion, the present study indicates in vitro anti-proliferative and apoptotic efficacy of CQ extract on A431 cells and suggests the possibility for applications of the CQ extract in related biomedical sciences. Further study is needed to investigate the mechanism of the anti-proliferative activity of CQ extract on cancer cell lines.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding information
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this Research group NO [RG-1435-076].
References
- Balasubramanian P, Jayalakshmi K, Vidhya N, Prasad R, Sheriff AK, Kathiravan G, Rajagopal K, Sureban SM. 2010. Antiviral activity of ancient system of Ayurvedic medicinal plant Vitis quadrangularis L. (Vitaceae). J Basic Clin Pharm. 1(1):37–40.
- Banu J, Varela E, Bahadur AN, Soomro R, Kazi N, Fernandes G. 2012. Inhibition of bone loss by Vitis quadrangularis in mice: a preliminary report. J Osteoporos. 2012:10p., 101206. doi:10.1155/2012/101206.
- Chidambara MKN, Vanitha A, Mahadeva SM, Ravishankar GA. 2003. Antioxidant and antimicrobial activity of Vitis quadrangularis L. J Med Food. 6(2):99–105.
- Diers AR, Broniowska KA, Chang CF, Hogg N. 2012. Pyruvate fuels mitochondrial respiration and proliferation of breast cancer cells: effect of monocarboxylate transporter inhibition. Biochem J. 444(3):561–571.
- Jainu M, Devi CSS, Vijaimohan K. 2006. Protective effect of Vitis quadrangularis on neutrophil mediated tissue injury induced by aspirin in rats. J Ethnopharmacol. 104(3):302–305.
- Keston AS, Brandt R. 1965. The fluorometric analysis of ultramicro quantities of hydrogen peroxide. Anal Biochem. 1:1–5.
- Lewandowska U, Szewczyk K, Owczarek K, Hrabec Z, Podsędek A, Koziołkiewicz M, Hrabec E. 2013. Flavanols from evening primrose (Oenothera paradoxa) defatted seeds inhibit prostate cells invasiveness and cause changes in Bcl-2/Bax mRNA ratio. J Agric Food Chem. 61(12):2987–2998.
- Mallika J, Shyamala CSD. 2005. In vitro and in vivo evaluation of free radical scavenging potential of Vitis quadrangularis. Afri J of Biomed Res. 8(2):95–99.
- Molassiotis A, Panteli V, Patiraki E, Ozden G, Platin N, Madsen E, Browall M, Fernandez-Ortega P, Pud D, Margulies A. 2006. Complementary and alternative medicine use in lung cancer patients in eight European countries. Complement Ther Clin Pract. 12(1):34–39.
- Mossman T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Method. 65(1–2):55–63.
- Muthusami S, Ramachandran I, Krishnamoorthy S, Govindan R, Narasimhan S. 2011. Vitis quadrangularis augments IGF system components in human osteoblast like SaOS-2 cells. Growth Hormone IGF Res. 21(6):343–348.
- Oben J, Kuate D, Agbor G, Momo C, Talla X. 2006. The use of a Vitis quadrangularis formulation in the management of weight loss and metabolic syndrome. Lipids Health Dis. 5:24.
- Panthong A, Supraditaporn W, Kanjanapothi D, Taesotikul T, Reutrakul V. 2007. Analgesic, anti-inflammatory and venotonic effects of Cissus quadrangularis Linn. J Ethnopharmacol. 110(2):264–270.
- Prajapati ND, Purohit SS, Sharma AK, Kumar T. 2007. A hand book of medicinal plants. Jodhpur, Section II:86.
- Rana SV. 2008. Metals and apoptosis: recent developments. J Trace Elements Med Biol. 22(4):262–284.
- Rastogi R, Mehrotra BN. 1995. Compendium of Indian medicinal plants. Part IV: Central Drug Research Institute. New Delhi: Lucknow and National Institute of Science Communication.
- Sotgia F, Outschoorn U, Lisanti MP. 2011. Mitochondrial oxidative stress drives tumor progression and metastasis: should we use antioxidants as a key component of cancer treatment and prevention? BMC Med. 9(62):1–5.
- Srisook K, Palachot M, Mongkol N, Srisook E, Sarapusit S. 2011. Anti-inflammatory effect of ethyl acetate extract from Vitis quadrangularis Linn may be involved with induction of heme oxygenase-1 and suppression of NF-κB activation. J Ethnopharmacol. 133:1008–1014.