Abstract
Chromosome studies have been carried out on two endemic species of the northern Western Ghats, India, viz. Barleria lawii and B. sepalosa. Both species showed 2n = 40 mitotic chromosomes. Chromosomes had median region centromeres and both species exhibited a karyotype formula of 20m. Karyotypes were moderately symmetrical and fell into category 4b of Stebbins’ asymmetry classes. Values for the intrachromosomal index (A1) for Barleria lawii and B. sepalosa were 0.23 and 0.28, respectively, while the interchromosomal index (A2) for the former was 0.19 and 0.23 for the latter. Meiosis was found to be normal and both species had 20 bivalents at diplotene/diakinesis. These data on cytogenetics add to the information about karyosystematics of Barleria section Chrysothrix and will be useful in understanding species interrelationships and also in undertaking hybridization programmes for producing hybrids of ornamental interest. Since gross chromosome morphology of the two species in particular, and all Barleria species for which chromosome data are available in general, is similar, it is suggested that further linear differentiation of chromosomes and better understanding of karyotype evolution in Barleria should be possible using banding techniques and fluorescence in situ hybridization (FISH) approaches.
Introduction
Barleria L. is one of the largest genera of the family Acanthaceae Juss. The genus comprises about 254 species and eight varieties (The Plant List 2013). It is divided into two subgenera, Barleria L. and Prionitis (Nees) Lindau, and seven sections. The former comprises two sections (Barleria L. and Chrysothrix M. Balkwill) and the latter five [Cavirostrata M. Balkwill, Fissimura C. B. Clarke, Prionitis (Nees) Lindau, Somalia (Oliv.) Lindau and Stellatohirta C. B. Clarke] (Balkwill and Balkwill Citation1997). Five sections are reported from India (Shendage and Yadav Citation2010) where there are 27 species, one subspecies and one variety (Shendage and Yadav Citation2010; Gosavi et al. Citation2013). Two sections, Barleria (Barleria acanthoides Vahl, B. buxifolia L., B. cristata L., B. longiflora L., B. mysorensis Roth., B. noctiflora L.f., B. pilosa B. Heyne ex Nees, B. repens Nees, B. tomentosa Roth) and Chrysothrix (Barleria acuminata Wight ex Nees, B. courtallica Nees, B. involucrata Nees, B. lawii T. Anderson, B. nitida Nees, B. sepalosa C. B. Clarke, B. strigosa Willd., B. terminalis (Nees) C. B. Clarke, B. vestita T. Anderson) have the largest representation in India with nine species each. Sections Cavirostrata and Prionitis are represented by four (Barleria gibsonii Dalzell, B. grandiflora Dalzell, B. montana Nees, B. prattensis Santapau) and three (Barleria cuspidata B. Heyne ex Nees, B. lupulina Lindl., B. prionitis L.) species, respectively. Somalia is the smallest section with two species (Barleria hochstetteri Nees and B. stocksii T. Anderson). Of the 29 taxa, 15 are endemic to India (Table ).
Table 1. Species (across subgenera and sections) of Barleria that occur in India and their diploid chromosome numbers (2n).
Barleria is well known for its large attractive flowers that come in many colours (purple, pink, white and yellow). This along with amenable chromosome size has prompted cytological investigation on the genus and, consequently, a total of 20 Indian species have been examined cytologically (Table ). Seven species, viz. Barleria hochstetteri, B. montana, B. nitida, B. pilosa, B. prattensis, B. stocksii and B. vestita, are yet to be investigated karyologically, owing to their restricted distribution. Of these, four species are endemic. Among the seven species, Barleria hochstetteri is very rare and it has not been collected in India for a long time (Shendage and Yadav Citation2010). It is distributed in North West India (Gujarat and Rajasthan). Barleria sepalosa has been recently collected by Gosavi et al. (Citation2013). Barleria stocksii is now known from Andhra Pradesh and Gadag district in Karnataka (Sankar et al. Citation2005; Kambhar et al. Citation2014). Barleria vestita is found in India as well as Sri Lanka. In India the species is only known from the collections of Lawson and Beddome housed at the Madras Herbarium (MH), Coimbatore (Shendage and Yadav Citation2010).
Cytogenetical data on endemic species are important from the perspective of understanding their biology. Karyological information such as chromosome number and the morphology and symmetry of chromosomes is useful in deducing evolution and interrelationships within the group (Gosavi et al. Citation2011). Chromosome information is an additional parameter that can refine the existing classification. We studied two endemic species, Barleria lawii and B. sepalosa, for which chromosomal data were previously lacking.
Material and methods
Barleria sepalosa was collected from the Torna fort (18°16.802′N, 073°37.397′E, 1165 m), Pune district, Maharashtra, India while B. lawii was collected from the Botanical Garden (16°40.547′N, 074°15.337′E, 587 m) of the Department of Botany, Shivaji University, Kolhapur. Both species are now being maintained in cultivation in the botanical garden. The voucher specimens are deposited in the Herbarium of the Department of Botany (SUK), Shivaji University, Kolhapur, Maharashtra, India. Mitosis was studied from root tips of germinated seeds. Root tips of 6–10 mm length were pretreated with saturated solution of para-dichlorobenzene for 3–4 h at 9 ± 3°C and then squashed in 2% propionic orcein. For meiotic studies, smears of developing anthers from appropriately sized flower buds were made after fixing in Carnoy’s fluid (3:1 ethanol and acetic acid) for 3 h using propionic orcein. Suitable mitotic and meiotic plates from freshly prepared slides were photographed with a LEICA DM 2000 fluorescence microscope with attached camera at 1000 × magnification. Ten well-separated somatic chromosome plates were selected for karyotype analysis for each species. Chromosomes were classified according to Levan et al. (Citation1964). Ideograms were constructed using the software Ideokar 1.2 (Mirzaghaderia and Marzangib Citation2015). The degree of karyotype asymmetry has been determined using intrachromosomal (A1) and interchromosomal (A2) indices (Romero Zarco 1986) and the categories of Stebbins (Citation1971).
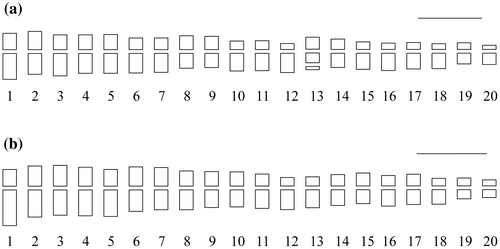
Results
Barleria lawii and B. sepalosa showed somatic counts (2n) of 40 (Figure 1a and f, respectively). The mean chromosome length (MCL) was 2.09 ± 0.40 μm in the former and 2.82 ± 0.66 μm for the latter. The longest chromosome measured 4.13 ± 0.74 μm in Barleria sepalosa while for B. lawii it was 2.88 ± 0.40 μm. The length of the shortest chromosome was 1.38 ± 0.22 μm and 1.71 ± 0.37 μm for Barleria lawii and B. sepalosa, respectively. The chromosome pair 13 in Barleria lawii showed secondary constriction. Total haploid genome length (TCL) of Barleria sepalosa was higher (56.35 ± 0.66 μm) than that of B. lawii (41.80 ± 0.40 μm). Chromosomes in both the species had median region centromeres and hence the karyotype formula 20m. Figure. shows the ideogram. Karyotypes were moderately symmetrical in both the species and fell in 4b category of Stebbins’s asymmetry classes. The values of intrachromosomal (A1) and interchromosomal (A2) indices were higher for Barleria sepalosa. A1 and A2 indices for Barleria lawii were 0.23 and 0.19, respectively while for Barleria sepalosa A1 was 0.28 and A2 0.23.
Figure 1. B. lawii: (a) mitotic metaphase (2n = 40); (b, c) PMCs at diplotene (n = 20); (d) PMC at metaphase-I showing precocious separation; (e) PMC at telophase-I. Barleria sepalosa: (f) mitotic metaphase (2n = 40); (g) PMC at diakinesis (n = 20); (h) PMC at metaphase-I; (i) PMC at anaphase-I. Scale bar = 10 μm.
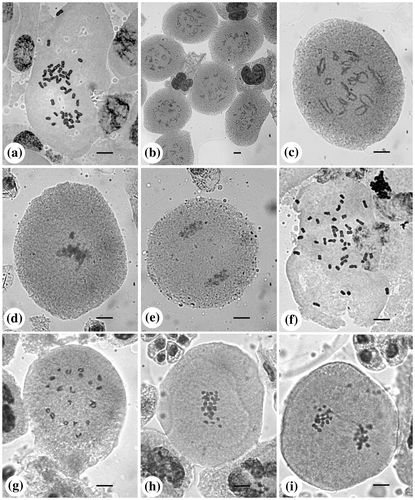
Meiosis was found to be normal in both the species. Pollen mother cells (PMCs) of Barleria lawii (Figure 1b and c) and B. sepalosa (Figure 1g) showed 20 bivalents at diplotene/diakinesis.
Discussion
The genus Barleria is the source of a number of ornamental species and has been studied well from the cytogenetics point of view in India (Table ). The diploid chromosome number ranges from 2n = 24 in Barleria noctiflora to 2n = 44 in B. grandiflora (Table ). The most common somatic number in Barleria is 2n = 40 which suggests that the base number (x) for the genus is 10. Somatic counts deviating from 2n = 40 (i.e. 2n = 30, 32, 34, 36, 38 and 42) in different horticultural varieties of Barleria cristata have been ascribed to aneuploid alterations and hybridization (Ranganath and Krishnappa Citation1990). Most of the species in genus Barleria have symmetrical (2b) karyotypes with m-type chromosomes, except B. acuminata which has an asymmetrical karyotype with sm, st and t-type of chromosomes, which falls in category 3b (Devi and Mathew Citation1991). However, Barleria gibsonii with 14 acrocentric chromosomes has been listed as having a more evolved karyotype than B. acuminata since it has no acrocentric chromosomes in its complement (Ranganath and Krishnappa Citation1990). The primitive nature of the karyotype of Barleria acuminata may further be corroborated by the presence of small chromosomes.
Ranganath and Krishnappa (Citation1990), while studying the karyotype of six taxa of Barleria (B. acuminata, B. buxifolia, B. gibsonii, Barleria involucrata var. elata, B. noctiflora and B. strigosa) observed the shortest chromosomes (1.73 μm) in Barleria acuminata. Barleria lawii and B. sepalosa also have symmetrical karyotypes falling in category 4b and all chromosomes have median region centromeres. Total chromatin length (TCL) value in Barleria ranges from 18.5 μm in Barleria noctiflora to 94.89 μm in Barleria involucrata var. involucrata (Govindarajan and Subramanian Citation1983). However, usually the TCL value ranges between 45 μm and 65 μm (Ranganath and Krishnappa Citation1990). This holds true for both Barleria lawii and B. sepalosa with TCL values 41.80 ± 0.40 μm and 56.35 ± 0.66 μm, respectively. Amongst Barleria acuminata, B. buxifolia, B. gibsonii, B. involucrata var. elata and B. strigosa, B. involucrata var. elata had the longest chromosomes, i.e. up to 7 μm (Ranganath and Krishnappa Citation1990). Longer chromosomes account for the higher TCL value of Barleria involucrata.
The values for intrachromosomal (A1) and interchromosomal (A2) asymmetry indices were higher in Barleria sepalosa than B. lawii. Intrachromosomal index is due to centromeric position while interchromosomal index depicts heterogeneity among chromosome sizes in a complement. Since both the species have symmetrical (4b) karyotype with m-type chromosomes, A1 and A2 indices aid the further differentiation of these species. Higher asymmetry values for Barleria sepalosa may be correlated to the advanced nature of its karyotype. Nevertheless, molecular phylogenetics is needed to deduce the karyotype evolution in Barleria. The karyotypes of most of the Barleria species are based on x = 10 (Devi and Mathew Citation1991) and hence are tetraploids. In order to ascertain the nature of ploidy, meiotic studies are a prerequisite. However, meiotic studies on Indian Barleria species are very few. Meiosis in Barleria lawii and B. sepalosa was regular and without any multivalent association, which suggests that they are allopolyploids. Multivalent associations have been observed in Barleria courtallica and it is suggested to be an autopolyploid (Devi and Mathew Citation1991).
Barleria lawii and B. sepalosa are currently classified in section Chrysothrix. Of the other Indian species, Barleria acuminata, B. courtallica, B. involucrata, B. nitida, B. sepalosa, B. strigosa, B. terminalis and B. vestita, B. nitida and B. vestita are yet to be studied karyologically while the remaining species show a uniform diploid number of 2n = 40, as do Barleria lawii and B. sepalosa, indicating that the section Chrysothrix is homogeneous. Karyotypes of Barleria lawii and B. sepalosa are most similar to B. courtallica as all possess m-type chromosomes. Barleria involucrata var. elata and B. strigosa have more asymmetrical karyotypes with 6 and 8 sub-terminal chromosomes, respectively (Ranganath and Krishnappa Citation1990).
Barleria species are potential ornamentals and since there is fairly good representation of the genus in India, hybridization studies hold great promise for developing commercially viable varieties. Krishnaswami and Menon (Citation1974) attempted hybridization in Barleria. They made crosses between Barleria cristata and B. mysorensis, B. cristata and B. noctiflora, and between B. prionitis and B. noctiflora. These six combinations involving four species yielded only one interspecific hybrid (B. prionitis × B. noctiflora). Nevertheless, the hybrid obtained was more vigorous than either parent and exhibited profuse branching and flowering (Krishnaswami and Menon Citation1974). Meiosis was regular and chromosome association showed only bivalents, indicating homology between the karyotyopes of B. prionitis and B. noctiflora (Krishnaswami and Menon Citation1974). Although this interspecific hybrid failed to set seed this does not undermine the potential of interspecific hybridization in Indian Barleria.
Hybridization studies are not only important for commercial exploitation of this ornamental genus but for revealing interrelationships amongst its species. Analysis of hybrid meiosis gives an opportunity to assess the homology of the two different genomes and hence their taxonomic affinities. Prior knowledge of the cytogenetics, particularly for those species which possess ornamental traits, will definitely prove handy in hybridization programmes. Furthermore, use of banding techniques and fluorescence in situ hybridization (FISH) would be useful to provide evidence for karyotype evolution in Barleria as the majority of the species have similar karyotype features (m-type chromosomes, symmetrical karyotypes), so conventional chromosome data (primary constriction, arm ratio, etc.) may not be sufficient in differentiating species.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgements
The authors are grateful to the Head, Department of Botany, Shivaji University, Kolhapur for providing research facilities.
References
- Balkwill MJ, Balkwill K. 1997. Delimitation and infra-generic classification of Barleria (Acanthaceae). Kew Bull. 52(3):535–573.
- Daniel TF, Chuang TI. 1998. Chromosome numbers of cultivated Acanthaceae and systematic implications. In: Mathew P, Sivadasan M, editors. Diversity and taxonomy of tropical flowering plants. Calicut (India): Mentor Books; p. 309–330.
- Datta PC, Maiti RK. 1970. Relationships of Justiceae (Acanthaceae) based on cytology. Genetica. 41(3):437–450.
- De A. 1963. Cyto and histogenetical studies in the family Acanthaceae. Report Bose Inst. 24:65–66.
- Devi GV, Mathew PM. 1991. Cytological studies in the south Indian Acanthaceae genus Barleria L. Cytologia. 56(3):353–357.
- Ellis JL. 1962. Chromosome numbers in some members of Acanthaceae. Sci Cult. 28:191–192.
- Gosavi KVC, Lekhak MM, Chandore AN, Yadav SR. 2011. Karyology of Barleria grandiflora Dalzell (Acanthaceae), a potential ornamental endemic to Northern-Western Ghats of India. Nucleus. 54(3):133–136.
- Gosavi KVC, Nalawade AD, Yadav SR. 2013. Taxonomic identity, rediscovery and epitypification of Barleria sepalosa (Acanthaceae) from Northern Western Ghats. India. Rheedea. 24(1):23–26.
- Govindarajan T, Subramanian D. 1983. Karyomorphological studies in south Indian Acanthaceae. Cytologia. 48(3):491–504.
- Govindarajan T, Subramanian D. 1985. Karyomorphological studies in south Indian Acanthaceae. Cytologia. 50(3):473–482.
- Grant WF. 1955. A cytogenetic study in the Acanthaceae. Brittonia. 8:121–149.
- Kambhar SV, Harihar NS, Katrahalli KS. 2014. Recollection of endemic species Barleria stocksii T. Anderson (Acanthaceae) in Karnataka State, India, after 140 years. Checklist. 10(2):419.
- Kaur J. 1969. Chromosome numbers in Acanthaceae IV. Sci Cult. 35:61–63.
- Khatoon S, Ali SI. 1993. Chromosome atlas of the angiosperms of Pakistan. Karachi: Department of Botany, University of Karachi; p. 232.
- Krishnaswami S, Menon MP. 1974. Cytomorphological study on some species and an interspecific hybrid of Barleria L. Cytologia. 39(3):397–402.
- Levan A, Fregda K, Sandberg AA. 1964. Nomenclature for centromeric position on chromosomes. Hereditas. 52:201–220.
- Mirzaghaderia G, Marzangi K. 2015. IdeoKar: an ideogram constructing and karyotype analyzing software. Caryologia. 68(1):31–35.
- Narayanan CR. 1951. Somatic chromosomes in Acanthaceae. J Madras Univ. 21(B):220–231.
- Pal M. 1964. Chromosome number in some Indian angiosperms. Proc Indian Acad Sci. 60(B):347–351.
- Ranganath RM, Krishnappa DG. 1990. Karyotypic studies in a few species of Barleria L. (Acanthaceae) from south India. Cytologia. 55(2):175–179.
- Romero Zarco C. 1986. A new method for estimating karyotype asymmetry. Taxon. 35:526–530.
- Sankar RV, Ravikumar K, Ganesh Babu NM. 2005. On the collection of a Peninsular endemic, Barleria stocksii (Acanthaceae), after a century. Zoo’s Print J. 20(3):1820.
- Shendage SM, Yadav SR. 2010. Revision of the genus Barleria (Acanthaceae) in India. Rheedea. 20(2):81–130.
- Stebbins G. 1971. Chromosomal evolution in higher plants. London: Edward Arnold.
- The Plant List. 2013. Version 1.1. [cited 2012 March 23]. Available from: http://www.theplantlist.org/