Abstract
Several cytogenetic studies in Prochilodus lineatus (Valenciennes 1836) describe a striking system of supernumerary microchromosomes revealing the occurrence of three morphological types visualized as acrocentric, metacentric and submetacentric. The present study aimed to compare the DNA homology among the B chromosome variants, as well as the components of the standard complement using total B specific probes obtained by microdissection. This technique, which allows direct isolation of DNA from each B chromosome variant of interest, was applied to obtain the probes. Accordingly, cross hybridizations targeting all types were performed by using the fluorescence in situ hybridization technique. The results obtained revealed signals of hybridization on all kinds of B chromosomes, indicating homology among the variants. Moreover, markings on the standard A complement were observed using the B submetacentric chromosome specific probe. Based on these data, we can infer that B chromosome variants in P. lineatus have homologous regions, suggesting a common origin from an ancestral variant. Furthermore, it can be also hypothesized that the primitive supernumerary was probably originated from elements of the standard complement, since the B submetacentric morphotype shares sequences with the centromeric region of some chromosomes of the A complement.
Introduction
Supernumerary chromosomes are extra chromosome elements present in the genome of various plants, fungal and animal species. It is assumed that the B chromosomes generally occur in approximately 15% of species. Nevertheless, the morphological and molecular heterogeneity found in different supernumerary chromosomes is an interesting problem, since the number of common characteristics is limited. Usually heterochromatic and dispensable nature identifies them better, while the absence of recombination during the meiotic process, both among themselves and with the other elements within the genome, in addition to a non-Mendelian mode of inheritance constitute strong particularities to recognize these genomic elements as supernumerary chromosomes (Vujošević et al. Citation2013).
There are several plausible hypotheses to explain the origin of B chromosomes. One proposal hypothesizes that supernumerary elements may have an intraspecific origin from the elements of the A standard complement, following their own evolutionary path (Camacho et al. Citation2000). Evidence of common ancestry among fish have given great support to the intraspecific origin of supernumeraries in several species, as derived from the formation of isochromosomes (Mestriner et al. Citation2000; Néo et al. Citation2000; Jesus et al. Citation2003; Artoni et al. Citation2006). Alternatively, an interspecific origin resulting from hybridizations of closely related species also can be considered as a mechanism for the origin of B chromosomes (Camacho et al. Citation2000; Sapre and Despanche Citation1987; Eickbush et al. Citation1992; McVean Citation1995; Perfectti and Werren Citation2001).
The fluorescence in situ hybridization (FISH) and chromosome microdissection techniques have become essential tools for the study of the origin of B chromosomes in various organisms (Voltolin, Pansonato-Alves, et al. Citation2013; Silva et al. Citation2014; Pansonato-Alves et al. Citation2014). However, very little is known about the composition, origin and maintenance of B chromosomes in the fish carriers.
Prochilodus lineatus has been one of the species most used in studies concerning the origin, behavior and evolution of supernumerary chromosomes in fish. In this species they generally are visualized as microchromosomes and present a wide frequency variation, which may involve the presence of individuals with zero to nine supernumeraries in the populations analyzed (Voltolin et al. Citation2011). Another interesting feature of this species is that these B chromosomes exhibit an interesting morphological polymorphism, which may result in three types, with acrocentric (small-sized), metacentric (medium-sized) and submetacentric B chromosomes (large-sized) (Artoni et al. Citation2006; Penitente et al. Citation2013).
The present study aimed to analyze the DNA composition and differentiation process of B chromosome variants in P. lineatus using B specific probes obtained by chromosome microdissection, along with the technique of fluorescence in situ hybridization. It is considered that the data obtained can provide new insights into the diversification mechanisms and particularities of the different forms presented by supernumerary chromosomes in this species.
Materials and methods
Chromosome preparations were obtained from 136 specimens of Prochilodus lineatus. The specimens were collected from the natural population of the Mogi-Guaçu River, Cachoeira de Emas, Pirassununga, SP, Brazil and maintained in the facilities of the ICMBio institution in Pirassununga, SP, Brazil.
The cytogenetic preparations were obtained through the lymphocyte culture technique described by Fenocchio and Bertollo (Citation1988) with some adjustments for this species. The best preparations of fish carrying each of the three supernumerary variants were selected, fixed in methanol and stored at 4°C. The technique described by Sumner (Citation1972) was applied for the characterization of constitutive heterochromatin.
Microdissection of B chromosomes was performed using a micromanipulator (Eppendorf-5171, Germany) with a glass needle coupled to an inverted microscope (Axiovert 100 – Zeiss, Germany). The cell suspensions were dropped onto 24 mm × 60 mm glass slides and stained with 5% Giemsa for 5 min. B chromosomes were easily identified in the metaphases due to their reduced size and distinct morphology from the chromosomes of the A complement.
Samples containing 10 microdissected B chromosomes of each variant from selected individuals were transferred to different microtubes (0.2 ml) with 9 μl ultrapure water, and the DNA was amplified using the kit GenomePlex® Single Cell Whole Genome Amplifition (WGA4 – Sigma, USA) (Gribble et al. Citation2004). Following the reactions, the products were verified by agarose gel electrophoresis, and after DNA amplification, probes for all three B chromosome morphotypes (acrocentric B [Ba], metacentric B [Bm] and submetacentric B [Bsm]) were generated from a reamplification of the amplified DNA using the GenomePlex® WGA Reamplification Kit (WGA3 – Sigma) associated with digoxigenin-11-dUTP (Roche Diagnostics, Indianapolis, USA).
Fluorescence in situ hybridization (FISH) using Ba, Bm and Bsm probes was performed on specimens bearing acrocentric, metacentric and submetacentric B chromosomes. The standard FISH procedure was adopted following the protocol established by Pinkel et al. (Citation1986). The chromosomes were counterstained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) and analyzed under an Olympus BX50 photomicroscope (Olympus Optical, Japan). Images were obtained using the QCapture Pro 5.1.1.14 software (http://www.qimaging.com/support/downloads/qcappro51.php).
Results
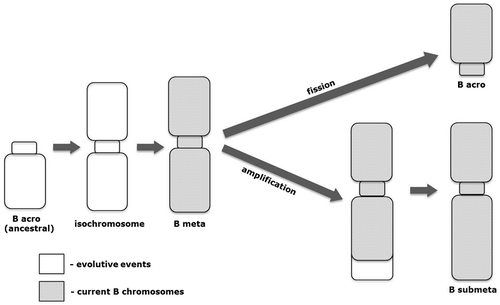
The first step of the chromosome staining experiments was to create a control group from the in situ hybridization of the probes combined with the metaphases that gave rise to each of them, in order to verify the functionality of the probes. The Ba probe (Figure ) was used for markings in control chromosomes (Figure a). The individual that originated this probe was carrying an acrocentric and two metacentric B chromosomes, and intense signals were observed on the three B chromosomes in this preparation, including the two metacentric chromosomes. Upon using the Ba probe in preparations of individuals carrying metacentric and submetacentric B chromosomes (Figure b and c, respectively), the probe revealed a definite homology among these B chromosomes.
Figure 1. Somatic chromosomes of Prochilodus lineatus after in situ hybridization using a B acrocentric (Ba) chromosome variant probe. In (a) metaphase cell with two metacentrics and one acrocentric variant B chromosome; (b) metaphase with one metacentric variant B chromosome; (c) metaphase with one submetacentric variant B chromosome. Arrowheads highlight metacentric, submetacentric and acrocentric B chromosome variants. Bar = 10 μm.
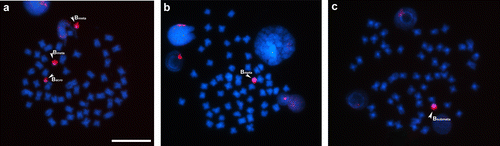
The Bm probe results were similar to those presented by the Ba probe, showing signals only on the metacentric control chromosome (Figure a). In addition, homology was observed when performing hybridization with carriers of acrocentric and submetacentric B chromosomes (Figures a, and b). However, no homology was identified with the standard components of the standard A complement. Finally, when assessing the results obtained by hybridization with the Bsm probe, homology was also observed between the control group and the other variants (Figure ), similarly to the results obtained by the Ba and Bm probes. Moreover, signals of hybridization were also detected in the centromeric and pericentromeric regions of 10 chromosomes of the standard A complement with the Bsm probe. It can be reinforced that heterochromatic blocks are generally observed in the centromeric and pericentromeric regions of the chromosomes in the A complement and in all B chromosomes in this species.
Figure 2. Somatic chromosomes of Prochilodus lineatus after in situ hybridization using a B metacentric (Bm) chromosome variant probe. In (a) metaphase cell with one metacentric variant B chromosome; (b) metaphase with two metacentrics and one acrocentric variant B chromosome; (c) metaphase with one submetacentric variant B chromosome. Arrowheads highlight metacentric, submetacentric and acrocentric B chromosome variants. Bar = 10 μm.
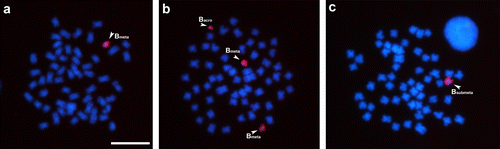
Figure 3. Somatic chromosomes of Prochilodus lineatus after in situ hybridization using a B acrocentric (Bsm) chromosome variant probe. In (a) metaphase cell with two metacentrics and one acrocentric variant B chromosome; (b) metaphase with one metacentric variant B chromosome; (c) metaphase with one submetacentric variant B chromosome; (d) metaphase after C-banding treatment showing heterochromatic blocks in the centromeric and pericentromeric regions on chromosomes of the normal complement. Arrowheads highlight metacentric, submetacentric and acrocentric B chromosomes and arrows points to marked sites located on chromosomes of the normal set. Bar = 10 μm.
Discussion
Cytogenetic studies performed by Voltolin, Penitente, et al. (Citation2013) on five species of the genus Prochilodus reported that P. lineatus presents heterochromatic blocks only in the centromeric and pericentromeric regions of the chromosomes in the A complement. Furthermore, the B microchromosomes found were fully heterochromatic. These data are confirmed by those presented in the present study (Figure d). Given the markings observed with the Bsm probe, it can be inferred that the A set chromosomes of P. lineatus present different patterns of heterochromatin composition in the pericentromeric region, since in 10 of 54 chromosomes of the standard complement were found sites of homology with the Bsm probe.
The chromosome painting experiments using the three Ba, Bm and Bsm probes showed homology of DNA sequences among all variants of B chromosomes in P. lineatus. These data indicate that such supernumeraries possibly originated from a common ancestral B variant, which arose in this species and developed expressive mutability, either by chromosomal rearrangements, duplications, deletions and inversions or through the invasion by repetitive sequences or transposable elements, due to its neutral and dispensable nature (Silva et al. Citation2014), and finally derived to three distinct morphological types of B microchromosomes actually found so far. The emergence of new types of B chromosomes was also recorded in the species of locust Eyprepocnemis plorans (Cabrero et al. Citation2013), where more than 50 variants have already been described. The frequent generation of new B variants indicates that the variation in size and morphology of the supernumerary chromosomes is an inherent characteristic of these elements in many species.
Furthermore, only the Bsm probe showed signals on several chromosomes of the standard A complement (Figure ), particularly in the centromeric and pericentromeric regions. Jesus et al. (Citation2003) studied the satellite DNA of P. lineatus from the Mogi-Guaçu River and reported that the SATH1 probe, composed of 900 bp showed marks on the B chromosomes and in the pericentromeric region of the A chromosomes. The data obtained in the present work using the Bsm probe are similar to those described by Jesus et al. (Citation2003) and it is possible that this variant may present sequences of the satellite DNA (SATH1), or a close homology among the sequences. The homology involving the Bsm variant probe sequences and the signals found in the centromeric and pericentromeric regions in chromosomes of the A complement could be evidence of a common origin. But the possibility cannot be discarded that the dispersed presence of these sequences in specific sites of the chromosomes in the normal set could be due to the saltatory nature of repetitive sequences in the genome of organisms. Another form of tandemly repeated DNA which has been frequently described from B chromosomes is rDNA, but recent studies performed by Voltolin et al. (Citation2009) on cytogenetic markers in the wild population of the Mogi-Guaçu River showed no signs of rDNA on B chromosomes in this species. Therefore, the hypothesis that those homologous fragments are rDNA sites can be discarded.
Most cytological and molecular studies support the notion that most B chromosomes seem to be derived from the A complement of their current host species. From this perspective, we could consider the origin of the Bsm chromosome as a simple byproduct of the evolution of the standard karyotype. For example, the Bsm chromosome variant could be derived from A chromosomes from centric fragments resulting from chromosome fissions or amplifications of the paracentromeric region.
On the other hand, when studying the origin of B chromosomes in P. lineatus using B chromosomes probes obtained by DOP-PCR, Voltolin et al. (Citation2010) and Voltolin, Pansonato-Alves, et al. (Citation2013) did not observe any homology involving B chromosome sequences and the standard complement elements. The results obtained in the present study using the Bsm probe contrast with those data. A variation in the frequency of B chromosome variants in P. lineatus studied by Penitente et al. (Citation2013) in the Mogi-Guaçu River was described, revealing the metacentric type as the most frequent. As in the experiments conducted by Voltolin et al. (Citation2010) and Voltolin, Pansonato-Alves, et al. (Citation2013) the polymorphism of the supernumeraries was not analyzed, and considering that the metacentric variant is the most common type found in this population, it is possible that these authors used a microdissected Bm or even a Ba variant as a probe, and did not observe any homology to the components of standard A complement. The use of different methodologies to obtain the probes may be another reason for this discrepancy as these authors used the DOP-PCR technique for amplification of the microdissected material.
Several hypotheses have been proposed to explain the intraspecific origin of supernumerary chromosomes from elements of the standard A complement (López-León et al. Citation1994; Vicente et al. Citation1996; Sharbel et al. Citation1998). According to Camacho et al. (Citation2000), the molecular characterization of repetitive DNA sequences to specific B chromosomes, which are also shared by chromosomes of the A complement in other species, supports the hypothesis of an interspecific origin for these genomic elements. Otherwise, the presence of molecular similarity between chromosomes of the A complement and the Bsm variant in P. lineatus seems to indicate an intraspecific origin for these elements.
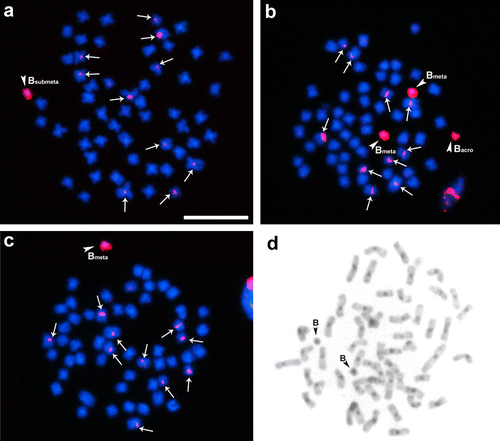
We can hypothesize about the origin of supernumerary elements in P. lineatus. An ancestral acrocentric B chromosome variant may have originated from fragments containing the centromere as a result of breaks occurring in pericentromeric and centromeric regions of a component of the A complement of this species. This element would have given rise to a B metacentric isochromosome similar to the metacentric type as can be found today. Subsequent fission events could then result in a B acrocentric chromosome type, just as amplification events in one arm of the metacentric chromosome could give rise to the corresponding submetacentric chromosome morphotype (Figure ). The high mutability and instability that characterize the supernumerary elements may have given these genomic elements their own evolutionary pattern, resulting in the variant morphotypes currently found in this species. It can be considered that during the evolutive process of each element, some sequences may have been lost while other sequences may have been added to the genome of each variant component, establishing their morphological and structural identity.
The results presented reinforce the hypothesis about the origin of supernumeraries through the formation of an isochromosome, as proposed by Mestriner et al. (Citation2000), Vicari et al. (Citation2011) and;Silva et al. (Citation2014). When studying B chromosomes in P. lineatus from two populations collected in the Mogi-Guaçu and Tibagi rivers, Artoni et al. (Citation2006) detected a symmetric localization of the SATH1 (900 bp) DNA satellite markers in the pericentromeric region of B chromosomes in individuals of the Tibagi River population.
As these chromosomes are susceptible to mutations and intense morphological and structural reorganizations due to their apparent neutrality and dispensable nature in the genome, other B variants may occur among different populations of P. lineatus, and also may be acting under the influence of distinct evolutionary factors and maintenance elements. However, based on the available data for B chromosomes in P. lineatus from the Mogi-Guaçu River population, there is strong evidence corroborating the intraspecific origin of these elements and the generation of different types of variants, possibly from a metacentric type ancestor in this species.
Funding information
The authors are grateful to the Brazilian funding agencies Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for providing financial support.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Artoni RF, Vicari MR, Endler AL, Cavallaro ZI, Jesus CM, Almeida MC, Moreira-Filho O, Bertollo LAC. 2006. Banding pattern of and B chromosome of Prochilodus lineatus (Characiformes, Prochilodontidae), with comments on B chromosome evolution. Genetica. 127:277–284.
- Cabrero J, López-Léon MD, Ruíz-Estévez M, Gómez R, Petitpierre E, Rufas JS, Massa B, Kamel Ben Halima M, Camacho JPM. 2013. B1 was the ancestor B chromosome variant in the western Mediterranean area in the grasshopper Eyprepocnemis plorans. Cytogenet Genome Res. 142(1):54–58. doi:10.1159/000356052.
- Camacho JPM, Sharbel TF, Beukeboom LW. 2000. B-chromosome evolution. R Soc Lond. 355:163–178.
- Eickbush DG, Eickbush TH, Werren JH. 1992. Molecular characterization of repetitive DNA sequences from a B chromosome. Chromosoma. 101:575–583.
- Fenocchio AS, Bertollo LAC. 1988. A simple method for fresh-water fish lymphocyte culture. Rev Brasil Genet. 11(4):847–852.
- Gribble S, Ng BL, Prigmore E, Burford DC, Carter NP. 2004. Chromosome paints from single copies of chromosomes. Chromosome Res. 12:143–151.
- Jesus CM, Galetti Jr PM, Valentini SR, Moreira-Filho O. 2003. Molecular characterization and chromosomal location of two families of satellite DNA in Prochilodus lineatus (Pisces, Prochilodontidae), a species with B chromosomes. Genetica. 118:25–32.
- López-León MD, Veves N, Schwarzacher JS, Heslop-Harrison JS, Hewitt GM, Camacho JPM. 1994. Possible origin of a B chromosome deduced from its DNA composition using double FISH technique. Chrom Res. 2:87–92.
- McVean GT. 1995. Fractious chromosomes hybrid disruption and the origin of selfish genetic elements. BioEssays. 17:579–582.
- Mestriner CA, Galetti PM Jr, Valentini SR, Ruiz IRG, Abel LDS, Moreira-Filho O, Camacho JPM. 2000. Structural and functional evidence that a B chromosome in the characid fish Astyanax scabripinnis is an isochromosome. Heredity. 85:1–9.
- Néo DM, Bertollo LAC, Moreira-Filho O. 2000. Morphological differentiation and possible origin of B chromosome in natural Brazilian population of Astyanax scabripinnis (Pisces, Characidae). Genetica. 108:211–215.
- Pansonato-Alves JC, Serrano EA, Utsunomia R, Camacho JPM, Costa Silva GJ, Vicari MR, Artoni RF, Oliveira C, Foresti F. 2014. Single origin of sex chromosomes and multiple origins of B chromosomes in fish genus Characidium. Plos One. doi:10.1371/journal.pone.0107169.
- Penitente M, Voltolin TA, Senhorini JA, Bortolozzi J, Foresti F, Porto-Foresti F. 2013. Transmission rate variation among three B chromosome variants in the fish Prochilodus lineatus (Characiformes, Prochilodontidae). An Acad Bras Ciênc. 85(4):1371–1377.
- Perfectti F, Werren JH. 2001. The interspecific origin of B chromosomes: experimental evidence. Evolution. 55:1069–1073.
- Pinkel D, Straume T, Gray JW. 1986. Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc Natl Acad Sci. 83:2934–2938.
- Sapre AB, Despanche DS. 1987. Origin of B chromosomes in Coix L. through spontaneous interspecific hybridization. J Hered. 78:191–196.
- Sharbel TF, Green DM, Houben A. 1998. B chromosome origin in the endemic New Zealand frog Leiopelma hochstetteri through sex chromosome devolution. Genome. 41:14–22.
- Silva DMZA, Pansonato-Alves JC, Utsunomia R, Araya-Jaime C, Ruiz-Ruano FJ, Daniel SN, Hashimoto DT, Oliveira C, Camacho JPM, Porto-Foresti F, Foresti F. 2014. Delimiting the origin of a B chromosome by FISH mapping, chromosome painting and DNA sequence analysis in Astyanax paranae (Teleostei, Characiformes). Plos One. 9(4):e94896. doi:10.1371/journal.pone.0094896.
- Sumner AT. 1972. A simple technique for demonstrating centromeric heterochromatin. Exp. Cell Res. 75(1):304–306.
- Vicari MR, Pistune HFM, Castro JP, Almeida MC, Bertollo LAC, Moreira-Filho O, Camacho JPM, Artoni RF. 2011. New insights on the origin of B chromosomes in Astyanax scabripinnis obtained by chromosome painting and FISH. Genetica. 139:1073–1081.
- Vicente VE, Moreira-Filho O, Camacho JPM. 1996. Sex-ratio distortion associated with the presence of a B chromosome in Astyanax scabripinnis (Teleostei, Characidae). Cytogenet Cell Genet. 74:70–75.
- Voltolin TA, Pansonato-Alves JC, Senhorini JA, Foresti F, Camacho JPM, Porto-Foresti F. 2013. Common descent of B chromosomes in two species of the fish genus Prochilodus (Characiformes, Prochilodontidae). Cytogenetic and Genome Research. 141:206–211.
- Voltolin TA, Penitente M, Mendonça BB, Senhorini JA, Foresti F, Porto-Foresti F. 2013. Karyotypic conservatism in five species of Prochilodus (Characiformes, Prochilodontidae) disclosed by cytogenetic markers. Genet Mol Biol. 36(3):347–352.
- Voltolin TA, Senhorini JA, Foresti F, Bortolozzi J, Porto-Foresti F. 2011. Intraspecific crosses resulting in the first occurrence of eight and nine B chromosomes in Prochilodus lineatus (Characiformes, Prochilodontidae). Genet Mol Biol. 34(2):220–224.
- Voltolin TA, Senhorini JA, Laudicina A, Oliveira C, Foresti F, Bortolozzi J, Porto-Foresti F. 2010. Origin and molecular organization of supernumerary chromosomes of Prochilodus lineatus (Characiformes. Prochilodontidae) obtained by DNA probes: Genetica. 138(11–12):1133–1139. doi:10.1007/s10709-010-9502-8.
- Voltolin TA, Senhorini JA, Oliveira C, Foresti F, Bortolozzi J, Porto-Foresti F. 2009. Cytogenetic markers in wild population of curimbatá (Prochilodus lineatus) from Mogi-Guaçu River. Cytologia. 74(3):281–287.
- Vujošević M, Jovanović V, Blagojević J. 2013. Polyploidy and B chromosomes in Allium flavum from Serbia. Arch Biol Sci. 65(1):23–32.