Abstract
A study of the cytogenetics of Pseudophoxinus battalgilae Bogutskaya, 1997, P. burduricus Küçük, Gülle, Güçlü, Çiftçi & Erdoğan, 2013, P. egridiri (Karaman, 1972), P. evliyae Freyhof & Özuluğ, 2009, P. fahrettini Freyhof & Özuluğ, 2009 and P. maeandri (Ladiges, 1960) was conducted by means of Giemsa staining, C- and AgNOR-banding procedures. Diploid chromosome numbers of analyzed species were found to be the same (2n = 50) but their karyotypes formulae were different. For all species examined, the largest chromosome pair of the complements was subtelo-acrocentric. Heteromorphic sex chromosomes were not detected in any of the studied species. C-bands were found on the centromeres of several chromosomes in all studied species. NORs were detected in one pair of submetacentric chromosomes in P. burduricus, P. egridiri and P. fahrettini, and in two pairs of submetacentric chromosomes in P. battalgilae, P. evliyae and P. maeandri. Further, NOR polymorphisms were observed in some specimens of P. battalgilae, P. burduricus, P. evliyae and P. fahrettini for number, location and size. This study may contribute to other leuciscine cytogenetic studies.
Introduction
The genus Pseudophoxinus Bleeker, 1860 belongs to the subfamily Leuciscinae. This genus has 29 species that are distributed in the Balkans, Anatolia, the Middle East, the Caspian Sea Basin and North Africa (Küçük et al. Citation2012; Eschmeyer Citation2015). The genus Pseudophoxinus is the fourth largest genus of Anatolian freshwater fishes, having 21 species in Anatolia (Kuru et al. Citation2014; Küçük and Güçlü Citation2014a; Ekmekçi et al. Citation2015). Of these species, 19 of them are endemic (Ekmekçi et al. Citation2015; Eschmeyer Citation2015). The species are small in size and are distributed in cold springs, small lakes and slow-flowing rivers. Due to their small size, taxonomic problems still exist in the species (Küçük et al. Citation2012, Citation2013). For this reason, some populations were re-examined and new species have been described from Anatolia, as mentioned in detail by Ekmekçi et al. (Citation2015). Changes in the systematic position of some species were also made. In this regard, P. fahirae was placed in the genus Chondrostoma (Freyhof and Özuluğ Citation2009). In addition, most of the Anatolian Pseudophoxinus species are affected especially by pollution, water abstraction and climate change and are, therefore, assessed as endangered (IUCN Citation2015).
A cytogenetic study may be useful to reveal phylogenetic relationships among species (Boron et al. Citation2009). An apparent conservation of the diploid chromosome number (2n = 50) and chromosome morphology was observed among 25 leuciscine genera that were cytogenetically examined (Rossi et al. Citation2012). Cytogenetic studies of Anatolian freshwater fishes have been initiated by Çolak et al. (Citation1985). With the exception of Chondrostoma meandrense, all Anatolian leuciscine species have the diploid number of 50 chromosomes; however, chromosome morphologies show some differences between the species (Uysal Citation2011; Arslan and Gündoğdu Citation2016). On the other hand, chromosomal band studies have been reported in fewer Anatolian leuciscine species and moderate variations were indicated in the distribution of C-bands and NORs (Arslan and Gündoğdu Citation2016).
Chromosomal studies in the genus Pseudophoxinus have been conducted in recent years (Ergene et al. Citation2010; Karasu et al. Citation2011; Gaffaroğlu et al. Citation2014; Ünal et al. Citation2014; Ünal Citation2015). However, these studies are considered inadequate in terms of richness of this genus. One study claims that conserved karyotypic evolution was observed in the genus Pseudophoxinus (Ünal et al. Citation2014). The aim of this study is to reveal the diploid chromosome number, chromosome morphology, C-band and NOR phenotypes of endemic leuciscins P. battalgilae, P. burduricus, P. egridiri, P. evliyae, P. fahrettini and P. maeandri from Anatolia.
Materials and methods
Fish sampling
In total 63 specimens of P. battalgilae, P. burduricus, P. egridiri, P. evliyae, P. fahrettini and P. maeandri were collected by electrofishing from Turkey in 2012 and 2013 (Table ). Specimens were transported alive to the laboratory and kept in well-aerated aquaria until analysis. After analysis, the specimens were deposited in 70% ethanol at the Cytogenetics Laboratory of the Department of Biology, Faculty of Arts and Sciences of the Ahi Evran University, Kırşehir, Turkey (M. K. A. 30-93).
Table 1. General information regarding collected specimens.
Chromosome preparations
Chromosome preparations were from the head kidney by using the air drying technique of Collares-Pereira (Citation1992). The fish were injected intraperitoneally with 0.1% colchicine solution (1 ml per 100 g body weight) and kept in aerated aquaria for 2 h. Then the head kidneys of the specimens were removed and placed in hypotonic KCl solution (0.075 M) for 40 min at 37 °C. After this step, the cell suspension was centrifuged for 10 min at 1200 rpm, after which the supernatant was discarded. The cells were fixed with 5 ml fixative solution (3:1, methanol:glacial acetic acid) for 30 min at 4 °C. Then the cells were centrifuged and supernatant was discarded again. These last two steps were repeated two to three times. The cell suspensions were then dropped onto cleaned and cold slides. Air-dried slides were stained by 10% Giemsa for 20 min. Then slides were rinsed with distilled water and allowed to dry at room temperature. 10 to 20 slides were prepared from each specimen.
Bandings
C-banding technique of Sumner (Citation1972) with some modifications was used for determining constitutive heterochromatin regions and the Ag-staining technique of Howell and Black (Citation1980) was used for determining NORs. For C-banding, slides were treated with 0.2 N HCl for 30 min at room temperature, then rinsed with distilled water and air-dried. The slides were then incubated with 5% Ba(OH)2 for 15–20 min at 37 °C, followed by rinsing and drying. Slides were incubated with 2 × SSC for 2 h at 70 °C and rinsed and dried once again. Then slides were stained by 10% Giemsa for 30 min. For Ag-staining, two drops of colloidal developer and four drops of 50% AgNO3 solution were added onto the slides. The coverslip was used to cover the slide and then placed in an incubator at 70 °C. When the slide color changed to golden brown, the coverslip was removed. Then slide was rinsed and dried.
Microscopy, image processing and karyotyping
All preparations were scanned with a Leica DM 3000 microscope (Leica Microsystems GmbH, Germany) and photographs of good metaphase plaques were taken with AKAS software (Argenit Mikrosistem, Turkey). At least 100 metaphase plaques were counted from each species to determine the diploid chromosome number. Chromosomes were classified according to Levan et al. (Citation1964) and karyotypes were prepared manually. For calculating fundamental arm number (FN), metacentric (m) and submetacentric (sm) chromosomes were taken as biarmed, while subtelo-acrocentric (st-a) chromosomes were taken as uniarmed.
Results
Diploid chromosome number and karyotypes
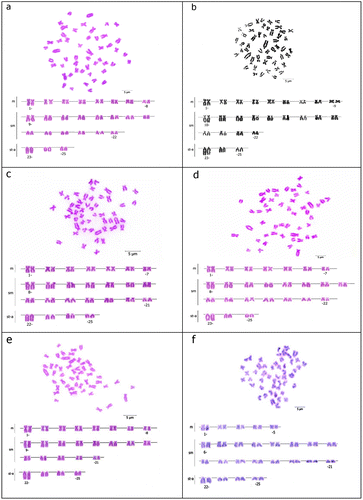
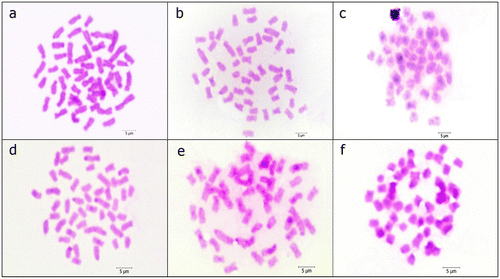
Diploid chromosome numbers of P. battalgilae, P. burduricus, P. egridiri, P. evliyae, P. fahrettini and P. maeandri were determined as 2n = 50. Karyotypes were as follows: eight pairs of m, 14 pairs of sm and three pairs of st-a for P. battalgilae; nine pairs of m, 13 pairs of sm and three pairs of st-a for P. burduricus; seven pairs of m, 14 pairs of sm and four pairs of st-a for P. egridiri; seven pairs of m, 15 pairs of sm and three pairs of st-a for P. evliyae; eight pairs of m, 13 pairs of sm and four pairs of st-a for P. fahrettini; five pairs of m, 16 pairs of sm and four pairs of st-a chromosomes for P. maeandri (Figure ). The largest chromosome pair of the complements was st-a in all studied species. FN was calculated as 92 in P. egridiri, P. fahrettini and P. maeandri, and 94 in P. battalgilae, P. burduricus and P. evliyae. Heteromorphic sex chromosomes were not detected in any of the studied species.
Constitutive heterochromatin regions
Constitutive heterochromatin regions were found on the centromeres of several chromosomes in P. battalgilae, P. burduricus, P. egridiri, P. evliyae, P. fahrettini and P. maeandri. Additionally, heterochromatic blocks were determined in the pericentromeres of some chromosome pairs in P. egridiri and P. fahrettini (Figure ).
NOR phenotypes
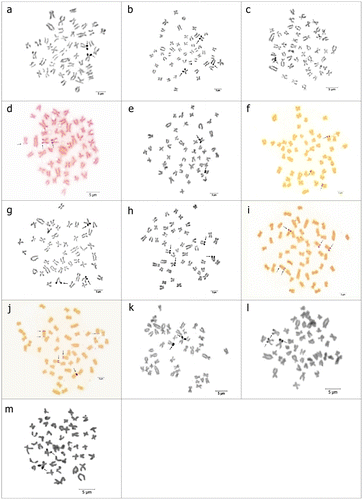
NORs were detected in the telomeres of the short arms of one pair of middle sized sm chromosomes in P. burduricus, P. egridiri and P. fahrettini, and in the telomeres of the short arms of two pairs of middle sized sm chromosomes in P. battalgilae, P. evliyae and P. maeandri (Figure ). Also NOR polymorphisms were observed on some specimens of P. battalgilae, P. burduricus, P. evliyae and P. fahrettini for number, location and size (Table , Figure ).
Figure 3. Ag-stained metaphases of (a) P. battalgilae; (b) P. burduricus; (c) P. egridiri; (d) P. evliyae; (e) P. fahrettini; (f) P. maeandri. Arrows indicate the NORs. Scale bar = 5 μm.
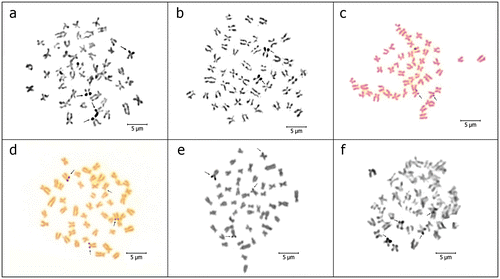
Table 2. NOR number polymorphism of the four studied species.
Figure 4. Ag-stained metaphases with number and/or location polymorphisms of (a, b) P. battalgilae showing two and three Ag-positive signals; (c, d) P. burduricus showing one and three Ag-positive signals; (e–j) P. evliyae showing from two to six Ag-positive signals; (k, l) P. fahrettini showing two and three Ag-positive signals; (m) P. fahrettini with size polymorphism. Arrows indicate the NORs. Scale bar = 5 μm.
Discussion
Despite the high diversity and endemism of Anatolian leuciscins only about 19% have been karyologically studied (Pekol Citation1999; Gaffaroğlu et al. Citation2006; Nur Citation2006; Kaya 2009; Uysal Citation2011; Ünal Citation2011; Arslan and Gündoğdu Citation2016; references in Table ). The diploid chromosome numbers and chromosome morphologies obtained for the six endemic species are considered to reflect the characteristic leuciscine pattern (2n = 50: dominance of meta- and submetacentrics) (Pereira et al. Citation2009). The obtained data indicate very low karyotypic variation with the chromosome sets of the species undertaken in this research. Other karyologically studied species of the genus Pseudophoxinus also have 2n = 50 chromosomes (Table ). However, their chromosome morphologies show little difference with the species in this study (except P. zekayi and P. fahrettini). In accordance with this discrepancy, the FNs of the some species are found to be different. Distinct FNs of these species may be the result of pericentromeric inversions (and/or translocations involving centromeres) as reported by Pereira et al. (Citation2012). This situation may be considered valid for the studied species in this research which have the same FNs but different chromosome morphologies. Further, the largest chromosome pair (st-a) of the complements are suggested to be a cytotaxonomic marker for leuciscins (Rab et al. Citation2008), as observed in this study. In regard of this marker chromosome, the Pseudophoxinus species in this study resemble P. crassus, P. elizavetae, P. firati, P. hittitorum and P. zekayi but differ to P. antalyae (references in Table ). In addition to the recognizable marker chromosome, the largest m pair and largest two sm pairs can be easily recognized in all metaphase plaques of six species such as Iberian leuciscine species (Pereira et al. Citation2012). On the other hand, no heteromorphic sex chromosomes are observed in this study as reported for P. firati (Karasu et al. Citation2011), P. crassus, P. hittitorum (Ünal et al. Citation2014) and P. zekayi (Ünal Citation2015).
Table 3. Karyological studies in the genus Pseudophoxinus.
One of the most common techniques successfully applied to fish chromosomes is C-banding. By using this technique, constitutive heterochromatin regions can be identified and this region is a useful tool for cytotaxonomy of cyprinids (Ren et al. Citation1992). In this study, constitutive heterochromatin regions were found to be associated to centromeric and pericentromeric regions as in the other Pseudophoxinus species (Ergene et al. Citation2010; Karasu et al. Citation2011; Gaffaroğlu et al. Citation2014; Ünal et al. Citation2014; Ünal Citation2015) and as in the other leuciscine genera such as Abramis (Ocalewicz et al. Citation2004), Acanthobrama (Gaffaroğlu and Yüksel Citation2009), Chondrostoma (Arslan and Gündoğdu Citation2016), Leuciscus (Boron et al. Citation2009) and Squalius (Ünal Citation2011; Rossi et al. Citation2012). Additionally, the heterochromatic blocks in P. egridiri and P. fahrettini were reported in P. antalyae (Ergene et al. Citation2010), Acanthobrama marmid (Gaffaroğlu and Yüksel Citation2009) and also in the other representatives of European leuciscins (Boron Citation2001). It is possible to form these heterochromatic blocks after pericentric inversions (Boron Citation1995).
NOR phenotype (number and location) is another useful tool which is generally used in cyprinid cytotaxonomy (Valic et al. Citation2010). The most studied Anatolian leuciscine species – P. crassus, P. hittitorum (Ünal et al. Citation2014), Squalius anatolicus (Ünal Citation2011), P. zekayi, Squalius seyhanensis (Ünal Citation2015) and Chondrostoma beysehirense (Arslan and Gündoğdu Citation2016) – have a single NOR in the sm pair, like P. burduricus, P. egridiri and P. fahrettini. This phenotype is observed in most leuciscins from Europe and considered as an ancestral character (Rab et al. Citation2000; Bianco et al. Citation2004; Rossi et al. Citation2012; Nabais et al. Citation2013). Further, differences in the location of single NORs have been reported in Anatolian and European leuciscins (Pekol Citation1999; Kalous et al. Citation2008; Ergene et al. Citation2010). In addition, multiple NORs are considered as a derived character (Valic et al. Citation2010). These NORs have been observed in some Anatolian leuciscine species (A. marmid (Gaffaroğlu et al. Citation2006), P. firati (Karasu et al. Citation2011) and P. elizavetae (Gaffaroğlu et al. Citation2014)) and also in several European leuciscine species (Boron Citation2001; Monteiro et al. Citation2009; Pereira et al. Citation2009) such as P. battalgilae, P. evliyae and P. maeandri.
In most metaphases, P. burduricus and P. fahrettini have a pair of NOR-bearing chromosomes; while P. battalgilae and P. evliyae have two pairs of NOR-bearing chromosomes. However, NOR number polymorphism was detected within and among the specimens of the so-mentioned species. Similar results have been reported for only P. zekayi (Ünal Citation2015) from the studied Anatolian leuciscine species and for several European leuciscins (Collares-Pereira and Rab Citation1999; Boron Citation2001; Boron et al. Citation2009; Monteiro et al. Citation2009; Pereira et al. Citation2009). Besides these, NORs were mainly located in the telomeres of the short arms of sm chromosomes in P. battalgilae, P. evliyae and P. fahrettini, while they were observed in the telomeres of the long arms of sm chromosomes on some metaphases. This condition has been detected in Eurasian leuciscins such as S. cephalus (Pekol Citation1999) and P. phoxinus (Boron Citation2001). Additionally, NOR size polymorphism that was observed in P. fahrettini has been reported in S. cephalus (Pekol Citation1999) and S. anatolicus (Ünal Citation2011) of the Anatolian leuciscins and in European leuciscins such as Chondrostoma lusitanicum (Collares-Pereira and Rab Citation1999), Rutilus aula (Bianco et al. Citation2004), Squalius lucumonis (Rossi et al. Citation2012) and the three species of the genus Vimba (Rabova et al. Citation2003). The observed NOR polymorphisms in this study may be considered as the result of rearrangements (unequal crossing over, duplication, translocation, amplification, etc.) of rDNA sites, as stated by other researchers (Collares-Pereira and Rab Citation1999; Gromicho and Collares-Pereira Citation2004). It was also suggested that complex NOR phenotypes could be either a hybrid situation or the possible involvement of an association between mobile elements and rDNA loci (Kalous et al. Citation2008; Silva et al. Citation2013). Thus, CMA3-staining and FISH with rDNA probes may be useful in understanding the detailed NOR phenotypes of the studied species.
This study showed that the karyological properties of the six species proved to be significantly similar at the macrostructural level. This suggests a close phylogenetic relationship between the species. Further, karyotypes with more biarmed chromosomes are regarded to represent a derived condition (Ganai et al. Citation2011). According to number of biarmed chromosomes and FNs, P. egridiri, P. fahrettini and P. maeandri can be considered to be primitive fishes when compared to P. battalgilae, P. burduricus and P. evliyae, which display a more derived karyotype.
As discussed in a study by Küçük and Güçlü (Citation2014b), the taxonomy of the genus Pseudophoxinus remains complex in light of results of morphological and molecular studies. Based on the findings of the study, it is considered that this study shall contribute to the cytotaxonomy of Anatolian Pseudophoxinus species.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding information
This study was supported by Gazi University Scientific Research Projects [project code: 05/2012-76].
Acknowledgments
Our gratitude is extended to Dr S. Serkan Güçlü (Süleyman Demirel University, Turkey) for his contribution of the valuable information about localities. Our thanks also to Ali Ayata for his assistance during the fieldwork, and to Dr Sevgi Ünal (Gazi University, Turkey) for her laboratory support.
References
- Arslan A, Gündoğdu H. 2016. Cytogenetic studies on the endemic Beyşehir nase, Chondrostoma beysehirense (Bogutskaya, 1997) in Turkey. Caryologia. In press.
- Bianco PG, Aprea G, Balletto E, Capriglione T, Fulgione D, Odierna G. 2004. The karyology of the cyprinid genera Scardinius and Rutilus in southern Europe. Ichthyol Res. 51:274–278.
- Boron A. 1995. Chromosome banding studies of Noemacheilus barbatulus (Linnaeus, 1758) from Poland. Caryologia. 48(3–4):239–246.
- Boron A. 2001. Comparative chromosomal studies on two minnow fish, Phoxinus phoxinus (Linnaeus, 1758) and Eupallasella perenurus (Pallas, 1814); an associated cytogenetic-taxonomic considerations. Genetica. 111:387–395.
- Boron A, Porycka K, Ito D, Abe S, Kirtiklis L. 2009. Comparative molecular cytogenetic analysis of three Leuciscus species (Pisces, Cyprinidae) using chromosome banding and FISH with rDNA. Genetica. 135:199–207.
- Çolak A, Sezgin İ, Süngü YS. 1985. Sazangiller familyasına (Cyprinidae) ait Beni balığında (Cyprinion macrostomum Heckel, 1843) kromozomal araştırmalar. Doğa Bilim Dergisi. A2:9–12.
- Collares-Pereira MJ. 1992. In vivo direct chromosome preparation (protocol for air drying technique). First International Workshop on Fish Cytogenetic Techniques; 14–24 September; France.
- Collares-Pereira MJ, Rab P. 1999. NOR polymorphism in the Iberian species Chondrostoma lusitanicum (Pisces:Cyprinidae) – re-examination by FISH. Genetica. 105:301–303.
- Ekmekçi FG, Atalay MA, Yoğurtçuoğlu B, Turan D, Küçük F. 2015. A new species of Pseudophoxinus (Teleostei: Cyprinidae) from Southwestern Anatolia, Turkey. Zootaxa. 4033(1):117–128.
- Ergene S, Karahan A, Kuru M. 2010. Cytogenetic analysis of Pseudophoxinus antalyae, Bogustkaya, 1992 (Pisces: Cyprinidae) from the Eastern Mediterranean River Basin. Turkey. Turk J Zool. 34(1):111–117.
- Eschmeyer WN. 2015. Catalog of fishes: Genera, species, references; [ cited 2015 September 20]. Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp.
- Freyhof J, Özuluğ M. 2009. Pseudophoxinus evliyae, a new species of spring minnow from Western Anatolia with remarks on the distribution of P. ninae and the systematic position of P. fahirae (Teleostei: Cyprinidae). Ichthyol Explor Freshw. 20(4):309–318.
- Gaffaroğlu M, Ayata MK, Ünal S, Yüksel, E. 2014. Chromosomal analysis of Pseudophoxinus elizavetae Bogutskaya, Kucuk and Atalay, 2007 (Teleostei, Cyprinidae) from Anatolia. FABA2014: International Symposium on Fisheries and Aquatic Science; 25–27 September; Trabzon.
- Gaffaroğlu M, Yüksel E. 2009. Constitutive heterochromatin in Acanthobrama marmid and Cyprinion macrostomus (Osteichthyes, Cyprinidae). Kafkas Univ Vet Fak Derg. 15(2):169–172.
- Gaffaroğlu M, Yüksel E, Rab P. 2006. Note on the karyotype and NOR phenotype of leuciscine fish Acanthobrama marmid (Osteichthyes, Cyprinidae). Biologia, Bratislava. 61(2):207–209.
- Ganai FA, Yousuf AR, Dar SA, Tripathi NK, Wani SU. 2011. Cytotaxonomic status of Schizothoracine fishes of Kashmir Himalaya (Teleostei: Cyprinidae). Caryologia. 64(4):435–445.
- Gromicho M, Collares-Pereira MJ. 2004. Polymorphism of major ribosomal gene chromosomal sites (NOR-phenotypes) in the hybridogenetic fish Squalius alburnoides complex (Cyprinidae) assessed through crossing experiments. Genetica. 122:291–302.
- Howell WM, Black DA. 1980. Controlled silver-staining of nucleolus organizer regions with a protective colloidal developer: a 1-step method. Experientia. 36:1014–1015.
- International Union for Conservation of Nature [IUCN]. 2015. The IUCN red list of threatened species; [ cited 2015 September 20]. Available from: http://www.iucnredlist.org/search.
- Kalous L, Doadrio I, Rabova M, Rab P. 2008. Note on the karyotype and NOR phenotype of the cyprinid fish Parachondrostoma arrigonis. Cybium. 32(3):211–215.
- Karasu M, Yüksel E, Gaffaroğlu M. 2011. Karyotype, NORs, and C-banding analysis of Pseudophoxinus firati Bogutskaya, Küçük & Atalay, 2007 (Actinopterygii, Cyprinidae) in the Euphrates River. Turkey. Turk J Zool. 35(6):865–868.
- Kaya F. 2009. Göksu Nehri’nde yaşayan bazı ekonomik balıkların karyolojilerinin incelenmesi [PhD thesis]. [Mersin (TR)]: Mersin University. [In Turkish].
- Küçük F, Atalay MA, Güçlü SS, Gülle İ. 2012. Türkiye’de yayılış gösteren Pseudophoxinus (Teleostei: Cyprinidae) türlerinin bazı morfolojik özellikleri ve zoocoğrafik dağılımları. Eğirdir Su Ürünleri Fak Derg. 8(2):1–9.
- Küçük F, Güçlü SS. 2014a. A new Pseudophoxinus (Teleostei, Cyprinidae) species from Asi River Drainage (Turkey). ZooKeys. 411:57–66.
- Küçük F, Güçlü SS. 2014b. Importance of characters in fish taxonomy and problems encountered in species descriptions. J Acad Doc Fish Aquacult. 3:121–129.
- Küçük F, Gülle İ, Güçlü SS, Çiftçi Y, Erdoğan Ö. 2013. A new Pseudophoxinus (Teleostei, Cyprinidae) species from Southwestern Anatolia, with remarks on the distribution of the genus in western Anatolia. ZooKeys. 320:29–41.
- Kuru M, Yerli SV, Mangıt F, Ünlü E, Alp A. 2014. Fish biodiversity in inland waters of Turkey. J Acad Doc Fish Aquacult. 3:93–120.
- Levan A, Fredga K, Sandberg AA. 1964. Nomenclature for centromeric position on chromosomes. Hereditas. 52(2):201–220.
- Monteiro R, Carvalho C, Collares-Pereira MJ. 2009. Karyotype and genome size of Iberochondrostoma almacai (Teleostei, Cyprinidae) and comparison with the sister-species I. lusitanicum. Genet Mol Biol. 32(2):268–275.
- Nabais C, Rampin M, Collares-Pereira, MJ. 2013. Comparative cytogenetics of two endangered leuciscine fish, Squalius aradensis and S. torgalensis (Teleostei, Cyprinidae), from the Iberian Peninsula. Compar Cytogenet. 7(1):33–42.
- Nur G. 2006. Kura-Aras havzasına endemik Acanthalburnus microlepis (De Filippi, 1863) ve Alburnus filippii (Kessler, 1877)’de kromozomal çalışmalar [Master’s thesis]. [Kars (TR)]: Kafkas University. [In Turkish].
- Ocalewicz K, Jankun M, Boron A. 2004. Karyotypic characterization of bream, Abramis brama (Pisces, Cyprinidae). Folia Zool. 53(3):329–334.
- Pekol S. 1999. Kastamonu Beyler ve Germeçtepe Barajlarındaki Cyprinus carpio (L., 1758) ve Leuciscus cephalus (L., 1758) populasyonlarının karşılaştırmalı karyotip analizi ve NOR fenotipleri [PhD thesis]. [Ankara (TR)]: Gazi University. [In Turkish].
- Pereira C, Neto A, Collares-Pereira MJ. 2009. Cytogenetic survey of species of two distinct genera of Iberian nases (Cyprinidae, Leuciscinae) that hybridize extensively in nature. I. Evidence of a similar and conserved chromosome pattern with some few species-specific markers at macro-structural level. Genetica. 137:285–291.
- Pereira CSA, Rab P, Collares-Pereira MJ. 2012. Chromosomes of European cyprinid fishes: comparative cytogenetics and chromosomal characteristics of ribosomal DNAs in nine Iberian chondrostomine species (Leuciscinae). Genetica. 140:485–495.
- Rab P, Rabova M, Economidis PS, Triantaphyllidis C. 2000. Banded karyotype of the Greek endemic cyprinid fish. Pachychilon macedonicum. Ichthyol Res. 47(1):107–110.
- Rab P, Rabova M, Pereira CS, Collares-Pereira MJ, Pelikanova S. 2008. Chromosome studies of European cyprinid fishes: interspecific homology of leuciscine cytotaxonomic marker = the largest subtelocentric chromosome pair as revealed by cross species painting. Chromosome Res. 16:863–873.
- Rabova M, Rab P, Ozouf-Costaz C, Ene C, Wanzeböck J. 2003. Comparative cytogenetics and chromosomal characteristics of ribosomal DNA in the fish genus Vimba (Cyprinidae). Genetica. 118:83–91.
- Ren X, Cui J, Yu Q. 1992. Chromosomal heterochromatin differentiation of cyprinid fishes. Genetica. 87:47–51.
- Rossi AR, Milana V, Hett AK, Tancioni L. 2012. Molecular cytogenetic analysis of the Appenine endemic cyprinid fish Squalius lucumonis and three other Italian leuciscines using chromosome banding and FISH with rDNA probes. Genetica. 140:469–476.
- Silva MZA, Pansonato-Alves JC, Utsunomia R, Daniel SN, Hashimoto DT, Oliveira C, Porto-Foresti F, Foresti F. 2013. Chromosomal organization of repetitive DNA sequences in Astyanax bockmanni (Teleostei, Characiformes): dispersive location, association and co-localization in the genome. Genetica. 141:329–336.
- Sumner AT. 1972. A simple technique for demonstrating centromeric heterochromatin. Exp Cell Res. 75:304–306.
- Ünal S. 2011. Squalius anatolicus (Bogutskaya, 1997) (Pisces, Cyprinidae)’un sitogenetik analizi [master’s thesis]. [Kırşehir (TR)]: Ahi Evran University. [In Turkish].
- Ünal S. 2015. Seyhan ve Ceyhan nehir sistemlerinde yaşayan bazı cyprinid türlerinde karyolojik incelemeler [PhD thesis]. [Kırşehir (TR)]: Ahi Evran University. [In Turkish].
- Ünal S, Gaffaroğlu M, Ayata MK, Yüksel E. 2014. Karyotype, C-banding and AgNORs of two endemic leuciscine fish, Pseudophoxinus crassus (Ladiges, 1960) and P. hittitorum Freyhof & Özulug, 2010 (Teleostei, Cyprinidae). Compar Cytogenet. 8(4):249–257.
- Uysal UE. 2011. Büyük Menderes nehrinden yakalanan Chondrostoma meandrense (Elvira, 1987) ve Acanthobrama mirabilis (Ladiges, 1960) (Cyprinidae)’in karyotip analizi [master’s thesis]. [Aydın (TR)]: Adnan Menderes University. [In Turkish].
- Valic D, Kapetanovıć D, Zanella D, Mrakovčıć M, Teskeredžıć E, Besendorfer V, Rábová M, Ráb P. 2010. The karyotype and NOR phenotype of Telestes ukliva (Cyprinidae). Folia Zool. 59(2):169–173.