Abstract
Pleurospermum Hoffm. (Apiaceae), a widely spread, heterogeneous genus of complex and controversial taxonomy, is poorly known for chromosome number and meiotic details from the Indian subcontinent. In the current study, we examined male meiosis, chromosome counts, and pollen fertility in two species, P. candollii and P. govanianum from alpine areas of north-west Indian Himalaya. Both the species exist at diploid level (P. candollii, n = 11 or 2n = 22, P. govanianum, n = 9 or 2n = 18) with two different basic chromosome numbers, x = 9 and 11. Meiotic course in the majority of pollen mother cells (PMCs) is normal; however, few meiocytes showed the occurrence of chromatin transfer among themselves which resulted into the formation of hypoploid and hyperploid PMCs. In addition, some PMCs depicted associated irregularities such as laggards, and chromatin bridges during meiosis-I and II. Microsporogenesis was also observed to be abnormal, and was characterized by the presence of micronuclei in the sporads. Owing to low frequency of meiotic irregularities, pollen fertility was not affected to greater extent; however, variable sized pollen grains were noticed in P. candollii. Analysis of previously published chromosome data revealed that there is no specific cytogeographic pattern formed for the genus on the basis of which we can construct any geographic segregation between the two basic numbers, x = 9 and 11. However, in a broader sense two overlapping zones seems to appear for the two basic numbers in the East, central and south Asia.
Introduction
Pleurospermum Hoffm. (Apiaceae) is a genus with 50 species distributed in Europe, Central Asia and Himalayas (Zehui and Watson Citation2005). It is a well-represented and diverse genus especially in the Himalayas. According to online data source “The Plant List” (http://www.theplantlist.org/1.1/browse/A/Apiaceae/Pleurospermum/) there are 161 scientific plant names of species rank for the genus Pleurospermum, of these 41 are accepted and 75 are synonym, while 45 are still under the status of unassessed. It is a widely spread, heterogeneous genus of complex and controversial taxonomy (Tutin Citation1968; Pimenov and Kljuykov Citation2000; Zehui and Watson Citation2005). Russian authors delimit Pleurospermum sensu stricto by only two species, P. austriacum and P. uralense, considering the other species to Aulacospermum, Hymenidium, Hymenolaena, Physospermopsis and Pterocyclus (Pimenov and Kljuykov Citation2000). However, this classification has not gained widespread acceptance and a more natural classification will only be possible following critical revision in the field, herbarium and possibly a more general approach, including the use of molecular, chemical, embryological and cytological parameters. Recently, Valiejo-Roman et al. (Citation2012) conducted a molecular phylogenetic analysis on 55 species using nuclear (nrITS) and cpDNA (psbA-trnH and trnL-trnF) sequences for representative species of Pleurospermum s. l. and closely related Hymenidium, Aulacospermum, Trachydium, Physospermopsis, Pseudotrachydium, Sinolimprichtia, Pterocyclus, and Hymenolaena, and also compared morphological characters. Only two traditional genera were supported as monophyletic groups in the molecular trees, namely Aulacospermum (including Pseudotrachydium) and Hymenolaena (Valiejo-Roman et al. Citation2012). Two stable groups were revealed within Hymenidium. The molecular data presented by Valiejo-Roman et al. (Citation2012) did not confirm an early divergence between northern and Sino-Himalayan species of Pleurospermum which indicated that Pleurospermum s. l. and most of the other genera included in their study are polyphyletic.
Members of the genus can be easily recognized by their broad, white margined bracts and bractlets. In India it is represented by 14 species (Aswal and Mehrotra Citation1994). Presently two species, P. candollii (DC.) Benth. ex C. B. Clarke (= Hymenolaena candollii DC.) and P. govanianum (Wall. ex DC.) Benth. ex C. B. Clarke (=Hymenolaena govanianum DC.) have been studied for chromosome number, meiotic behaviour and pollen fertility.
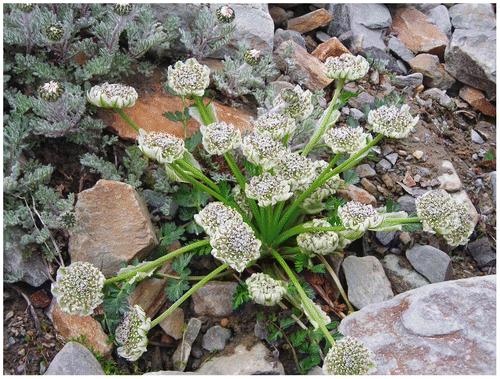
P. candollii (DC.) Benth. ex C. B. Clarke (= Hymenolaena candollii DC.) is a perennial, robust, aromatic herb with hollow stem, and base covered with persistent leaf bases. Leaves are petiolate, pinnate (pinnae are like Adiantum leaflets), opposite and ovate. Leaf base is sheathing and involucral bracts are white and leaf-like (Figures , ). The species is commonly distributed in Indian Himalayas and western Pakistan at altitudes of 3500–5000 m (Zehui and Watson Citation2005). It grows among the rocky boulders and moist alpine slopes along with Waldhemia tomentosa, Geum elatum and Ranunculus sp. It has been observed by the senior author in the current study that there are two habits for this plant species, acaulescent (stem not visible, (Figure ) and caulescent (stem visible, Figure ). The differences between the lengths of peduncles bearing the umbel have also been observed between the two habits of the species (Figures , ).
Figure 1. Pleurospermum candollii growing in its natural habitat on moist alpine slopes among stones, acaulescent habit.
Recently, Acharya Balkrishna, a key associate of Baba Ramdev (Pitanjali Yogpeeth in Haridwar in Uttarakhand State of India) claimed that the species (P. candollii) is one of the constituents of “Sanjeevani Booti” or the “life giving herb”. As per the majestic Hindu epic Ramayana, the Sanjeevani Booti is found in the Indian Himalayas and has the remarkable property of bioluminescence, a feature which facilitates it to be identified even in the darkness (Sah Citation2008). According to Hindu mythology, Sanjeevani Booti has the power to heal every recognized or mysterious disease and can even breathe life into a dead person. Sanjeevani Booti finds its mention in the Ramayana when Lakshamana (Laxmana, the younger brother of Lord Shree Rama) was wounded and was almost killed by Meghnaad (Ravana’s son). Hanuman was called upon to bring this herb from the Dronagiri parvat (mountain, now in Uttarakhand State of India) in the Himalayas. Sanjeevani Booti, according to popular faith, gave life to the dying Laxmana. Now the question crop up, which is this plant, so far a number of attempts had been made to know the exact plant which has these revitalizing properties. Acharya Balkrishna claimed that Sanjeevani Booti is a group of medicinal plants, which includes “Van” (P. candollii) and “Kasturi kamal” (Saussurea gossipiphora D. Don) and the two collectively make “Mrit Sanjeevani”. The tribal people living in the Dhauladhar hills used to call it “Bana” or “Shiva” (P. candollii). They used to bring both the plants to their homes with the belief that both of these are life saving. Both the above-mentioned herbs are used by the locals to cure unconsciousness, cerebral disorders, respiratory problems, body pains and other problems (Kumar Citation2004). In Tibetan medicine, roots of P. candollii are used for curing fever, particularly in food poisons and other poisons (Singh et al. Citation2008). The species is also used to heal stomach ache, renal pain, dyspepsia and flatulence (Singh et al. Citation2008). The aroma of the plant is said to make people dizzy (N.C. Nair unpublished).
P. govanianum (Wall. ex DC.) Benth. ex C. B. Clarke is a tall plant with fibrous stem base and prominent greenish umbell growing at high altitudes of 3000–4500 m in India and western Pakistan. Leaves are radical and cauline, 1–2-pinnate and pinnae pinnatifid (Figure ). It has an involucre of 5–6 divided foliaceous bracts, long, white margined, and an involucel of many pinnately divided, white margined bractlets, longer than the flowering umbellet. The species grows along with Geum elatum, Potentilla sp. and Polygonum sp. in alpine meadows at moist places.
A thorough review of the available literature (Darlington and Wyile Citation1955; Fedorov Citation1969; Kumar and Subramanian Citation1986; Khatoon and Ali Citation1993; Rice et al. Citation2015; IPCN Citation2015) revealed that members of the genus are poorly known for chromosome number and meiotic details from the Indian subcontinent and elsewhere. Information on chromosome numbers and ploidy is vital for the elucidation of taxonomic affiliations and modes of their evolution such as crossing barriers or historical and contemporary gene flow (Levin Citation2002; Stuessy Citation2009; Sánchez-Jiménez et al. Citation2012). The aim of the current paper is to report chromosome number, meiotic behaviour and pollen fertility in two species of Pleurospermum from unexplored alpine regions of north-west Himalaya. An attempt has also been made to find out a general cytogeographic pattern in the genus based on published data.
Material and methods
During the present investigation, materials for meiotic studies were collected from wild plants growing in alpine areas of north-west Himalaya, India. For cytological investigations, young unopened flower buds of suitable sizes were collected during the peak flowering period. These flower buds were fixed in Carnoy’s fixative (6 absolute alcohol: 3 chloroform: 1 glacial acetic acid v:v:v) for 24 h. Subsequently, the materials were transferred to 70% alcohol and stored in a refrigerator at about 4°C until analysis. For meiotic preparations, smears of the fixed anthers were made in 1% acetocarmine as per the technique of Belling (Citation1921). Pollen fertility was determined through the stainability test of pollen grains in mixture of glycerin and acetocarmine (1:1) (Marks Citation1954). Only well-filled pollen grains with well-stained nuclei were taken as apparently fertile and viable. Photomicrographs of pollen mother cells (PMCs) for chromosomal counts at different stages, meiotic irregularities, sporads and pollen grains were made from the freshly prepared slides using a Nikon Eclipse 80i microscope (Melville, NY, USA). The cytologically studied plants were identified using regional floras and compared with the specimens deposited at the Herbarium of Botanical Survey of India (BSD), Northern Regional Centre, Dehra Dun. The voucher specimens (PUN 51480, 51540, 51548, 51549) were deposited in the Herbarium, Department of Botany, Punjabi University, Patiala (PUN).
Results and discussion
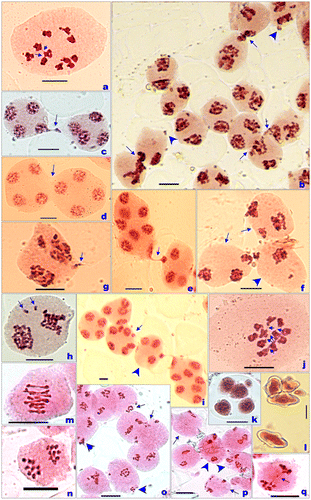
During meiosis, the presently studied individuals of P. candollii collected from Batal (3960 m) in the Lahaul Valley showed the meiotic chromosome count of n = 11 as confirmed from the presence of 11 equal sized bivalents at metaphase-I (Figure a). The phenomenon of cytomixis involving transfer of chromatin material among 2–3 adjacent meiocytes (2.91% PMCs) was observed during anaphase-I (A-I)/telophase-I (T-I) (Figure b) and metaphase-II (M-II)/telophase-II (T-II) (Figure c). A few PMCs (2.07%) showing broad cytomictic channels without any chromatin material transfer were also noticed (Figure d). Transfer of chromatin material among PMCs was noticed to be unidirectional forming one or more narrow chromatin strands (Figure e). Transfer of chromatin resulted into hypoploid and hyperploid PMCs (Figures f, g), and PMCs with extra chromatin masses (Figure g). These extra chromatin masses lagged behind during anaphases/telophases (Figure h) resulting in the formation of micronuclei in 4.45% of the observed PMCs. Partial transfer of chromatin material at A-I/T-II also resulted in formation of PMCs with varied number of nuclei of different sizes (Figure i). Interbivalent connections involving two or more bivalents were also observed in few PMCs at M-I (Figure j). Microsporogenesis was abnormal, and was characterized by the presence of micronuclei in 4.73% sporads (Figure k). Cytomixis and associated meiotic irregularities caused some pollen malformation (9%) and unequal-sized pollen grains (Figure l).
Figure 4. PMCs (pollen mother cells) showing meiotic chromosome numbers and meiotic abnormalities, and pollen grains. (a) Metaphase-I, n = 11; (b) chromatin transfer (arrows) and broken chromatin strands at A-I (arrowheads); (c) chromatin transfer at T-II; (d) broad cytomictic channels (arrow) without chromatin material transfer at T-II; (e) unidirectional chromatin transfer at T-II by forming single narrow strand with broad head; (f) hypoploid (arrowhead) and hyperploid (arrows) PMCs; (g) hyperploid PMC with (arrows) broken chromatin strands at A-I; (h) laggards (arrows) at A-I; (i) a PMC at T-II having seven different sized nuclei, and adjacent one with only three nuclei receiving chromatin from another PMC; (j) interbivalent connections (arrows); (k) sporad with a micronucleus; (l) heterogeneously sized stained pollen grains; (m) metaphase-I, n = 9; (n) 9:9 chromosome distributions at M-II; (o) chromatin transfer (arrows) and broken chromatin strands at A-I I (arrowheads); (p) hypoploid (arrows) and hyperploid (arrowhead) PMCs; (q) chromatin bridge at A-I. Scale bar =10 µm.
The present haploid chromosome count of n = 11 in the species agrees to the earlier report of 2n = 22 from India Himalayas by Russian workers (Pimenov et al. Citation2006) and by authors (Kumar and Singhal Citation2011). However, previous studies were restricted to mere counting of the chromosome number in the species and till now no details regarding the meiotic behaviour and pollen fertility is available.
Cytological studies have been performed in the accession of P. govanianum collected from Manimahesh Lake, 4300 m (Manimahesh hills). During meiosis, nine bivalents at M-I (Figure m), and 9:9 chromosomes distributions were observed at M-II (Figure n). The phenomenon of cytomixis was observed involving transfer of chromatin material through narrow as well as broad cytomictic channels forming a single chromatin strand in 1.04% PMCs during meiosis-I (Figures o, p). Chromatin material transfer resulted into the formation of hypoploid and hyperploid PMCs at M-II (Figures o, p). Chromosome bridges were observed in PMCs at A-I in 1.39% of the observed PMCs (Figure q). Consequently, the individuals of this accession also showed some pollen malformation (3%). The species was analysed chromosomally for the first time from India by Kumar and Singhal (Citation2011).
Cytomixis and associated meiotic abnormalities
The present study adds two more species to the list of angiosperms depicting the phenomenon of cytomixis from high altitude north-west Himalayas. Since the last decade, this unique phenomenon of inter PMC trans-migration of chromosomes/chromatin material has been reported quite frequently in the plants of north-west Himalaya by the authors (Kumar and Singhal Citation2011; Kumar et al. Citation2011, Citation2014, Citation2015; Singhal and Kumar Citation2008; Singhal et al. Citation2011, Citation2014; Kumar Citation2010; Rana et al. Citation2013, Citation2014) and other workers engaged in chromosomal studies from high altitude north-west Himalayas (Malik et al. Citation2010; Saggoo et al. Citation2011). The phenomenon had been correlated with the formation of hypoploid and hyperploid PMCs, unreduced pollen grains (2n gametes) and high pollen sterility (Lattoo et al. Citation2006; Negrón-Ortiz Citation2007; Singhal and Kumar Citation2008; Mursalimov et al. Citation2010; Mursalimov and Deineko Citation2015; Kumar et al. Citation2014, Citation2015). Cytomixis has been emphasized as a process of evolutionary importance because it results in changes in the gametic chromosome numbers (Falistocco et al. Citation1995; Liu et al. Citation2012). The 2n gamete production as a result of cytomixis and subsequent fusion of such gametes could serve as a mean of polyploid formation (Kumar et al. Citation2014). Polyploidy has been recognized to play a significant and pivotal role in speciation and evolution of plants. However, in the current study, owing to low frequencies of cytomixis and associated meiotic abnormalities, pollen fertility and pollen grain size was not affected. A number of causes have be suggested for the occurrence of cytomixis in plants (see Kumar Citation2010); however, in the present case, cytomixis seems to be a natural process of intercellular interaction under direct genetic control (De and Sharma Citation1983; Lattoo et al. Citation2006; Singhal and Kumar Citation2008; Kumar et al. Citation2010; Kravets Citation2012; Mandal et al. Citation2013).
Chromosomal status and cytogeographic pattern
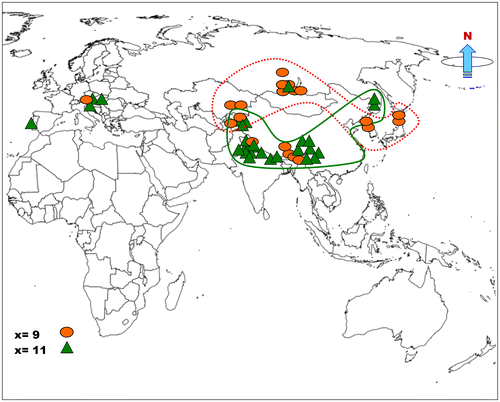
Chromosome number has been considered as a part of the multidisciplinary approach to the characterization of taxa. As has already been mentioned, Pleurospermum is a heterogeneous genus of complex and controversial taxonomy. In addition to the taxonomic data, information from other branches of systematics is of paramount importance to solve the taxonomic controversies existing in the genus. The genus includes 161 scientific plant names of species rank for the genus Pleurospermum; of these 41 are accepted while 75 are synonyms and 45 are under the status of unassessed (http://www.theplantlist.org/1.1/browse/A/Apiaceae/Pleurospermum/). The importance of cytological data in the study of plant evolution and diversification has long been recognized (Stebbins Citation1950, Citation1971; Hong Citation1990; Stace Citation2000). Chromosome surveys provide useful insight into population structure of both rare and common plants. Shner et al. (Citation2011) worked on the cytology of South African Umbelliferae and concluded that chromosome information can be of value in critical taxonomic revisions of the interesting and anomalous lineages of family. In Pleurospermum, chromosome counts are only available for a limited number of species. Among the 41 accepted species only 21 (Table ) have had their chromosome number counted, including the two presently studied species; about 50% of species are still to be determined cytologically. In India the genus is rather poorly studied; of the 14 species, chromosome numbers are available for four (28.57%) species (Mehra and Dhawan Citation1971; Pimenov et al. Citation2006; Jeelani et al. Citation2011; Kumar and Singhal Citation2011).
Table 1. Chromosome number, ploidy and geographical distribution 21 species of genus Pleurospermum.
The currently studied species of the genus, P. candollii and P. govanianum, revealed two different meiotic chromosome numbers, n = 11 and n = 9, respectively. The chromosome count of n = 11 for P. candollii is the second report in the species (Table ). The species has already been counted previously from India (Pimenov et al. Citation2006; Kumar and Singhal Citation2011). The meiotic chromosome count of 2n = 18 for P. govanianum was recorded for the first time by Kumar and Singhal (Citation2011). However, all previous studies remained primarily confined to mere counting of chromosomes and no information was available on the chromosome pairing and meiotic behaviour in these species.
Both P. candollii and P. govanianum exist at diploid level based on the basic chromosome number of x = 9 and 11, respectively, reported for the genus. Thus, the genus exhibits a dysploid series of 2n chromosome numbers. Rani et al. (Citation2014) compiled chromosome counts for 17 species in the genus and were of the opinion that x = 11 is the established basic number. A basic chromosome number of x = 11 constitute euploid series in some species of genus viz., P. apiolens (2n = 2x = 22, 2n = 3x = 33), P. hookeri var. thomsonii and P. uralense (2n=2x=22, 2n = 4x = 44) and P. heterosciadium (2n = 4x = 44). A perusal of available literature from various chromosome indexes (Darlington and Wyile Citation1955; Fedorov Citation1969; Kumar and Subramanian Citation1986; Khatoon and Ali Citation1993; Rice et al. Citation2015; IPCN Citation2015) revealed that of the total of 41 known species in the genus, chromosomes have been counted in only 21 species, and in 12 species ploidy level is strictly based on x = 11 (Table ), while in seven species the ploidy level is based on x = 9. Surprisingly, the ploidy level in P. austriacum is based on both the basic numbers, x = 9 and 11. In P. austriacum only the count from Austria (Wetschnig and Leute Citation1991) is based on x = 9; the other four counts are based on x = 11. Furthermore, a stray count of 2n = c.50 is also available from Russian region (Sokolovskaya Citation1963) in P. austriacum. In another species, P. uralense, of the 12 chromosome counts available, nine are based on x = 9 and three on x = 11. Coincidently, Russian worker (Pimenov and Kljuykov Citation2000) delimit Pleurospermum sensu stricto to two species, P. austriacum and P. uralense and the rest to genera Aulacospermum (x = 9), Hymenidium (x = 9, 11), Hymenolaena (x = 9), Physospermopsis x = 9) and Pterocyclus (x = 9, 11). The chromosome counts in the above-mentioned allied genera are also based on either x = 9 or 11 or on both. Rani et al. (Citation2014) considered x = 11 as the basic chromosome number for the genus and x = 9 as another proposed basic number for the genus. The chromosome number in all the species of the genus should be recorded first, and in some cases needs reconfirmation before suggesting segregation of these taxa based on chromosomal studies. However, x = 12 or x = 6 is regarded as the most plausible basic and ancestral chromosome number in Apiales (Sharma and Chatterji Citation1964; Yi et al. Citation2004). It is now established that chromosome number variation does exist in the genus (2n = 18, 22, 33, 44, c.50; see Table ). Polyploidy seems to have also played role in the evolution, but to little extent, as only two species, P. hookeri var. thomsonii C. B. Clarke (China: 2n = 2x = 22, Pu et al. Citation2006; 2n = 4x = 44, Alexeeva et al. Citation2000), P. uralense (Russia: 2n = 2x = 22, 2n = 4x = 44, Gurzenkov and Gorovoy Citation1971, 2n = c.50 aneuploid, Sokolovskaya Citation1963) and P. apiolens (Nepal and Kyrgyzstan: 2n = 2x = 22, Nepal 2n = 3x = 33) harbour intraspecific chromosomal variation. P. heterosciadium is strictly polyploid (China; 2n = 4x = 44, Pimenov et al. Citation1999). We are of the opinion that the base number x = 11 seems to have been much favoured in the genus. Further, intraspecific polyploid cytotypes in the above-mentioned species are based on x = 11, indicating the probability of most favoured base numbers in the genus. We here cautiously (based on the available chromosomal information for the genus) suggest that x = 11 may be considered as the original basic numbers from which x = 9 has been derived through descending aneuploidy.
Cytogeographic patterns found in a particular genus may provide insight to chromosome evolution (Jones and Smith Citation1966). Most chromosome counts in Pleurospermum are from the Asian region (Table ). Scrutiny of the published chromosome reports from different areas of the world revealed no clear cytogeographic pattern in the genus, particularly for base number. However, there are two overlapping zones noticeable for the two base numbers x = 9 and 11 (Figure ). The well-established basic number x = 11 is mainly found to be distributed in north-west Indian Himalayas and Nepal Himalayas in South Asia, Hengduan Mountains, Yunnan and Sichuan (south-west China) in the East Asia, Far East Russia and parts of the Europe. On the other hand, x = 9 clearly prevails in the Japanese and Korean part of East Asia and to some extent in Siberian region (Russia) in North Asia and Kazakhstan in Central Asia. If we assess the overall situation x = 11 is most widely spread basic chromosome number in the genus throughout its range. However, the geographic boundary between the two base numbers is not very obvious and a mixed situation can be noticed in East and North Asia.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding information
The authors are thankful to the University Grants Commission (UGC), New Delhi, for providing financial assistance under the DRS SAP I, II & III, ASIST programme, and Dr D.S. Kothari Postdoctoral Fellowship to Dr Puneet Kumar [award letter no. F.4-2/2006(BSR)/13-427/2011(BSR)].
Acknowledgements
The authors are very grateful to the Head of the Department of Botany, Punjabi University, Patiala and Dr Paramjit Singh, Director, Botanical Survey of India, Headquarters Kolkata and Joint Director, NRC, Dehradun, for necessary laboratory, Herbarium (PUN, BSD) and library facilities.
References
- Alexeeva TV, Pimenov MG, Kljuykov EV, Hao HZ. 2000. In IOPB chromosome data 16. Int Organ Pl Biosyst Newslett. (Pruhonice). 32(1):11–12.
- Aswal BS, Mehrotra BN. 1994. Flora of Lahaul-Spiti (A cold desert in northwest Himalaya). Dehra Dun, India: Bishen Singh Mahendra Pal Singh.
- Baksay L. 1958. The chromosome numbers of Ponto-Mediterranean plant species. Ann Hist Nat Mus Nation Hung ser nov. 9(1):121–125.
- Belling J. 1921. On counting chromosomes in pollen mother cells. Am Nat. 55(641):573–574.
- Byung-Yun S, Park JH, Kwak MJ, Kim CH, Kim KS. 1996. Chromosome counts from the flora of Korea with emphasis on Apiaceae. J Plant Biol. 39(1):15–22.
- Cauwet-Marc AM. 1982. In IOPB chromosome number reports LXXVII. Taxon. 31(5):771–772.
- Chepinoga VV, Gnutikov AA, Enushchenko IV. 2010. In IAPT/IOPB chromosome data 9. Taxon. 59(4):1298–1302.
- Chin HC, Pan ZH, Sheh ML, Wu CJ. 1989. A report on chromosome numbers of Chinese Umbelliferae. Acta Phytotax Sin. 27(2):268–272.
- Darlington CD, Wyile AP. 1955. Chromosome atlas of flowering plants. London: George Allen and Unwin.
- De M, Sharma AK. 1983. Cytomixis in pollen mother cells of an apomictic ornamental Ervatamia divaricata (L.) Alston. Cytologia. 48(1):201–207.
- Druskovic B. 1995. In IOPB chromosome data 9. Int Organ Pl Biosyst Newslett (Zurich). 24(1):11–14.
- Falistocco E, Tosti N, Falcinelli M. 1995. Cytomixis in pollen mother cells of diploid Dactylis, one of the origins of 2n gametes. J. Hered. 86(6):448–453.
- Fedorov ANA. 1969. Chromosome numbers of flowering plants. (Reprint 1974). Leningrad: Academy of Sciences of the USSR. Komarov Botanical Institute.
- Gurzenkov NN, Gorovoy PG. 1971. Chromosome numbers of Umbelliferae of the Far East. (In Russian) Bot Žhurn. 56(12):1805–1815.
- Hong DY. 1990. Plant cytotaxonomy. Beijing: Science Press.
- IPCN [Internet]. 2015. Index to plant chromosome numbers. [cited 2015 Jul 15]. Available from: http://www.tropicos.org/NameSearch.aspx?projectid=9
- Jeelani SM, Kumari S, Gupta RC. 2011. In IAPT/IOPB chromosome data 12. Taxon. 60(6):1784–1796.
- ]ones K, Smith JB. 1966. The cytogeography of Impatiens L. (Balsaminaceae). Kew Bull. 20(1):63–72.
- Khatoon S, Ali SI. 1993. Chromosome atlas of the angiosperms of Pakistan. Karachi: Department of Botany. University of Karachi.
- Kravets EA. 2012. Nature, significance, and cytological consequences of cytomixis. Cytol Genet. 46(3):188–195.
- Krogulevich RE. 1976. Chromosome numbers of plant species from the Tunkinsky Alpes (East Sayan). News Sib Depart Acad Sci USSR Ser Biol. 15(3):46–52.
- Krogulevich RE. 1978. Karyological analysis of the species of the flora of eastern Sayana. In Malyshev LI, Peshlcova GA, editors. Flora of the Prebaikal. Novosibirsk: Nauka Publishers. p. 19–48.
- Kumar K. 2004. Bharat ki divya vanaspatiyan. Lucknow, India: National Botanical Research Institute.
- Kumar P. 2010. Exploration of cytomorphological diversity in the members of Polypetalae from Lahaul-Spiti and adjoining areas [PhD thesis]; [cited 2015 Jul 25]. Punjabi University, Patiala, Punjab, India. Available from: http://hdl.handle.net/10603/2872
- Kumar V, Subramanian B. 1986. Chromosome atlas of flowering plants of the Indian subcontinent. Vol I. Dicotyledon. Calcutta: Botanical Survey of India.
- Kumar P, Rana PK, Himshikha Singhal VK, Gupta RC. 2014. Cytogeography and phenomenon of cytomixis in Silene vulgaris from cold regions of Northwest Himalayas (India). Plant Syst Evol. 300(5):831–842.
- Kumar P, Singhal VK. 2011. Chromosome number, male meiosis and pollen fertility in selected angiosperms of the cold deserts of Lahaul-Spiti and adjoining areas (Himachal Pradesh, India). Plant Syst Evol. 297(3):271–297.
- Kumar P, Singhal VK, Kaur D, Kaur S. 2010. Cytomixis and associated meiotic abnormalities affecting pollen fertility in Clematis orientalis. Biol Pl. 54(1):181–184.
- Kumar P, Singhal VK, Rana PK, Kaur S, Kaur D. 2011. Cytology of Ranunculus laetus Wall. ex Royle from cold desert regions and adjoining hills of North-west Himalayas. Caryologia. 64(1):25–32.
- Kumar R, Rana PK, Himshikha Kaur D, Kaur M, Singhal VK, Gupta RC, Kumar P. 2015. Structural heterozygosity and cytomixis driven pollen sterility in Anemone rivularis Buch.-Ham. ex DC. from Western Himalaya (India). Caryologia. 68(5):246–253.
- Lattoo SK, Khan S, Bamotra S, Dhar AK. 2006. Cytomixis imparis meiosis and influences reproductive success in Chlorophytum comosum (Thunb) Jacq. – An additional strategy and possible implications. J Biosci. 31(5):629–637.
- Liu Y, Hui RK, Deng RN, Wang JJ, Wang M, Li ZY. 2012. Abnormal male meiosis explains pollen sterility in the polyploid medicinal plant Pinellia ternata (Araceae). Genet Mol Res. 11(1):112–120.
- Levin DA. 2002. The role of chromosomal change in plant evolution. Oxford: Oxford University Press.
- Malik RA, Gupta RC, Kumari S. 2010. Genetic diversity in different populations of Artemisia absinthium Linn. from Kashmir Himalaya. Cytologia. 75(3):273–276.
- Mandal A, Datta AK, Gupta S, Paul R, Saha A, Ghosh BK, Bhattacharya A, Iqbal M. 2013. Cytomixis – a unique phenomenon in animal and plant. Protoplasma. 250(5):985–996.
- Marks GE. 1954. An acetocarmine glycerol jelly for use in pollen fertility counts. Stain Technol. 29(5):277.
- Mehra PN, Dhawan H. 1971. In IOPB chromosome number reports XXXIV. Taxon. 20(5/6):785–791.
- Murín A. 1978. In Index of chromosome numbers of Slovakian flora. Part 6. Acta Fac Rerum Nat Univ Comenianae Bot. 26(1):1–42.
- Murín A, Májovský J. 1978. In IOPB chromosome number reports LXI. Taxon. 27(4):376–378.
- Mursalimov SR, Baiborodin SI, Sidorchuk YV, Shummy VK, Deineko EV. 2010. Characteristics of the cytomictic channel formation in Nicotiana tabacum L. pollen mother cells. Cytol Genet. 44(1):14–18.
- Mursalimov SR, Deineko EV. 2015. How cytomixis can form unreduced gametes in tobacco Plant Syst Evol. 301(4):1293–1297.
- Negrón-Ortiz V. 2007. Chromosome numbers, nuclear DNA content, and polyploidy in Consolea (Cactaceae), an endemic cactus of the Caribbean islands. Am J Bot. 94(8):1360–1370.
- Nishikawa T. 1988. Chromosome counts of flowering plants of Hokkaido (11). J Hokkaido Univ Educ Sect. 2B(38):33–40.
- Pimenov MG, Alexeeva TV, Artem’eva GM, Kljuykov EV. 1998. In IOPB chromosome data 13. Int Organ Pl Biosyst Newslett (Oslo). 29(1):23–24.
- Pimenov MG, Alexeeva TV, Kljuykov EV. 2001. In IOPB chromosome data 17. Int Organ Pl Biosyst Newslett (Pruhonice). 33(1):24–25.
- Pimenov MG, Alexeeva TV, Kljuykov EV. 2006. In IAPT/IOPB chromosome data 2. Taxon. 55(3):757–758.
- Pimenov MG Alexeeva TV, Kljuykov EV, Bokova OM, Xin LQ. 1999. In IOPB chromosome data 15. Int Organ Pl Biosyst Newslett (Pruhonice). 31(1):13–16.
- Pimenov MG, Kljuykov EV. 2000. Taxonomic revision of Pleurospermum and related genera of the Umbelliferae. II. The genera Pleurospermum, Pterocyclus, Trachydium, Keraymonia, Pseudotrachydium. Aulacospermum and Hymenolaena. Feddes Rep. 111(7–8):517–534.
- Pimenov MG, Vassil’eva MG. 1983. In IOPB chromosome number reports LXXXI. Taxon. 32(4):663–664.
- Pu J-X, He X-J, Zhang X-M, Chen W-W. 2006. Karyotypes of seven populations belonging to four species of Umbelliferae in Hengduan Mountains. Acta Bot Boreal Occid Sin. 26(10):1989–1995.
- Rana PK, Kumar P, Singhal VK. 2013. Spindle irregularities, chromatin transfer and chromatin stickiness on male meiosis and pollen grain formation in Anemone tetrasepala. Turk J Bot. 37(1):167–176.
- Rana PK, Kumar P, Singhal VK. 2014. Cytomixis and associated abnormalities during male meiosis in Lindelofia longiflora var. falconeri (Boraginaceae). Cytologia. 79(4):535–540.
- Rani S, Jeelani SM, Kumar S, Kumari S, Gupta RC. 2014. An overview of chromosome and basic numbers diversity in cytologically investigated polypetalous genera from the Western Himalayas (India). Caryologia. 67(1):1–24.
- Rice A, Glick L, Abadi S, Einhorn M, Kopelman NM, Salman-Minkov A, Mayzel J, Chay O, Mayrose I. 2015. The Chromosome Counts Database (CCDB) – a community resource of plant chromosome numbers. New Phytol. 206(1):19–26.
- Rostovtseva TS. 1976. Chromosome numbers of some species of the family Apiaceae in South Siberia. Bot Žhurn (Moscow & Leningrad). 61(1):93–99.
- Rostovtseva TS. 1979. Chromosome numbers of some species of the family Apiaceae Lindl. II. Bot Žhurn. 64(2):227–232.
- Saggoo MIS, Srivastava DK, Grewal P. 2011. Meiotic studies in 14 species of the Nepeta L. (Lamiaceae) From cold desert regions of Lahaul-Spiti and adjoining areas of northwest-Himalaya. India. Cytologia. 76(3):231–236.
- Sah P. 2008. Does the magical Himalayan herb “Sanjeevani Booti” really exist in Nature? J Am Sci. 4(3):65–67.
- Sánchez-Jiménez I, Hidalgo O, Canela MÁ, Siljak-Yakovlev S, Šolić ME, Vallès J, Garnatje T. 2012. Genome size and chromosome number in Echinops (Asteraceae, Cardueae) in the Aegean and Balkan regions: technical aspects of nuclear DNA amount assessment and genome evolution in a phylogenetic frame. Pl Syst Evol. 298(6):1085–1099.
- Sharma AK, Chatterji AK. 1964. Cytological study as an aid in the interpretation of the systematic status of different genera of Araliaceae. Cytologia. 29(5):1–2.
- Shner JV, Alexeeva TV, Pimenov MG, Wyk BEV. 2011. Chromosome numbers of South African Umbelliferae (Apiaceae). S Afr J Bot. 77(2):497–502.
- Shner JV, Alexeeva TV, Pimenov MG, Kljuykov EV, Ukrainskaya UA, Zakharova EA. 2012. In IAPT/IOPB chromosome data 14. Taxon. 61(6):1336–1345.
- Shner JV, Alexeeva TV, Pimenov MG, Kljuykov EV, Ukrainskaya UA, Zakharova EA. 2014. IAPT/IOPB chromosome data 18. Taxon. 63(6):1393.
- Shner JV, Pimenov MG, Kljuykov EV. 2009. In IAPT/IOPB chromosome data 8. Taxon. 58(4):1281–1289.
- Singh KN, Gopichand Kumar A, Lal B. 2008. Species diversity and population status of threatened plants in different landscape elements of the Rohtang Pass. Western Himalaya. J Mt Sci. 5(1):73–83.
- Singhal VK, Kumar P. 2008. Impact of cytomixis on meiosis, pollen viability and pollen size in wild populations of Himalayan poppy (Meconopsis aculeata Royle). J Biosci. 33(3):371–380.
- Singhal VK, Kumari V, Kumar P. 2014. Cytomorphological diversity in some selected members of Poaceae from Parvati Valley in Kullu district of Himachal Pradesh. India. Plant Syst Evol. 300(6):1385–1408.
- Singhal VK, Rana PK, Kumar P, Kaur D. 2011. Persistent occurrence of meiotic abnormalities in a new hexaploid cytotype of Thalictrum foetidum L. from Indian cold deserts. Biologia. 66(3):458–464.
- Sokolovskaya AP. 1963. Geographical distribution of polyploid plant species. Study on the flora of Kamchatka Peninsula. Proc Leningrad Univ Biol. 3(1):38–52 (In Russian).
- Stace CA. 2000. Cytology and cytogenetics as a fundamental taxonomic resource for the 20th and 21st centuries. Taxon. 49(3):451–477.
- Stebbins GL. 1950. Variation and evolution in plants. New York, NY: Columbia University Press.
- Stebbins GL. 1971. Chromosomal evolution in higher plants. London: Edward Arnold.
- Stuessy TF. 2009. Plant taxonomy: the systematic evaluation of comparative data. 2nd ed. New York, NY: Columbia University Press.
- Tutin TG. 1968. In: Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine DH, Walters SM, Webb DA, editors. Flora Europaea 2. London: Cambridge University Press; p. 343.
- Valiejo-Roman CM, Terentieva EI, Pimenov MG, Kljuykov EV, Samigullin TH, Tilney PM. 2012. Broad polyphyly in Pleurospermum s. l. (Umbelliferae-Apioideae) as inferred from nrDNA ITS and chloroplast sequences. Syst Bot. 37(2):573–581.
- Vassil’eva MG, Alexeeva TV, Pimenov MG, Kljuykov EV. 1991. In IOPB chromosome data 3. Int Organ Pl Biosyst Newslett (Zurich). 17(1):10–13.
- Vassil’eva MG, Daushkevich JV, Alexeeva TV, Pimenov MG. 1993. IOPB chromosome data 5. Int Organ Pl Biosyst Newslett (Zurich). 20(1):7–9.
- Wetschnig W, Leute GH. 1991. Chromosomenzahlen Kärntner Gefässflanzen (Teil 2, Doldenblütler Apiaceae = Umbelliferae). Linzer Biol Beitr. 23(2):457–481.
- Yi T, Lowry IIPP, Plunkett GM, Wen J. 2004. Chromosomal evolution in Araliaceae and close relatives. Taxon. 53(4):987–1005.
- Zehui P, Watson MF. 2005. Pleurospermum Hoffmann. Flora of China. 14:40–51.