Abstract
Banana (Musa spp.) is the world’s fourth most important food crop after rice, wheat and maize. The Valery banana cultivar is imported in Iran and well known as economic bananas in southern Iran. Shoots (with 2–3 cm stems) of the “Valery” cultivar of banana (Musa sp.) were selected, and meristematic tissue explants were isolated and cultured. Following embryo development, germination and differentiation, samples of regenerated plantlets were randomly taken and fixed in Formaldehyde, Glycial Acetic Acid, Alchol Ethylic (FAA) solution. Serial sections (10 μm) were prepared and stained in hematoxiline and eosine. An aqueous solution of 8-hydroxyquinoline was used to pre-treat the root tips. A solution of absolute ethanol and glacial acetic acid was used to fix the root tips. The mitotic cells identified with metaphase or prometaphase stages were used for chromosome counting. The meristematic tissue segments became swollen two weeks after cultures were initiated. Calli of the embryogenic line were kept separately; these tissues released embryogenic cells. The highest number of embryos (globular, torpedo and mature) were obtained with embryogenic medium (BM). The mature embryos and regenerated plantlets were cultured on regenerated medium (GM). The percentage of germination and development of completely regenerated banana plants was 90–96%. Histological study of regenerated shoots showed that there were many vascular tissues but of a small size; also that the vascular bundles were large and dispersed. The chromosomes were aggregated in metaphase. The regenerated plants from somatic embryos showed 60% triploidy, 10% diploidy and 30% aneuploidy and the chromosomes are more contracted and spread around the cell.
Introduction
Banana (Musa spp.) is the world’s fourth most important food crop after rice, wheat and maize (Kauther et al. Citation2013). It is a staple food for nearly 400 million people throughout the developing world (Sasson Citation1997) and an export commodity which contributes to the food security of millions of people in the developing world, and when traded in local markets provides income and employments to rural populations. More than 58 million tons of bananas are produced worldwide (FAO Citation2003). The average global yield is 15 tons ha−1 per year.
Traditionally, most of the edible clones are seedless, sterile and propagated by suckers. For these reasons, classical breeding is difficult. The multiplication of a clone is slow, laborious, and time-consuming as far as to obtain a large number of homogeneous plants (Sasson Citation1997). Recent advances in biotechnology for crop improvement have had a great impact on banana cultivation.
Plant regeneration via somatic embryogenesis culture has been described by a number of authors. Immature male flowers have been used to initiate cultures of several banana cultivars (Escalant et al. Citation1994; Morroquin et al. Citation1993). Somatic embryogenesis has been reported from leaf sheaths, rhizome fragments from in vitro plants (Khalil et al. Citation2004), thin sections from proliferating buds (Meenakshi et al. Citation2011), immature zygotic embryos (Samson Citation1982) and male flower bud cultures. Somatic embryos were obtained from callus derived from leaf base cultured in liquid medium (Sandoval et al. Citation1991; Yeung Citation1995). However, these embryos regenerated only roots. The conversion of the embryos into plantlets is frequently low, thus limiting genetic transformation techniques.
In vitro regeneration via callus and somatic embryogenesis cultures is not only important for an integral part of genetic transformation but also for selection of useful somaclonal variants. Embryogenic callus culture of the “Valery” cultivar of banana was initiated from shoot tips. The Valery banana cultivar is imported in Iran and well known as economic bananas in southern Iran. It is seedless, tasty, aromatic and highly priced. Histological knowledge of callus induction can provide important information for improving the somatic embryogenesis for this crop. However, to date no precise histological study in Valery cultivar has been performed.
Materials and methods
Plant material and culture initiation
The initial plant material used was the Valery cultivar of banana (Musa sp.) belonging to the AAA group. Shoots (with 2–3 cm stems) were selected, washed with 1% (v/v) detergent solution for 5 min and surface sterilized by 10% NaOCl2 for 15 min and finally rinsed three times with sterile water. In a sterile Petri dish, the outer leaves were peeled with forceps until meristematic tissues of 0.5–1 cm in length were obtained (Farahani et al. Citation2012). The meristematic tissues were isolated for cultures.
Callus initiation
Meristematic tissues explants were isolated and cultured on solid embryogenic medium (BM) at two strengths, consisting of MS basal medium (Murashige and Skoog Citation1962) plus benzyl amino purine (BA) (22.5 mg l−1) and 30 g l−1 sucrose with pH adjusted to 5.8. The cultures were incubated in the growth chamber at 25°C for four weeks. The meristematic tissues became swollen four weeks after cultures were initiated in BM medium; this could be seen as whitish tissue protruding from tissues. Primary somatic embryos were produced after tissues were transferred to fresh media for 12 weeks. Compact white calluses and friable embryogenic tissues with globular structures containing primary somatic embryos were formed.
Differentiation of embryos, embryo germination and plantlet formation
Embryos were developed and germinated on BM medium then transferred to regenerated medium (GM) for embryo differentiation. GM medium (Farahani et al. Citation2012) contains MS salts, MS vitamin, BA (3 mg l−1), indole acetic acid (IAA) (2 mg l−1), charcoal (0.5 g l−1), sucrose (30 g l−1), and agar (7 g l−1). The cultures were incubated at 25°C with a photoperiod of 16 h light and 8 h dark for 10–13 weeks.
Histological investigation
Morphological characteristics of calli were examined using an Olympus stereo microscope SZH10 (Olympus, Germany). The paraffin method was used for light microscopy following the method described by Majd et al. (Citation2010) and Johansen (Citation1940). The samples of regenerated plantlets were randomly taken and fixed in Formaldehyde, Glycial Acetic Acid, Alchol Ethylic (FAA) II solution containing 95% ethyl alcohol: glacial acetic acid: formaldehyde: water (10:1:2:7 v/v). Fixed specimens were dehydrated through a tertiary-butyl alcohol series, embedded in Paraplast plus (melting point 56°C) using Histo-embedder (Jung, Leica, Akhn, Germany). Sections were cut using a rotary microtome and mounted in Permount (Fisher Scientific International Inc., California, USA) (Kanchapoom and Korapatchaikul Citation2012). Serial sections (10 μm) were prepared in a rotary microtome (Leica RM-2155) with a tungsten-carbide knife, the sections floated in water drops, dried on a hot plate (40°C), and stained either in hematoxiline (0.05%) or eosine (0.1%), and counterstained with hematoxiline (0.5%) (Majd et al. Citation2010).
Cytogenetic investigation
Roots from mother plants and somatic embryos of regenerated plantlets were used as a source of root tips for chromosome preparation. This was to prevent oxidation, which results in discoloration of the roots, while the buffer solution ensured cell division until fixation was carried out. An aqueous solution of 0.02% (w/v) 8-hydroxyquinoline was used to pre-treat the root tips for 45–60 min in the dark since this chemical is photosensitive. A solution of absolute ethanol and glacial acetic acid in the ratio of 3:1 (v/v) was used to fix the root tips for at least 24 h at room temperature. Fixed roots were then stored in 70% ethanol until used for slide preparation (Osuji et al. Citation1996; Dole Zel et al. Citation1998; Bakry et al. Citation2008).
The preparations were covered with coverslips. Slides were observed with an oil immersion objective lens and a Leitz Diaplan phase contrast microscope. The mitotic cells identified with metaphase or prometaphase stages were used for chromosome counting, both for the normal and somaclonal regenerants for the chromosome information used for assessment of materials.
Good root cells at prometaphase were photomicrographed to reflect chromosome number and morphology. A Leica Wild MPS 52 microscope camera was used to photograph good plates, using appropriate filters.
Results and discussion
Culture initiation and callus development
Meristematic tissues explants were isolated and cultured on BM medium. The meristematic tissues segments became swollen four weeks after cultures were initiated. Meristematic tissues could be seen as whitish tissue protruding from the apical and axillary buds. The swollen tissues were in BM medium carefully excised from the mother tissue, transferred to fresh medium. These were kept in the same as medium culture for 12 weeks. Initially, the callus that formed was yellowish and compact, with white friable embryogenic tissues developing 10–13 weeks after initiation (Figure a).
Figure 1. (a) The yellowish and compact callus; (b) somatic embryos developed to globular and torpedo shapes of embryos; (c) somatic embryos differentiated to small shoots on GM medium; (d) mature somatic embryos differentiated to small plantlets.
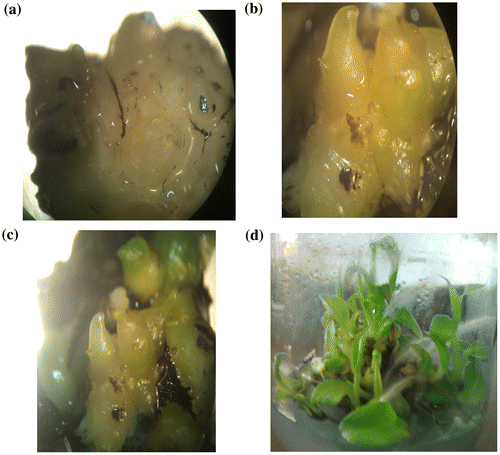
Calli of embryogenic line were kept separately, these tissues released embryogenic cells. Some proembryos and nodular structures were also present in the medium culture. When BM was used the highest number of embryos (globular, torpedo and mature) were obtained at 25°C. Somatic embryos initially appeared small and rapidly enlarged into a distinct globular structure, passing through a recognizable torpedo structure (Figure b). Somatic embryos initially globular and torpedo observed was noted within 18–21 days after aspirated on BM regeneration medium.
Embryo germination and plantlet formation
The mature embryos and regenerated plantlets were obtained when BM were cultured onto GM medium. Vuylsteke (Citation1989), Lee (Citation1992) and Okole and Schulz (Citation1996) reported that high BA concentrations induced shoot formation.
The effects of BM, developed by Khalil et al. (Citation2002), and a differentiation medium (GM) on the maturation of embryos and development of regenerated banana plants were studied. Figure c illustrates that the somatic embryos which developed on BM and then were transferred to GM produced the highest number of shoots (96%). Culture on GM medium allows these embryos to be differentiated with small shoot.
Mature somatic embryos which differentiated on GM medium gave rise to small plantlets (shoots and roots) within 10 days (Figure d). The small plantlets were subcultured onto GM for elongation and development of new leaves. The percentages of germination and development of completely regenerated banana plants from embryogenic solid medium reported in this study are 90–96% (Figure ), i.e. higher than those reported by Novak et al. (Citation1989) (1.5–12%); Dedh’a et al. (Citation1991) (10–23%), Cote et al. (Citation1996) (3–20%); Grapin et al. (Citation1996) (10–20%) and Navarro et al. (Citation1997) (13–25%). Our results are even higher than those of Kosky et al. (Citation2002) who reported 89.3% germination cell suspension using a bioreactor and Khalil et al. (Citation2002) who reported 89.5% germination percentage using secondary somatic embryos. The established protocol in this paper for regeneration takes 5.5–6.5 months from initiation of solid medium culture, but a longer time in other studies, e.g. 16 months (Cote et al. Citation1996); 18 months (Becker et al. Citation2000) and 10–11 months (Ganapathi et al. Citation2001). We have successfully regenerated plants using the system described in this paper via meristem cell culture. The high efficiency of regeneration reported in this paper is useful for plant mass propagation and for genetic transformation experiments, indicating that this protocol is reproducible and needs a short time.
The explants responded positively to embryogenesis induction, when cultured in cytokinin hormone. In preliminary tests, the thickness of the explants influenced the response. Thickness of approximately 1–2 mm gave best results. Embryogenic response was more intense in the shoot meristem, leaf bases and central cylinder. These regions retain more meristematic ability, which facilitates the onset of embryogenesis.
Presence of BA increased in vitro shoot multiplication of plantlets regenerated from embryogenic callus (Kauther et al. Citation2013). The possible use of a low concentration of auxin enhanced the formation of embryogenic callus. Generally, reduction of auxin concentration or its complete omission leads to the germination of somatic embryos. The presence of BA was necessary for banana colonial propagation since cytokinins induce multiple shoot formation by supporting apical dominance of the main meristem (Dole Zel et al. Citation1998).
After 12 weeks in induction medium and transfer to GM medium with auxin, somatic embryos detached from the explant and initial development of a root primordium was observed.
Histological investigation
Histological sections of the explants at the time of introduction in vitro showed cortical parenchyma surrounding the central cylinder region. This region was constituted by parenchyma tissue interspersed with vascular strands.
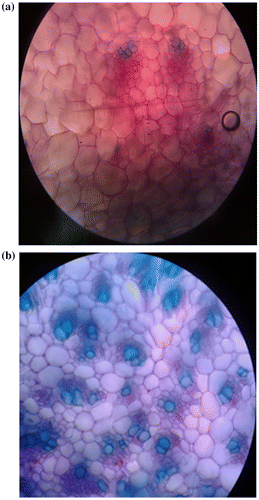
Histological study of regenerated shoots showed that the number of vascular tissues was large but the size of them was small. Phloem and xylem vascular tissues were initiated on several cambium rings. Disarrangement was observed in vascular tissues. In regenerated shoots, meta-xylem cells were larger and parenchyma cells between vascular tissues were smaller than in the shoots of mother plants (Figure a, b).
Figure 2. Cross section of the shoot of (a) regenerated plantlet from somatic embryo; (b) mother plant (×10).
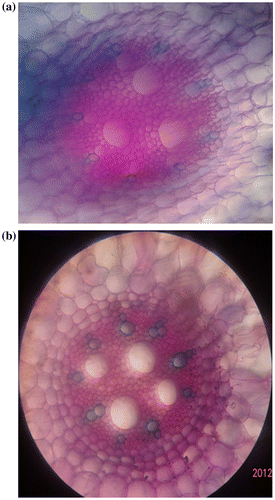
The regenerated roots showed that the vascular bundles were large and dispersed. Phloem and xylem vascular tissues were initiated on vascular cambium. In regenerated root, meta-xylem cells and parenchyma cells between vascular tissues were larger than the root of mother plants (Figure a, b).
Figure 3. Cross section of the root of (a) regenerated plantlet from somatic embryo; (b) mother plant (×10).
McGahan (Citation1961) presented details of the zygotic embryo of M. balbisiana. A distinct shoot apical meristem and the first leaf primordium were observed and a single procambial strand bifurcates several times through all the embryo extension. Lee et al. (Citation1997) observed regions with more intense mitotic activity in banana somatic embryos after 50 days in induction medium, which could be the initial stage of plumule development; however, further embryo development was not observed.
Cytogenetic investigation
Approximately 100% of the chromosome counts showed a count of 3x = 2n = 33 in the mother plants. Although there were phenotypically distinguishable descriptors for the somaclonal variants in each case, none were associated with any structural or numerical chromosomal abnormality. The chromosomes were aggregated in the metaphase of mitotic division. There were a few cases of variation in number, which were judged to be aneuploid cases in regenerated plants.
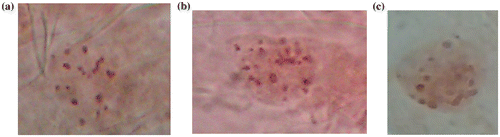
Prometaphase and metaphase chromosomes were differences in number of chromosome within and between the materials used in this study. The regenerated plants from somatic embryos showed 60% triploidy and the chromosomes are not very contracted and well spread around the cell with a small vertical dispersion, 10% diploidy and 30% aneuploidy and the chromosomes are more contracted and spread around the cell (Figure a, b–c). The mother plants were 100% triploid, the chromosomes are not very contracted and well spread around the cell in the same plane with no overlaps.
Figure 4. The chromosome of regenerated plants from somatic embryos (a) 60% triploidy (2n = 3x = 33); (b) 10% diploidy (2n = 2x − 2 = 20); (c) 30% aneuploidy (2n = 2x + 1 = 23).
Variability in chromosome number and morphology is more common in somatic cells cultured in vitro than in the natural environment (Bayliss Citation1980; Larkin and Scowcroft Citation1981; Levan et al. Citation1964; Choy et al. Citation2001; Gordian et al. Citation2007). This is one of the possible reasons for somaclonal variation occurring in tissue culture. The in vitro environment affected mitotic instability in bananas, as the mean frequency of aberrant metaphase cells was significantly higher in somaclonal variants than in mother plants.
Vuylsteke et al. (Citation1992) reported that tissue culture leads to somaclonal variation in Musa. Our results showed that visible numerical changes were observed between the mother plants and somaclonal regenerants of the banana cv. Valery used in this study. It is possible that the length of time the materials remained in culture was too long to induce these abnormalities.
Funding
This work was supported by Islamic Azad University [grant number 123]; Shahid Beheshti University of biology Sciences (IR) [grant number 789]; Islamic Azad University (IR) [grant number 456].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bakry F, Shepherd K. 2008. Chromosome count on banana root tip squashes. Fruits. 63(3):179–181. doi:10.1051/fruits:2008008.
- Bayliss MW. 1980. Chromosomal variation in plant tissues in culture. Int Rev Cytol. 11a (Suppl.):113–114.
- Becker DK, Dugdale B, Smith MK, Harding RM, Dale JL. 2000. Genetic transformation of Cavendish banana (Musa spp. AAA group) cv Grand Nain via microprojectile bombardment. Plant Cell Rep. 19(3):229–234. doi:10.1007/s002990050004.
- Choy MK, Teoh SB. 2001. Mitotic instability in two wild species of bananas (Musa acuminata and M. balbisiana) and their common cultivars in Malaysia. Caryologia. 54(3):261–266. doi:10.1080/00087114.2001.10589234.
- Cote FX, Domergue R, Monmarson S, Schwendiman J, Teisson C, Escalant JV. 1996. Embryogenic cell suspensions from the male flower of Musa AAA cv. Grand nain. Physiol Plant. 97(2):285–290. doi:10.1034/j.1399-3054.1996.970211.x.
- Cote FX, Domergue R, Monmarson S, Schwendiman J, Teisson C, Escalant JV. 1996. Embryogenic cell suspensions from the male flower of Musa AAA CV. Grand nain. Physiol Plant. 97(2):285–290. doi:10.1034/j.1399-3054.1996.970211.x.
- Dedh’a D, Dumortier F, Panis B, Vuylsteke D, Langgue DE. 1991. Plant regeneration in cell suspension cultures of the cooking banana cv. Bluggoe (Musa spp. ABB group). Fruits. 46:125–135.
- Dole Zel J, Dole Zelva M, Van Den Houwe I, Roux N. 1998. A novel method to prepare slides for high resolution chromosome analysis in Musa. Infomusa. 7(1):3–4.
- Escalant JV, Teisson C, Cote FX. 1994. Amplified somatic embryogenesis from male flowers of triploid banana and plantain cultivars (Musa spp.). In Vitro Cell Dev Biol Plant. 30(4):181–186. doi:10.1007/BF02823029.
- FAO. 2003. The World Banana Economy 1985–2002. Rome, Italy: Food and Agriculture Organization.
- Farahani F, Majd A. 2012. Comparison of liquid culture methods and effect of temporary immersion bioreactor on growth and multiplication of banana (Musa, cv. Dwarf Cavendish). Afr J Biotechnol. 11(33):8302–8308. doi:10.5897/AJB11.2020.
- Ganapathi TR, Higgs NS, Balint-Kurti PJ, Arntzen CJ, May GD, Van Eck JM. 2001. Agrobacterium?-mediated transformation of embryogenic cell suspensions of the banana cultivar Rasthali (AAB). Plant Cell Rep. 20(2):157–162. doi:10.1007/s002990000287.
- Gordian C, Obute P, Aziagba C. 2007. Evaluation of karyotype status of Musa L. Somaclonal Variants (Musaceae: Zingiberales). Turk J Bot. 31:143–147.
- Grapin A, Schwendiman J, Teisson C. 1996. Somatic embryogenesis in plantain banana. Vitro Cell Dev Biol Plant. 32(2):66–71. doi:10.1007/BF02823133.
- Johansen DA. 1940. Plant Microtechnique. New York, NY: McGraw-Hill.
- Kanchapoom K, Korapatchaikul K. 2012. Histology of callogenesis in diploid bananas (Musa acuminata, AA Group) ‘Kluai Sa’ and ‘Kluai Leb Mu Nang’. Not Sci Biol. 4(1):94–97.
- Kauther SA, Elhassan AA, Ehiweris SO, Maki HE. 2013. Embryogenesis and plantlet regeneration via immature male flower culture of banana (Musa sp.) cv. Grand nain. Journal Forest Products & Industries. 2(3):48–52.
- Khalil SM, Elbanna AAM. 2004. Highly efficient somatic embryogenesis and plant regeneration via suspension cultures of banana (Musa spp.). Arab. J Biotechnol. 7(1):99–110.
- Kosky RG, Silva M, De F, Perez LP, Gilliard T, Martnez FB, Vega MR, Milian RC, Mendoza EQ. 2002. Somatic embryogenesis of the banana hybrid cultivar FHIA-18 (AAAB) in liquid medium and scaled up in a bioreactor. Plant Cell Tissue Organ Cult. 68:21–26.
- Larkin PJ, Scowcroft WR. 1981. Somaclonal variation – a novel source of variability from cell cultures for plant improvement. Theor Appl Genet. 60(4):197–214. doi:10.1007/BF02342540.
- Lee KS, Zapata-Arias FJ, Brunner H, Afza R. 1997. Histology of somatic embryo initiation and organogenesis from rhizome explants of Musa spp. Plant Cell Tissue Organ Cult. 51(1):1–8. doi:10.1023/A:1005985401756.
- Lee SW. 1992. Improvement of methods used in the regeneration of micropropagated banana plantlets. In: Valmayor RV, Hawang SC, Ploetz R, Lee SW, Rao NV, editors. Proc Int Symp Recent Dev Banana Cultivation Technol. Pingtung, Taiwan: INIBAP/ASPNET; p. 179–192.
- Levan A, Fredga K, Sandberg AA. 1964. Nomenclature for centromeric position on chromosomes. Hereditas. 52(2):201–220. doi:10.1111/j.1601-5223.1964.tb01953.x.
- Majd A, Poor Mohammad Fatali, S, Mirzaei M. 2010. Study effects of some plant growth regulators on somatic embryogenesis and its histological stages in tomato (Lycopersicum esculentum L. var. Y). J Plant Sci Res, Ser 20. 4:49–56.
- McGahan MW. 1961. Studies on the seed of banana. I. Anatomy of the seed and embryo of Musa balbisiana. Am J Bot. 48(3):230–238. doi:10.2307/2439401.
- Meenakshi M, Shinde BN, Suprasanna P. 2011. Somatic embryogenesis from immature male flowers and molecular analysis of regenerated plants in banana, LAL KELA (AAA). J Fruit Ornamental Plant Res. 19(2):15–23.
- Morroquin CG, Paduscheeck C, Esalant JV. 1993. Somatic embryogenesis and plant regeneration through cell suspension in Musa acuminate. In Vitro Cell Dev Biol. 29:43–46.
- Murashige T, Skoog F. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 15(3):473–497. doi:10.1111/j.1399-3054.1962.tb08052.x.
- Navarro C, Escobedo RM, Mayo A. 1997. In vitro plant regeneration from embryogenic cultures of a diploid and a triploid, Cavendish banana. Plant Cell Tissue Organ Cult. 51(1):17–25. doi:10.1023/A:1005965030075.
- Novak FJ, Afza R, Vanuren M, Perea-Dallos M, Conger BV, Xiolang T. 1989. Somatic embryogenesis and plant regeneration in suspension cultures of dessert (AA, AAA) and cooking (AAB) bananas. Biotechnology. 46:125–135.
- Okole BN, Schulz FA. 1996. Micro-cross sections of banana and plantains (Musa spp.): morphogenesis and regeneration of callus and shoot buds. Plant Science. 116(2):185–195. doi:10.1016/0168-9452(96)04381-6.
- Osuji JO, Okoli BE, Ortiz R. 1996. An improved procedure for mitotic studies of the Eumusa section of the genus Musa L. (Musaceae). Infomusa. 5:12–14.
- Khalil SM, Cheah KT, Perez EM, Perez DA, Hu JS. 2002. Regeneration of banana (Musa spp. AAB cv. Dwarf Brazilian) via secondary somatic embryogenesis. Plant Cell Rep. 20(12):1128–1134. doi:10.1007/s00299-002-0461-0.
- Samson JA. 1982. Tropical Fruits. New York, NY: Longman.
- Sandoval J, Tapia A, Muller L, Villabobos V. 1991. Obervaciones sobre la variabilidad encontrada en plantas micropropagades de Musa cv. Falso Cuerno AAB. Fruits. 46:533–539.
- Sasson A. 1997. Importance of tropical and subtropical horticulture, future prospects of biotechnology in tropical and subtropical horticulture species. International Society for Horticultural Sciences (ISHS) Leiden. Acta Hort. 460:12–26.
- Stuessy TF. 1990. Plant Taxonomy. New York: Columbia University Press.
- Vuylsteke D. 1989. Shoot-Tip Culture for the Propagation, Conservation and Exchange of Musa germplasm. Practical Manuals for Handing Crops in Vitro. 2. Rome: The International Board for Plant Genetic Resources (INPGR).
- Vuylsteke D, Swenne R In:. 1992. Biotechnology approaches to plantain and banana improvement at IITA. In: Biotechnology Enhancing Research on Tropical Crops in Africa.
- Yeung EC. 1995. Structural and developmental patterns in somatic embryogenesis. In: Thorpe TA, editor. In Vitro Embryogenesis in Plants. Dordrecht: Kluwer Academic; p. 205–24.
